Abstract
Introduction
Patients with fibromyalgia syndrome (FMS) generally present with chronic widespread pain, accompanied by a range of additional and non-specific symptoms, such as fatigue, disturbed sleep, and cognitive dysfunction, which tend to increase with overall severity. Previous studies have shown moderate cognitive impairment in patients with FMS, but there are few valid data explicitly assessing the relevance of these findings to everyday functions, such as driving ability. Therefore, we studied patients with FMS to assess the impact of FMS on tests that predict driving ability.
Methods
Female patients with FMS were prospectively compared to a historical control group of healthy volunteers. The test battery comprised assessments of visual orientation, concentration, attention, vigilance, motor coordination, performance under stress, and reaction time.
Results
A total of 43 patients were matched to 129 controls. The results indicated that the patients’ psychomotor and cognitive performances were significantly non-inferior when compared to healthy controls (with 0.05% alcohol), with the exception of motor coordination. Patients and healthy controls showed an age-related decline in test performance. Correlations were smaller in patients and reversed for vigilance which was linked to a greater FMS symptom load in younger patients.
Conclusion
The results of the present study demonstrate that, in general, the driving ability of patients with FMS was not inferior to that of healthy volunteers based on a standardized computer-based test battery. However, variables, such as younger age, depression, anxiety, fatigue, pain, and poor motor coordination, likely contribute to the subjective perception of cognitive dysfunction in FMS.
Electronic supplementary material
The online version of this article (doi:10.1007/s40122-014-0028-0) contains supplementary material, which is available to authorized users.
Keywords: Chronic pain, Driving ability, Fibromyalgia, Neurocognitive function, Vienna test system
Introduction
Patients with fibromyalgia syndrome (FMS), a chronic widespread pain syndrome of unknown pathophysiology, generally show diffuse and generalized musculoskeletal pain accompanied by reduced pain thresholds (i.e., the presence of tender points) which may represent evidence of central sensitization. Other typical clinical features include sleep disturbance, fatigue, and morning stiffness [1]. Although the traditional classification criteria (American College of Rheumatology 2010) remain controversial [2], in agreement with other countries, 3–4% of the German population is affected with FMS [3].
In addition to pain and fatigue, FMS patients often present with substantial yet subjective physical and cognitive impairments [4–7]. Several studies have replicated these subjective reports using more objective and standard tests and have identified subtle cognitive impairments in patients with FMS [7–10]. No study data have explicitly assessed the clinical relevance of these findings, although significant impairments in the functions of daily living have been convincingly demonstrated for patients with FMS [11]. In addition, these patients are often treated in clinical practice with centrally acting analgesics and co-analgesics [12, 13], despite overall mixed evidence for their long-term effectiveness [14]. Thus, the cognitive functions of these patients may be impaired by both the syndrome and by its pharmacological treatment.
Safely driving a motor vehicle requires a complex interaction of operational, physical, cognitive, perceptual, and psychological skills. Because driving ability depends on complex psychomotor and cognitive skills, national and international recommendations indicate that tests should examine concentration, attention, reaction time, and an individual’s performance and orientation while under stress. These skills may be assessed by computerized neuropsychological test batteries, such as the Vienna test system [15], by driving simulators [16], or by road tests [17].
Because driving ability is considered an important aspect of self-determination and social participation, previous studies in the pain field using this methodology have focused on the effect of opioids on the complex psychomotor and cognitive functions of non-cancer patients under long-term opioid therapy and in drug addicts in opioid substitution programs [15, 18–23]. Only a few studies have examined the impact of chronic pain on complex psychomotor and cognitive tasks. According to a systematic review of the quality and generalizability of studies on the effects of opioids on driving and cognitive/psychomotor performance [24], high levels of pain may also affect aspects of these complex tasks. Moreover, it is important to note that cognitive impairment may occur in patients only when pain intensity levels exceed a certain threshold, which has been located between 64 and 71 of 100 [25]. However, even low-level pain may interfere with complex tasks, resulting in less accuracy and reduced performance speed [26]. Furthermore, any pain-related disruptions of attention seem to be worsened through such factors such as pain catastrophizing [27].
Driving ability can be compromised if one or more of the aspects involved in cognitive and psychomotor functions, such as attention, reaction time, visual orientation, perception, vigilance, and motor coordination, are impaired [15, 19, 20, 22]. To assess all of these factors, a battery of tests and statistical analyses complying with international recommendations, as well as with German legislation, were applied [28–33]. We included only patients with FMS currently off medication to assess the impact of FMS itself on tests that predict driving ability.
Methods
This was a prospective comparison of patients with FMS and a historical group of healthy volunteers. Subjects and controls were matched for age and sex, with three controls selected for every patient with FMS. The controls were not matched for educational level and social status, as the corresponding data for the control group (CG) were not available.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study. The study protocol and the consent form were approved by the ethics committee of the University of Cologne (Ethics Committee number 04-005).
Patients
Female outpatients older than 18 years suffering from FMS were enrolled. All patients included were required to fulfill the ACR 1990 criteria by Wolfe [1]. Patients were also questioned about the onset of chronic pain and when a diagnosis of FMS was made first. In addition, the diagnosis was confirmed by an experienced physician (FP) with regard to FMS when patients presented to our pain clinic. All patients had to stop pharmacological pain treatment with centrally acting agents after screening and at least 1 week prior to the actual computer test. Participants also required a valid driver’s license and the ability to speak, read and write German fluently. Patients were excluded from the study if they were receiving strong opioid analgesics, benzodiazepines, barbiturates, pharmacotherapy for a diagnosis of depression, or regular antihistamines. Patients with physical disabilities, severe psychiatric or neurological diseases or visual disorders that would prevent them from performing the tests were also excluded. All patients gave written, informed consent for their participation prior to any study procedures. The study protocol and the consent form were approved by the ethics committee of the University of Cologne (Ethics Committee number 04-005).
Control Group
Controls were randomly selected from a pool of healthy volunteers who had been tested between March 1996 and March 1998 (between 2 and 5 p.m.) at the Institute for Traffic Safety of the German Technical Monitoring Association in Cologne, Germany. This pool was part of a larger sample composed of healthy volunteers, with five men and five women for each year of age from 18 to 80 years. The control sample was described as representative of the normal German population with regard to activity, autonomy, and driving experience [30, 34].
