Abstract
Introduction
In treatment of prosthetic vascular graft infection (PVGI), appropriate antimicrobial treatment is crucial for controlling the septic process and preventing re-infection of the new graft. Glycopeptides are the mainstay of treatment for device-related infections by methicillin-resistant Staphylococcus aureus strains, but with some limitations, especially concerning vancomycin-intermediate and glycopeptide-intermediate S. aureus. We report our experience using a high dose of daptomycin (DAP) for treatment of PVGI.
Methods
We reviewed medical reports of 26 patients treated with high doses of DAP (>8 mg/kg) and beta-lactams/aminosides for PVGI, defined as positive bacterial culture of intraoperative specimens or blood samples and/or clinical, biological, and radiological signs of infection. Clinical success was defined by resolution of all clinical signs at the end of follow-up, without the need for additional antibiotic therapy, and/or negative culture in case of new surgery.
Results
Cultures of intraoperative samples were positive in 21 patients (80.8%). Blood and intraoperative cultures were concomitantly positive in 10 patients. The main microorganism identified in microbiological samples was S. aureus (n = 18). Surgery was performed in 23 patients (88.4%). The mean duration of the DAP regimen was 12.3 ± 11.9 days. DAP was discontinued in 26 patients [need to switch to microbiological results (n = 19), bacterial pneumonia (n = 2), and increased creatine phosphokinase levels (n = 4)]. One patient had myalgia, while 9 received concomitant statins.
Conclusion
High-dose DAP therapy shows a satisfactory toxicity profile even in severely ill patients with multiple comorbidities, and may favorably compete with vancomycin, especially concerning the risk of induced nephrotoxicity.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-014-0035-9) contains supplementary material, which is available to authorized users.
Keywords: Biofilm, Daptomycin, Prosthetic vascular graft infection, Staphylococcus aureus, Staphylococcal infection
Introduction
Prosthetic vascular graft infection (PVGI) is a severe complication of vascular surgery that carries high morbidity and mortality rates [1–3]. A surgical approach, combining excision of the graft, complete debridement of devitalized and infected tissues and maintenance of vascular flow to the distal bed, is consistently required, although some patients are not fit for operative intervention [1, 4]. The choice of antimicrobial agents as empirical or definitive therapy and the duration of treatment remain unclear. One major challenge lies in the rapid development of bacterial biofilms on intravascular leads that are relatively impermeable to antimicrobial treatment [5, 6]. Thus, the appropriate antimicrobial treatment is crucial for controlling the septic process and the risk of graft rupture. Since most PVGIs are due to staphylococci or, to a lesser extent, to Gram-negative bacilli, daptomycin (DAP) could be an option in association with beta-lactams.
DAP is a cyclic lipopeptide with rapid concentration-dependent bacterial activity against Gram-positive pathogens. DAP has been approved in Europe and USA for treatment of complicated skin and soft tissue infections, bacteremia and right-sided infective endocarditis caused by Staphylococcus spp. at 4 and 6 mg/kg once daily, respectively [7, 8]. At 6 mg/kg/day, DAP does not perform better than standard therapy for endocarditis [7]. Despite its recent arrival on the market of anti-infective agents, DAP-resistant mutants have already been reported [9–12]. To prevent the occurrence of resistant mutants (especially in the presence of foreign bodies) [13–16] and to limit the increase in staphylococcal minimum inhibitory concentration (MIC) [17, 18], some authors [19, 20] suggest increasing the daily dose of DAP. Clinical experience with a dose >6 mg/kg is limited, but data reported to date suggest that DAP is safe and well-tolerated [20–22].
In this report, we present our center’s experience with high-dose DAP for empirical treatment of PVGI during the very crucial post-operative period, and as treatment adapted to microbiological results.