Course of the Study
Initially, personal details (age, gender, etc.) and medical histories were recorded, including full details of their pain disease and the treatments they were receiving. Participants were also asked about their driving experience. Testing was performed between 12 and 3 p.m. at least 1 week after screening. Prior to testing, a urine sample was taken to screen for the use of drugs possibly not reported by the patients at the time of screening and to verify the discontinuation of medical treatment (urine was screened for opioids, antidepressants and anticonvulsants as well as benzodiazepines and hypnotics). Pain intensity was rated immediately before testing using a visual analog scale (VAS) ranging from 0 mm (no pain) to 100 mm (worst pain that can be imagined).
Questionnaires
For the assessment of relevant patient characteristics, we applied various questionnaires. The long form of Beck Depression Inventory (BDI) was used for the detection and assessment of depression, and the overall summary score was used for analysis [35].
The Short Form (SF)-36 contains 36 items and measures eight domains of health: physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF-36 yields a score for each of these domains, as well as summary scores for both physical and mental health and a single health utility index [36].
The short form of the McGill Pain Questionnaire (SF-MPQ) is one of the most widely used tests for the measurement of pain. It provides valuable information on the sensory, affective and evaluative dimensions of the pain experience and is capable of discriminating between different pain problems. The main component of the SF-MPQ consists of 15 descriptors (11 sensory; 4 affective), which are rated on an intensity scale as 0 = none, 1 = mild, 2 = moderate, or 3 = severe. Three pain scores are derived from the sum of the intensity rank values of the words chosen for sensory, affective, and total descriptors [37].
The Brief Pain Inventory (BPI) is a comprehensive instrument for pain assessment and has been validated in the German language [38]. The BPI measures both the intensity of pain (average, worst, and recurrent pain) and the interference of pain in the patient’s life (reactive dimension) [39].
The Brief Fatigue Inventory (BFI) was constructed to assess the severity and impairment from fatigue and has been validated in the German language [40]. The BFI measures the intensity of fatigue (average, worst, and current fatigue) and is interference with function.
The State-Trait Anxiety Inventory (STAI) is composed of separate self-reported scales to measure state anxiety (A-State) and trait anxiety (A-Trait). The A-Trait scale discriminates between subjects based on their disposition to respond to psychological stress with different levels of current or A-State intensity. Trait anxiety refers to relatively stable individual differences in anxiety proneness; in other words, this concept refers to differences among subjects in their tendencies to respond to situations perceived as threatening with elevations in A-State intensity [41].
Test Battery
The test battery followed the German national recommendations for tests used to determine driving ability [42], which require the assessment of (1) performance under pressure, (2) orientation, (3) concentration, (4) attention and (5) reaction time. Using our tests (described subsequently), each of these five domains was covered. Test batteries similar to the ones used in this study are commonly used for traffic delinquents in Germany; in this cases, permission to drive is usually denied if subjects fail on one or more of these tests, i.e., if the test result is below the 16th percentile of the age-independent reference range (based on data from a group of young healthy volunteers) [33]. In addition, previously validated tests for motor coordination and vigilance were also used in this study.
All tests were performed under standardized conditions with standardized instructions and in the same sequence using computerized test system (Vienna test system). There was no interaction between the tester and the tested subject, except in instances of failure understanding the test procedures, in which case the test instructions could be repeated and explained. Raw data, as well as combined scores, were measured. The entire test battery normally takes approximately 75 min to perform, with the vigilance test at the end taking 25 min.
Test for Reaction Time Under Pressure (Determination Test; DT)
Subjects were given a series of different audio–visual signals. Color symbols were presented on the screen and acoustic signals had to be answered using the corresponding buttons on the panel, while the symbols on the right or left sides of the screen also had to be answered using the corresponding pedals. The frequency of the stimuli was automatically adapted to the subject’s response. This test took 240 s, and the mean time to a correct response [i.e., mean reaction time (MRT)] was used as the score [43].
Attention Test (Cognitrone Test; COG)
Four pictures (numbers, letters, figures, etc.) were presented in a row, with another picture presented below. Subjects had to decide whether the lower picture matched any of the four pictures above. A new set of pictures was presented either after a response or automatically after 1.8 s. Up to 200 sets of pictures were used in this test. The number of correct and incorrect responses, and the MRT were recorded for a given subject. The overall score was calculated as the sum of the MRT and the square root of the product of the MRT and the wrong answers [44].
Test for Motor Coordination (2-Hand)
The subjects had to keep a signal on a track by simultaneously turning two steering wheels: one controlling horizontal movements and the other controlling vertical movements. The track consisted of three different sections (circle, V-shape, and L-shape) and had to be negotiated 19 times. The mean time taken to negotiate the track (T, in seconds) and the mean percentage of the total time during which the signal was off the track (Off %) were recorded. The score was calculated as [45].
Vigilance Test (VIG)
Subjects were presented with a circle consisting of separate small white spots on a dark monitor. A bright spot moved stepwise around this circle, similar to the hand of a watch. At long but irregular intervals, the spot sometimes missed one of the positions (i.e., jumped over one of the marker spots). When this occurred, the subjects had to press a button as quickly as possible. The number of mistakes (incorrect responses or undetected jumps) and the MRT were recorded. The score was calculated as the sum of the MRT and the square root of the product of the MRT and the sum of the missed and wrong answers [46].
Statistical Methods
Non-Inferiority
The study was designed as a non-inferiority trial; i.e., the objective was to demonstrate that patients with FMS do not perform significantly worse in the tests than controls. This means, that their performance was not inferior when compared to the control group.
In such non-inferiority trials, a clinically significant difference (delta, δ) from the standard outcome or performance must be defined. Typically, cognitive performance under the influence of defined levels of alcohol has been used as a standard to assess the degree of impairment induced by several drugs [47]. A blood alcohol level of ≥0.05% has been shown to cause a marked impairment in driving ability and is the threshold for being unfit to drive under German law [48–50]. In a previous study, the effect of different antidepressants on cognitive and psychomotor functions was compared using a computerized test battery similar to our study. During this study, patients received alcohol orally with a targeted blood concentration of 0.05%. The strongest impairment was observed in the testing of vigilance [49]; from the data in this previous study, an effect size of δ = 0.57 for the alcohol-related impairment of vigilance was calculated. Using this effect size, the raw values of the control group in our study were transformed to obtain virtual values that would be equivalent to test performance under the influence of a 0.05% blood alcohol level.
Using this assumption, non-inferiority in the test battery results of the fibromyalgia patients compared to controls could be interpreted as a performance significantly better than that of the CG with a blood alcohol concentration of 0.05%, which likely reflects sufficient fitness to drive. In accordance with prior studies from our group with patients on acute and chronic opioid therapy [15, 19, 20, 22, 51], we used 1:3 randomizations to increase the power of the study. With 43 patients and 129 controls, the power of this study was calculated to be close to 1 (one-sided t test, α = 0.05).
Each of the five tests used involved the recording of several parameters. To reduce the problem of multiple testing, one ‘relevant score’ for each test was defined prior to the analysis of the study data.
Passed Tests
Another method for evaluating driving ability using the various cognitive tests previously described is to assume unimpaired driving ability only if all test results are above the 16th percentile of the age-independent reference range [33]. Accordingly, for both subjects with FMS and healthy controls, the passing of the 16th percentile was recorded using the original raw data and not the transformed data reflecting the influence of alcohol.
Secondary Analysis
In the secondary analysis, the effects of age on the test results of the two groups were explored, as well as the rates of correct versus incorrect responses.
Statistical Testing
The Mann–Whitney U test was used because a normal distribution of the data could not be ascertained for all parameters. Because the direction of the expected change in a non-inferiority trial is defined, a one-sided P value <0.05 was regarded as significant. Data on passing rates or the 16th percentile were compared using the χ 2 square test. Significance tests for parameters other than the primary endpoints were exploratory in nature and were performed two-sided without adjustment for multiple comparisons. Correlational analyses were performed using Spearman’s rho correlation coefficient for non-parametric analysis. Unless stated otherwise, the results are presented as arithmetic means ± standard deviation (SD).
Results
A total of 43 female outpatients were enrolled in the study and matched with 129 controls in a 3:1 fashion. As an expected result of matching, the study and control populations included only women with similar age (Table 1). In patients, the mean duration of pain was 248 ± 164 months (range 36–720). The mean duration of time after the initial diagnosis of FMS was 71 ± 49 months (range 1–192). The mean current pain intensity was rated as 54 ± 21 mm with the VAS (0 mm: no pain; 100 mm: worst pain that can be imagined). Urine screening detected no unreported use of drugs and compliance with the withdrawal procedures. The data from all 43 patients were analyzed in accordance with the study protocol and the results of the tests are displayed in Table 2.
Table 1.
Demographic data
| Characteristic | FMS group (n = 43) | Control group (n = 129) |
|---|---|---|
| Gender (female), n (%) | 43 (100) | 138 (100) |
| Age (years), mean ± SD (range) | 55 ± 9 (38–75) | 55 ± 10 (38–75) |
| Duration of chronic pain (months), mean ± SD (range) | 248 ± 164 (36–720) | |
| Duration of FMS (months), mean ± SD (range) | 71 ± 49 (1–192) | |
| Current pain intensity (BPI), mean ± SD (range) | 54 ± 21 (1–89) | |
| Current driving (km/year), mean (range) | 8,349 (200–30,000) | |
| Driving experience (years), mean ± SD (range) | 32 ± 9 (4–45) | |
| BDI score, mean ± SD (range) | 16 ± 9 (3–45) | |
| BPI—average pain score, mean ± SD (range) | 95 ± 47 (16–168) | |
| BPI—interference score, mean ± SD (range) | 34 ± 13 (4–61) | |
| BFI—average score, mean ± SD (range) | 16 ± 6 (3–27) | |
| BFI—interference score, mean ± SD (range) | 27 ± 14 (1–52) | |
| SF-36 physical summary score, mean ± SD (range) | 28 ± 7 (16–45) | |
| SF-36 mental summary score, mean ± SD (range) | 40 ± 13 (17–69) | |
| STAI A-state score, mean ± SD (range) | 44 ± 10 (24–77) | |
| STAI A-trait score, mean ± SD (range) | 48 ± 12 (29–76) |
A-state state anxiety, A-trait trait anxiety, BFI Brief Fatigue Inventory, BPI Brief Pain Inventory, FMS fibromyalgia syndrome, SF-36 short form 36, STAI State-Trait Anxiety Inventory
Table 2.
Psychomotor and cognitive performance measures including the calculated score of the different tests
| Parameter | FMS group | Control group | |
|---|---|---|---|
| Raw values | Raw values + δ | ||
| DT (n) | 43 | 129 | 129 |
| Correct answers (n) | 210.8 ± 36.987 # | 214.2 ± 37.3 | 192.9 ± 36.5 |
| Wrong answers (n) | 6.7 ± 5.5 ## | 9.4 ± 9.0 | 14.5 ± 9.0 |
| Omitted answers (n) | 11.3 ± 6.2 | 9.4 ± 5.2 | 12.3 ± 5.2 |
| Score = MRT (s) score | 0.9 ± 0.2 # | 0.9 ± 0.1 | 1.0 ± 0.1 |
| COG (n) | 43 | 129 | 129 |
| Correct answers (n) | 48.3 ± 10.5 | 52.4 ± 10.9 | 46.2 ± 10.9 |
| Wrong answers (n) | 18.6 ± 7.8 ## | 31.6 ± 16.7 | 41.1 ± 16.7 |
| MRT (s) | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Score | 5.8 ± 1.0 ## | 6.8 ± 1.3 | 7.6 ± 1.3 |
| 2-Hand (n) | 43 | 129 | 129 |
| Mean time (s) | 46.6 ± 24.0 ## | 47.0 ± 19.8 | 58.3 ± 19.8 |
| Time off track (%) | 3.8 ± 3.3 | 2.0 ± 1.6 | 2.9 ± 1.6 |
| Score | 8.4 ± 4.5 | 6.7 ± 3.2 | 8.5 ± 3.2 |
| VIG (n) | 43 | 129 | 129 |
| Correct answers (n) | 95.4 ± 8.8 ## | 97.3 ± 4.2 | 94.9 ± 4.2 |
| Wrong answers (n) | 2.2 ± 3.0 ## | 4.9 ± 6.8 | 8.8 ± 6.8 |
| Omitted answers (n) | 4.6 ± 8.8 ## | 2.7 ± 4.2 | 5.1 ± 4.2 |
| MRT (s) | 0.5 ± 0.1 ## | 0.5 ± 0.1 | 0.6 ± 0.1 |
| Score | 2.0 ± 1.5 ## | 2.2 ± 1.2 | 2.8 ± 1.2 |
2-Hand test for motor coordination, COG Cognitrone test, DT determination test, FMS fibromyalgia syndrome, MRT mean reaction time, VIG vigilance test
The results are presented as arithmetic means ± the standard deviation. The results of the control group are presented as raw values, as are the calculated results of the effect of impairment due to an alcohol level of 0.05% [raw values transformed by δ (calculated effect size for alcohol-related impairment of vigilance) and the variance of the item in the whole sample]
Bold: Significant non-inferiority of the FMS group compared to the control group + δ (# P < 0.05, ## P < 0.01)
DT
Significant non-inferiority of the FMS group was detected with regards to the number of “correct answers”, the number of “wrong answers” and the “MRT” (=summary score) compared to the CG + δ (P < 0.05). The number of “omitted answers” was higher in the CG + δ than the FMS group, but this difference was not statistically significant (Table 2).