Methods
The present study was retrospectively conducted from January 2008 to December 2010 and included all patients treated with DAP for PVGI at our regional referral centers for these infections (University Hospital of Lille, Lille, France and Dron Hospital, Tourcoing, France). The objective of this study was to evaluate the safety of DAP at daily dosages >8 mg/kg in patients with PVGI. This study was approved by the institutional review boards of Dron Hospital and the University Hospital of Lille. All patients included in this study were informed and gave their consent. As in our previous studies [3], as there is no standard definition for diagnosis of definite or suspected PVGI, we used criteria proposed by FitzGerald et al. [1]. A patient was considered as suffering from clear-cut PVGI if at least two of the following three criteria were present: (a) positive bacterial culture of intraoperative specimens or blood samples (for potentially contaminant bacteria, such as coagulase-negative staphylococci, Propionibacterium acnes, or corynebacteria, at least two intraoperative specimens or blood samples or at least one intraoperative specimen and one blood culture were required); (b) clinical signs of infection in the area of the prosthesis; (c) biological or other radiological signs of infection (perigraft air or fluid persisting for more than 8 weeks post-operatively; abscess). Each case of definite infection was classified as early-onset infection when occurring within 4 months after surgery or as late-onset infection when occurring more than 4 months after surgery. PVGI or stent infection was suspected when bacteremia involving a site other than the surgical site occurred in the early post-operative period (within 4 weeks of graft or stent implantation) [23, 24]. PVGI was documented only by intraoperative or blood samples. Superficial samples were excluded. Multiple intraoperative samples were cultured on blood agar plates with standard aerobic and anaerobic methods. Antibiotic susceptibility patterns were interpreted in accordance with recommendations of the “Comité de l’Antibiogramme de la Société Française de Microbiologie” [25]. DAP was started in combination with broad-spectrum beta-lactams (e.g., piperacilline tazobactam) with or without aminoglycosides as first-line empiric antibiotic treatment in patients who had suspected or definite PVGI immediately after intraoperative samples were taken, or as second-line treatment in those who experienced adverse effects with a prior antibiotic regimen. To treat infection and to maintain or re-establish vascular flow to the distal bed, optimal surgical treatment included complete debridement of devitalized and infected tissues around the prosthesis, total graft excision, and in situ reconstruction with a new prosthesis, autogenous vein, or arterial allograft/homograft. Debridement without graft excision was proposed to patients with very early PVGI or to patients with severe comorbidities. Finally, when revascularization was not possible, amputation was proposed to the patient. Patients were evaluated at the end of DAP therapy and at the end of culture-guided therapy; in the case of prosthetic or homograft, they were followed up for 1 year after the end of treatment and, in case of venous graft, for 3 months after the end of treatment. Clinical success was defined by resolution of all clinical signs at the end of follow-up, with no need for additional antibiotic therapy, and/or negative culture in case of new surgery. Failure was defined as any other outcome. The safety of DAP was assessed on renal function and creatine phosphokinase (CPK) blood levels during treatment. For statistical analysis, numerical data are presented as mean (SD) or median and range. Categorical data are presented as number and percentage. Statistical analysis was performed using Stata® (version 9; StataCorp LP, College Station, TX, USA).