COG
Significant non-inferiority of the FMS group was detected with regards to the number of “wrong answers” and the overall score compared to the CG + δ (P < 0.01). Although the number of “correct answers” for the FMS group was higher compared to the CG + δ, this difference did not reach statistical significance. Therefore, non-inferiority could not be demonstrated for this item. The MRT for performing this test was higher and therefore slower in the FMS group compared to the CG + δ (P < 0.01 with a two-sided Mann–Whitney U test, Table 2). The low number of wrong answers likely explains the non-inferiority in the overall score, as it compensated for the slowest reaction times.
2-Hand
The average time needed to complete the test was significantly non-inferior in the FMS group (P < 0.01). However, the analysis of the percentage of “time off track” failed to show a significant non-inferiority of the FMS group compared to the CG + δ. In fact, FMS patients even tended to have a longer time off track (in %) when compared to the CG + δ. Accordingly, the calculated test score also failed to demonstrate statistically significant non-inferiority when compared to the CG + δ (Table 2).
VIG
The difference in the number of “correct answers”, “wrong answers”, “omitted answers”, the MRT and the scores for the FMS group proved to be significantly non-inferior compared to the CG + δ (P < 0.01; Table 2).
Passed Tests
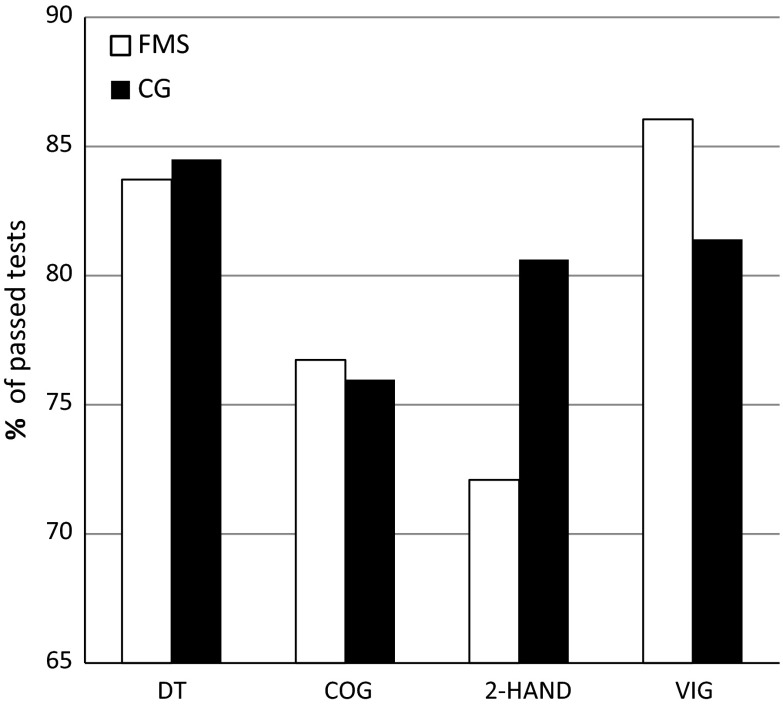
The percentages of patients who passed the single tests, that is, those whose relevant test scores were above the 16th percentile compared to an age-independent reference group (RG), are displayed in Fig. 1. The subjects from the RG passed an average of 3.2 (SD = 1.4) of the four tests, which was similar to the patients from the FMS group, with 3.2 (SD = 1.3) passed tests. The percentage of subjects passing all four tests was 60% for the RG and 51% for the FMS group (P = 0.08). In the DT and 2-Hand, fewer patients from the FMS group reached the 16th percentile compared to the RG. The percentage of patients passing the test was higher for the FMS group in terms of the COG but the same for the VIG compared to the RG. None of these differences were statistically significant.
Fig. 1.
Percentage of passed tests. Percentage of all passed tests (test score above the 16th percentile). 2-Hand test for motor coordination, CG age-independent reference group, COG Cognitrone test, DT determination test, FMS fibromyalgia syndrome, MRT mean reaction time, VIG vigilance test
Secondary Analysis
To assess the potentially different effects of age on the results of the cognitive testing, age was correlated with all four primary scores in patients with FMS and in the CG + δ (Table 3). The correlational relationships were generally positive for the two groups, with coefficients being smaller in the FMS group. The VIG score showed a negative relationship in patients with FMS compared to a weak, but positive relationship in the CG + δ. This indicates decreases in test performance (greater scores) with increasing age for most parameters, independent of the group status. However, the relatively greater overall disease burden in younger FMS patients compared to both older patients and age-matched healthy controls may explain the discrepant VIG finding and the smaller correlation coefficients. This notion is supported by the significantly negative correlations between age and BDI score (ρ = −0.44, P < 0.003), trait anxiety (STAI-T, ρ = −0.43, P < 0.004), and fatigue scores (BFI, ρ = −0.42, P < 0.004) but not pain scores (BPI, ρ = −0.25, P = 0.15) in patients with FMS.
Table 3.
Correlation of age with the four main scores for the two groups (Spearman Rho, level of significance)
| Score | FMS group | Control group |
|---|---|---|
| DT score | 0.44, P < 0.003 | 0.75, P < 0.0001 |
| COG score | 0.30, P < 0.05 | 0.42, P < 0.0001 |
| 2-Hand score | 0.43, P < 0.004 | 0.55, P < 0.0001 |
| VIG (n) | −0.38, P < 0.013 | 0.20, P < 0.025 |
2-Hand test for motor coordination, COG Cognitrone test, DT determination test, FMS fibromyalgia syndrome, VIG vigilance test
The ratio of wrong to correct answers is shown in Table 4. Patients with FMS showed overall fewer false responses in the VIG, COG, and DT tests, even when corrected for the number of correct responses. This result was in contrast to the increase observed for the time and percentage of “time off track” observed in FMS patients in the 2-Hand test.
Table 4.
Percentage of wrong answers in % of correct answers
| Parameter | FMS group | Control group | |
|---|---|---|---|
| Raw values | Raw values + δ | ||
| DT (n) | 43 | 129 | 129 |
| Wrong/correct (%) | 6.7 ± 5.5 # | 9.4 ± 9.0 | 14.5 ± 9.0 |
| COG (n) | 43 | 129 | 129 |
| Wrong/correct (%) | 18.6 ± 7.8 ## | 31.6 ± 16.7 | 41.1 ± 16.7 |
| VIG (n) | 43 | 129 | 129 |
| Wrong/correct (%) | 2.2 ± 3.0 ## | 4.9 ± 6.8 | 8.8 ± 6.8 |
δ calculated effect size for alcohol-related impairment of vigilance, COG Cognitrone test, DT determination test, FMS fibromyalgia syndrome, VIG vigilance test
Bold: Significant non-inferiority of the FMS group compared to the control group + δ (# P < 0.05, ## P < 0.01)
Discussion
The present study examined the psychomotor and cognitive performance of FMS patients to predict their driving ability. Using tests to assess impairment of driving ability in clinical practice for all sorts of conditions we failed to show a large and relevant problem in our sample of patients with FMS. The relevant summary scores of the DT (reaction time), COG (attention), and the vigilance score were significantly non-inferior compared to the control group (CG + δ). Only in the 2-Hand test (motor coordination) could significant non-inferiority of the score not be reached due to the results for “time off track” in the FMS group. In addition, patients with FMS and control subjects showed similar rates of passed tests.
The impairment of cognitive functions in FMS patients is a core symptom reported by patients, but the severity and clinical relevance of these impairments have been questioned [52]. Several authors reported a significant impairment in cognitive performance in patients with FMS, with greatest agreement in tests of working memory performance [7, 53–55]; however, other studies did not find evidence of cognitive impairment in patients with FMS [56–58] and highlighted the importance of examining the influence of co-variables, such as pain severity, fatigue, depression, and required effort, on cognitive performance in FMS [10, 58].
Despite the evidence for poor performance in functions of daily living in FMS [11], the contribution of cognitive dysfunction has not been established. This study of driving-related abilities demonstrates one potential avenue to address this clinically relevant interaction. In contrast to many other studies, and following the recommendations of some authors [47], the effect of alcohol was used in our study as a “real-world” endpoint and an important benchmark for clinical relevance, as its impact on complex psychomotor performance is well documented and quantifiable. The blood alcohol concentration of 0.05% blood alcohol was chosen as a benchmark according to the German legislation and previous studies [15, 19, 22, 34]. From this perspective, the current results indicated that irrespective of the levels of individual symptomatology and severity, patients with FMS as a group did not perform worse than the normal population under the influence of a 0.05% blood alcohol level. This finding does not exclude the presence of cognitive dysfunction in individual patients with FMS, but rather indicates that these changes do not result in relevant impairment of driving ability.
Published studies examining FMS and its influence on cognition and psychomotor performance show high variability related to the various methodological approaches used, ranging from neuropsychological and computerized tests to neuroimaging, etc. [8, 53–55, 57, 59]. Computerized test batteries can be designed to measure different aspects related to driving ability (e.g., reaction time, vigilance, psychomotor coordination), and their high capacity for standardization helps to minimize observer biases. On the other hand, the most of the neuropsychological tests, especially when using single tests, are not sufficient for the effective prediction of potential outcome measures like, for example, fatalities while driving a car [60]. However, as proposed by Lincoln et al. [61] a combination of cognitive tests might overcome this limitation. In our study, a combination of different tests, as proposed by the German legislation, has been used to assess fitness of driving. To our knowledge, there has only been one other study using a computerized neuropsychological test battery in patients with FMS, and it did not demonstrate significant differences between FMS patients and controls [57].
A small yet interesting finding of this study relates to the poorer results in the 2-Hand test. A recent study [62] described impaired dexterity and fine motor control of the hands in patients with FMS with likewise an increased time demand and poorer hand function. This dysfunction was not linked to individual symptom load and was related to altered central motor control.
Several objective measures of cognitive function have shown subtle differences between FMS patients and controls. Most authors agree that memory deficits in FMS patients are evident and result in subjective impairments [4, 7–9, 53–55]. Patients with FMS have been shown to have poorer cognitive performance than carefully matched adults of similar age and to perform more like to people 20 years older [63].
Interestingly, in our study age had an overall negative effect in both groups but correlation coefficients tended to be smaller in FMS patients compared to healthy controls for most parameters. Vigilance even demonstrated a different pattern with greater relative impairment in younger FMS patients and more pronounced symptoms that may indirectly affect memory function, which was not tested in our study. This interpretation of a possible differential effect of age is supported by findings that older patients show lower reductions of health-related quality of life than younger and middle-aged patients, especially on physical and social dimensions [64].
The results of previous studies on attention are not uniform [65]. In our study, the FMS patients’ performance in attention tests (COG and VIG) was significantly non-inferior to that of the CG. The tests used in our study evaluated attention from different perspectives; the VIG test is based on the assessment of attention in the form of sustained vigilance in a low-stimulus observation situation, whereas the COG test assesses attention and concentration through comparisons of figures with regard to their congruence. Glass et al. [8] tested the processing speed of patients with FMS during a task (similar to the COG, e.g., “are two strings of letters identical or different?”), and these authors found that patients with FMS performed just as well as the age-matched controls and were significantly faster than the older controls. This result was supported by the study of Dick et al. [53], which compared the attentional functioning of FMS patients to that of pain-free controls and of rheumatoid arthritis and musculoskeletal pain patients. FMS patients performed similar to other patient groups in any of the investigated domains of attentional and cognitive functioning, indicating comparable patterns of attentional performance among chronic pain patients with different rheumatologic disorders. Observed attentional deficits compared to controls may thus be related more to the presence of chronic pain than to any specific disease-related factor [53].
The speed of processing is fundamental to nearly all cognitive abilities and is viewed as a global indicator of neurobiological function [7]. Park et al. [7] found no evidence for a deficit in the speed of processing in their study and concluded that cognitive dysfunction associated with FMS thus cannot be viewed simply as an accelerated form of cognitive aging. The only conflicting finding in this study with regard to speed of processing concerned the MRT in the COG test, which was slowest (or worst) in the FMS group. However, exploratory analysis revealed fewer mistakes in most of the tests by the patients with FMS, which may reflect cognitive strategies that result in a slower MRT for the most complex tasks (COG), which require most attention. This may also reflect a “rising to the occasion” or marshaling of resources to perform well on relatively short cognitive tests [66].
Pain and many of the common comorbid symptoms of FMS, such as depression, fatigue, and sleep disturbances, can have potentially negative impacts on cognitive function [63]. Due to the lack of data available for the CG in our study and general non-inferiority, we were unable to address this relationship. However, Suhr [10] found that depression was significantly related to memory performance and that self-reported fatigue was related to psychomotor speed. Miro et al. [59] reported slower overall reaction time, greater interference and disturbed vigilance in part related to depression, anxiety, and sleep quality. Similarly, Dick et al. [53] found a relationship between depression, anxiety, and cognitive function. In contrast, in the study of Luerding et al. [55], neither BDI scores nor pain scores were significantly correlated with neuropsychological performance. Furthermore, Park et al. [7] failed to detect a correlation between depression and performance on any of the cognitive measures. The exploratory results of the current study indicate a potentially greater negative effect of these comorbid factors (on vigilance) in younger patients and provide a potential explanation for the overall discrepant findings. This highlights the problem of subject selection and characterization in studies of cognitive function in FMS.
In a recent study, Landrø et al. [67] reported of the subjective and objective neuropsychological functioning measured by a questionnaire and a neuropsychological test battery in a heterogeneous sample of 72 patients from a tertiary multidisciplinary pain clinic. More than 20% of patients scored below cut-off in the objective tests. A larger proportion of patients with generalized and neuropathic pain performed below this cut-off, whereas patients with localized pain exhibited impaired function to a lesser degree. Five out of seven tests were significantly correlated to subjective impairment. Unfortunately, in the present study no specific assessment of subjective neuropsychological symptoms was performed. In addition, comparison of the two populations is difficult, as our FMS patients were off medication and patient’s in the study of Landrø et al. [67] had different underlying pain diagnoses and were in part on an analgesic treatment. All FMS patients were able to withdraw medication and were driving actively, with 75% driving more than 4,000 km/year.
The generalizability of the results of our study for FMS patients thus is limited for several reasons. First, patients willing to withdraw from medication or those who do not require any medication may represent a subgroup with less severe disease. Second, the unknown educational level of the CG may have had an impact on the test results of our study, as we could not exclude a relevant group difference (patients vs. control) in educational level. Third, variability in results does indicate greater (possibly more relevant) impairment in selected individuals. Fourth, standardized test batteries only represent selected aspects of driving ability. Actual driving performance in a real-world setting may require practical driving tasks. Longer testing duration may have revealed more cognitive dysfunction due to greater fatigability.
Conclusion
Using typical tests to assess impairment of driving ability in clinical practice for all sorts of conditions, we failed to show a large and general problem in sample of unmedicated patients suffering from FMS still actively driving. However, the significant variability of the test results indicates relevant FMS-related impairments in some individuals. Variables such as younger age, depression, anxiety, fatigue, pain, and poor motor coordination likely contribute to the subjective perception of cognitive dysfunction in FMS patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by the KölnFortune Program, Faculty of Medicine, University of Cologne, Cologne, Germany. The authors wish to thank Natalie Schlegel and Monika Thomm for their technical assistance. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Rainer Sabatowski and Frank Petzke contributed equally to this work.
Conflict of interest
Sergey Shmygalev, Oguzhan Dagtekin, Hans Jürgen Gerbershagen, Hanke Marcus, Martin Jübner, Rainer Sabatowski, and Frank Petzke declare that they have no conflicts of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study. The study protocol and the consent form were approved by the ethics committee of the University of Cologne (Ethics Committee number 04-005).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Contributor Information
Sergey Shmygalev, Email: sergey.shmygalev@uniklinikum-dresden.de.
Frank Petzke, Email: frank.petzke@med.uni-goettingen.de.
References
- 1.Wolfe F, Smythe HA, Yunus MB, The American College of Rheumatology et al. Criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.Eich W, Hauser W, Arnold B, et al. Fibromyalgia syndrome. Definition, classification, clinical diagnosis and prognosis. Schmerz. 2012;26(3):247–258. doi: 10.1007/s00482-012-1169-x. [DOI] [PubMed] [Google Scholar]
- 3.Schochat T, Raspe H. Descriptive and analytic epidemiology of criteria for generalized fibromyalgia syndrome in the female population of Bad Sackingen. Z Rheumatol. 1998;57:262–264. doi: 10.1007/s003930050108. [DOI] [Google Scholar]
- 4.Grisart J, Van der Linden M, Masquelier E. Controlled processes and automaticity in memory functioning in fibromyalgia patients: relation with emotional distress and hypervigilance. J Clin Exp Neuropsychol. 2002;24(8):994–1009. doi: 10.1076/jcen.24.8.994.8380. [DOI] [PubMed] [Google Scholar]
- 5.Komaroff AL, Fagioli LR, Doolittle TH, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med. 1996;101(3):281–290. doi: 10.1016/S0002-9343(96)00174-X. [DOI] [PubMed] [Google Scholar]
- 6.Neeck G, Crofford LJ. Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin N Am. 2000;26(4):989–1002. doi: 10.1016/S0889-857X(05)70180-0. [DOI] [PubMed] [Google Scholar]
- 7.Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44(9):2125–2133. doi: 10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Glass JM, Park DC. Cognitive dysfunction in fibromyalgia. Curr Rheumatol Rep. 2001;3(2):123–127. doi: 10.1007/s11926-001-0007-4. [DOI] [PubMed] [Google Scholar]
- 9.Grace GM, Nielson WR, Hopkins M, Berg MA. Concentration and memory deficits in patients with fibromyalgia syndrome. J Clin Exp Neuropsychol. 1999;21(4):477–487. doi: 10.1076/jcen.21.4.477.876. [DOI] [PubMed] [Google Scholar]
- 10.Suhr JA. Neuropsychological impairment in fibromyalgia: relation to depression, fatigue, and pain. J Psychosom Res. 2003;55(4):321–329. doi: 10.1016/S0022-3999(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 11.Amris K, Waehrens EE, Jespersen A, Bliddal H, Danneskiold-Samsoe B. Observation-based assessment of functional ability in patients with chronic widespread pain: a cross-sectional study. Pain. 2011;152(11):2470–2476. doi: 10.1016/j.pain.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Berger A, Sadosky A, Dukes E, Martin S, Edelsberg J, Oster G. Characteristics and patterns of healthcare utilization of patients with fibromyalgia in general practitioner settings in Germany. Curr Med Res Opin. 2008;24(9):2489–2499. doi: 10.1185/03007990802316550. [DOI] [PubMed] [Google Scholar]
- 13.Marschall U, Arnold B, Hauser W. Treatment and healthcare costs of fibromyalgia syndrome in Germany: analysis of the data of the Barmer health insurance (BEK) from 2008–2009. Schmerz. 2011;25(4):402–404 (pp. 406–410). [DOI] [PubMed]
- 14.Sommer C, Hauser W, Alten R, et al. Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline. Schmerz. 2012;26(3):297–310. doi: 10.1007/s00482-012-1172-2. [DOI] [PubMed] [Google Scholar]
- 15.Sabatowski R, Schwalen S, Rettig K, Herberg KW, Kasper SM, Radbruch L. Driving ability under long-term treatment with transdermal fentanyl. J Pain Symptom Manag. 2003;25(1):38–47. doi: 10.1016/S0885-3924(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 16.Strumpf M, Köhler A, Zenz M, Willweber-Strumpf A, Dertwinkel R, Donner B. Opioide und Fahrtüchtigkeit. Schmerz. 1997;11(4):233–240. doi: 10.1007/s004820050091. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuijzen DS, van Wijck AJ, Wille F, et al. Effect of chronic nonmalignant pain on highway driving performance. Pain. 2006;122(1–2):28–35. doi: 10.1016/j.pain.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain. 2005;21(4):345–352. doi: 10.1097/01.ajp.0000125244.29279.c1. [DOI] [PubMed] [Google Scholar]
- 19.Dagtekin O, Gerbershagen HJ, Wagner W, Petzke F, Radbruch L, Sabatowski R. Assessing cognitive and psychomotor performance under long-term treatment with transdermal buprenorphine in chronic noncancer pain patients. Anesth Analg. 2007;105(5):1442–1448. doi: 10.1213/01.ane.0000281078.65585.1e. [DOI] [PubMed] [Google Scholar]
- 20.Gaertner J, Radbruch L, Giesecke T, et al. Assessing cognition and psychomotor function under long-term treatment with controlled release oxycodone in non-cancer pain patients. Acta Anaesthesiol Scand. 2006;50(6):664–672. doi: 10.1111/j.1399-6576.2006.01027.x. [DOI] [PubMed] [Google Scholar]
- 21.Jamison RN, Schein JR, Vallow S, Ascher S, Vorsanger GJ, Katz NP. Neuropsychological effects of long-term opioid use in chronic pain patients. J Pain Symptom Manag. 2003;26(4):913–921. doi: 10.1016/S0885-3924(03)00310-5. [DOI] [PubMed] [Google Scholar]
- 22.Shmygalev S, Damm M, Weckbecker K, Berghaus G, Petzke F, Sabatowski R. The impact of long-term maintenance treatment with buprenorphine on complex psychomotor and cognitive function. Drug Alcohol Depend. 2011;117(2–3):190–197. doi: 10.1016/j.drugalcdep.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Tassain V, Attal N, Fletcher D, et al. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non-cancer pain. Pain. 2003;104(1–2):389–400. doi: 10.1016/S0304-3959(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 24.Mailis-Gagnon A, Lakha SF, Furlan A, Nicholson K, Yegneswaran B, Sabatowski R. Systematic review of the quality and generalizability of studies on the effects of opioids on driving and cognitive/psychomotor performance. Clin J Pain. 2012;28(6):542–555. doi: 10.1097/AJP.0b013e3182385332. [DOI] [PubMed] [Google Scholar]
- 25.Kuhajda MC, Thorn BE, Klinger MR, Rubin NJ. The effect of headache pain on attention (encoding) and memory (recognition) Pain. 2002;97(3):213–221. doi: 10.1016/S0304-3959(01)00488-2. [DOI] [PubMed] [Google Scholar]
- 26.Harman K, Ruyak P. Working through the pain: a controlled study of the impact of persistent pain on performing a computer task. Clin J Pain. 2005;21(3):216–222. doi: 10.1097/00002508-200505000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Vancleef LM, Peters ML. Pain catastrophizing, but not injury/illness sensitivity or anxiety sensitivity, enhances attentional interference by pain. J Pain. 2006;7(1):23–30. doi: 10.1016/j.jpain.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.International Council on Alcohol Drugs and Traffic Safety (ICADTS). Guidelines on experimental studies undertaken to determine a medicinal drug’s effect on driving or skills related to driving. Ontario: ICADTS; 1999.
- 29.Bundesanstalt für Straßenwesen . Begutachtungs-Leitlinien zur Kraftfahrereignung. Bergisch Gladbach: Wirtschaftsverlag NW; 2000. [Google Scholar]
- 30.Herberg K. Untersuchung der Entwicklung der sicherheitsrelevanten Leistungsfähigkeit mit dem Lebensalter. Cologne: TÜV Kraftfahrt GmbH-Institut für Verkehrssicherheit; 1998. [Google Scholar]
- 31.Hilgers R, Friedel B, Berghaus G. Äquivalenztestung im Rahmen experimenteller Untersuchungen zur Fahrtüchtigkeit. Bundesanstalt für Straßenwesen Kongreßbericht 1997 der Deutschen Gesellschaft für Verkehrsmedizin eV. Bergisch Gladbach: Wirtschaftsverlag NW; 1998. pp. 130–134.
- 32.Kroj G. Perspektiven für Forschung und Begutachtung aus psychologischer Sicht. Drogen und Verkehrssicherheit. Bergisch Gladbach: Wirtschaftsverlag NW; 1995. pp. 78–82.
- 33.Kroj G. Psychologisches Gutachten Kraftfahreignung. Bonn: Deutscher Psychologenverlag; 1995. [Google Scholar]
- 34.Herberg K. Age-related changes in aspects of performance relevant to safety. Euro J Geriatr. 2000;2:18–26. [Google Scholar]
- 35.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 36.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 38.Radbruch L, Loick G, Kiencke P, et al. Validation of the German version of the Brief Pain Inventory. J Pain Symptom Manag. 1999;18(3):180–187. doi: 10.1016/S0885-3924(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 39.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 40.Radbruch L, Sabatowski R, Elsner F, Everts J, Mendoza T, Cleeland C. Validation of the German version of the brief fatigue inventory. J Pain Symptom Manag. 2003;25(5):449–458. doi: 10.1016/S0885-3924(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 41.Spielberg C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 42.Bundesregierung Deutschland. Verordnung über die Zulassung von Personen zum Straßenverkehr und zur Änderung straßenverkehrsrechtlicher Vorschriften: Bundesgesetzblatt; 1998. pp. 2214–2262.
- 43.Wiener Determinationstest, version 2.00. In: Schuhfried GmbH, editor. Mödling: Dr. Schuhfried GmbH; 1996.
- 44.Cognitrone-Handbuch. In: Schuhfried GmbH, editor. Mödling: Dr. Schuhfried GmbH; 1994.
- 45.Zweihand-Koordination, version 4.00. In: Schuhfried GmbH, editor. Mödling: Dr. Schuhfried GmbH, 1995.
- 46.Vigilanz, Version 5.00. In: S GmbH, editor. Mödling: Dr. Schuhfried GmbH, 1995.
- 47.Thapar P, Zacny JP, Thompson W, Apfelbaum JL. Using alcohol as a standard to assess the degree of impairment induced by sedative and analgesic drugs used in ambulatory surgery. Anesthesiology. 1995;82(1):53–59. doi: 10.1097/00000542-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Bartl G, Lager F, Domesle L. Test performance with minimal alcoholic intoxication. Blutalkohol. 1996;33(1):1–16. [PubMed] [Google Scholar]
- 49.Herberg KW. Antidepressives and traffic safety. Fortschr Neurol Psychiatr. 1994;62(Suppl 1):24–28. doi: 10.1055/s-2007-1002358. [DOI] [PubMed] [Google Scholar]
- 50.Richter R, Hobi V. The impairment of the ability to drive with blood alcohol concentrations of 0.5 per mille. A review of the literature. Schweiz Med Wochenschr. 1975;105:884–890. [PubMed] [Google Scholar]
- 51.Sabatowski R, Scharnagel R, Gyllensvard A, Steigerwald I. Driving ability in patients with severe chronic low back or osteoarthritis knee pain on stable treatment with tapentadol prolonged release: a multicenter, open-label, phase 3b trial. Pain Ther. 2014;3(1):17–29. doi: 10.1007/s40122-014-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 53.Dick B, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002;47(6):639–644. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- 54.Leavitt F, Katz RS. Normalizing memory recall in fibromyalgia with rehearsal: a distraction-counteracting effect. Arthritis Rheum. 2009;61(6):740–744. doi: 10.1002/art.24559. [DOI] [PubMed] [Google Scholar]
- 55.Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain–cognition interaction. Brain. 2008;131(Pt 12):3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 56.Mohs R, Mease P, Arnold LM, et al. The effect of duloxetine treatment on cognition in patients with fibromyalgia. Psychosom Med. 2012;74(6):628–634. doi: 10.1097/PSY.0b013e31825b9855. [DOI] [PubMed] [Google Scholar]
- 57.Walitt B, Roebuck-Spencer T, Bleiberg J, Foster G, Weinstein A. Automated neuropsychiatric measurements of information processing in fibromyalgia. Rheumatol Int. 2008;28(6):561–566. doi: 10.1007/s00296-007-0487-2. [DOI] [PubMed] [Google Scholar]
- 58.Walteros C, Sanchez-Navarro JP, Munoz MA, Martinez-Selva JM, Chialvo D, Montoya P. Altered associative learning and emotional decision making in fibromyalgia. J Psychosom Res. 2011;70(3):294–301. doi: 10.1016/j.jpsychores.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 59.Miro E, Lupianez J, Hita E, Martinez MP, Sanchez AI, Buela-Casal G. Attentional deficits in fibromyalgia and its relationships with pain, emotional distress and sleep dysfunction complaints. Psychol Health. 2011;26(6):765–780. doi: 10.1080/08870446.2010.493611. [DOI] [PubMed] [Google Scholar]
- 60.Sabatowski R, Mordenti G, Miceli L. Opioids and driving ability: current data do not support one opioid being more favorable than another. Pain Pract. 2014;14(2):196–197. doi: 10.1111/papr.12149. [DOI] [PubMed] [Google Scholar]
- 61.Lincoln NB, Radford KA, Lee E, Reay AC. The assessment of fitness to drive in people with dementia. Int J Geriatr Psychiatry. 2006;21(11):1044–1051. doi: 10.1002/gps.1604. [DOI] [PubMed] [Google Scholar]
- 62.Perez-de-Heredia-Torres M, Martinez-Piedrola RM, Cigaran-Mendez M, Ortega-Santiago R, Fernandez-de-Las-Penas C. Bilateral deficits in fine motor control ability and manual dexterity in women with fibromyalgia syndrome. Exp Brain Res. 2013;226(1):137–143. doi: 10.1007/s00221-013-3417-4. [DOI] [PubMed] [Google Scholar]
- 63.Glass JM. Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum Dis Clin N Am. 2009;35(2):299–311. doi: 10.1016/j.rdc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Campos RP, Vazquez MI. The impact of Fibromyalgia on health-related quality of life in patients according to age. Rheumatol Int. 2013;33(6):1419–1424. doi: 10.1007/s00296-012-2568-0. [DOI] [PubMed] [Google Scholar]
- 65.Reyes Del Paso GA, Pulgar A, Duschek S, Garrido S. Cognitive impairment in fibromyalgia syndrome: the impact of cardiovascular regulation, pain, emotional disorders and medication. Eur J Pain. 2012;16(3):421–429. doi: 10.1002/j.1532-2149.2011.00032.x. [DOI] [PubMed] [Google Scholar]
- 66.Ambrose KR, Gracely RH, Glass JM. Fibromyalgia dyscognition: concepts and issues. Reumatismo. 2012;64(4):206–215. doi: 10.4081/reumatismo.2012.206. [DOI] [PubMed] [Google Scholar]
- 67.Landrø NI, Fors EA, Vapenstad LL, Holthe O, Stiles TC, Borchgrevink PC. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? Pain. 2013;154(7):972–977. doi: 10.1016/j.pain.2013.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.