Results
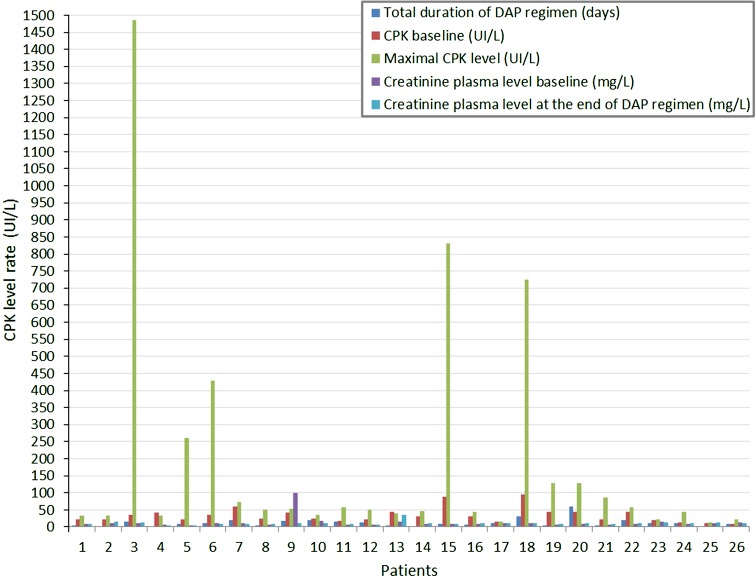
Among the 128 patients with suspected or definite PVGI from January 2008 to December 2010 at our two referral centers, 30 (23.4%) were treated with DAP doses >8 mg/kg per day in association with broad-spectrum beta-lactams for PVGI and gave their written consent for treatment. Four patients were excluded because of missing data or suspected PVGIon follow-up. Finally, 26 patients were included in our study. Patient demographic and clinical characteristics are listed in Table 1. Most of patients had intracavitary PVGI (69.2%). Half of the patients had early post-operative PVGI. Radiological signs included false aneurysm (n = 1), disruption of PVGI (n = 3), thrombosis (n = 2), and periprosthetic collection (n = 24). Microbiological documentation was obtained in 21 patients (80.1%) despite previous antibiotic administration (n = 16) within the 2 days prior to DAP treatment: penicillin (n = 12), carbapenems (n = 1), glycopeptides (n = 6), fluoroquinolones (n = 4), glycylcyclines (n = 1), aminoglycosides (n = 2), or miscellaneous agents (n = 3). Cultures of intraoperative samples were positive in 21 patients (80.1%). Blood and intraoperative cultures were concomitantly positive in 10 patients. The main microorganism identified in microbiological samples was Staphylococcus sp. (n = 18), including 11 methicillin-sensitive S. aureus (MSSA), 5 methicillin-resistant S. aureus strains (MRSA), and 2 methicillin-sensitive coagulase-negative staphylococci. All MRSA strains were susceptible to glycopeptides. No MIC for DAP was performed. The initial treatment options were: graft excision and replacement of the infected prosthesis by an in situ allo/homograft (n = 10), autologous vein (n = 1), or new prosthesis (n = 6); debridement without removed prosthesis (n = 6) and medical treatment without surgery (n = 3). All patients were treated with DAP as empirical treatment after intraoperative samples and/or blood cultures were taken. The mean DAP daily dosage was 729 ± 151 mg (9.5 mg/kg), except for 2 patients under hemodialysis who received 850 mg/48 h. Mean duration of the DAP regimen was 12.3 ± 11.9 days. The agents most frequently associated with DAP were piperacillin tazobactam (n = 16), imipenem (n = 4), caspofungin (n = 5), or other (n = 2). The empirical antibiotic was adequate in 100% of patients included in the study. Fourteen patients (53.8%) were admitted to the intensive care unit. The main complications were septic shock (n = 6), acute renal failure (n = 5) including those requiring hemodialysis (n = 2), graft disruption (n = 4), and pneumonia (n = 2). A second surgical procedure was necessary for 10 patients during the same hospital stay, with a mean interval of 5.6 days, due to persistent infection in most cases. In 6 patients, vascular graft was removed and replaced by allo/homograft. For the others, debridement was performed. New microorganisms were identified in 3 patients (Enterococcus sp. n = 1; Enterobacter sp. n = 2, E. coli n = 2, Candida sp. n = 1). During hospitalization, five patients died of a cause directly related to PVGI. Deaths were not directly related to the DAP regimen, but rather to the general condition of patients and disruption of the graft. For the 21 survivors, mean follow-up was 394 ± 265 days (123–1,376). No relapse was observed, but two patients died of pulmonary cancer during follow-up. No dosage of DAP was performed. No neutropenia or eosinophilic pneumonia was observed. Mean CPK blood levels at baseline and at the end of DAP therapy were, respectively, 38 ± 23 UI/L and 287 ± 221 UI/L, whereas creatinine blood levels were quite similar (13.1 ± 1.2 vs. 10.8 ± 5.5 mg/L) (Fig. 1). One of these patients had myalgia without renal impairment. Among the 9 patients who received concomitant statins, 3 of them had increased CPK blood levels. The reasons for discontinuing DAP was the use of antibiotic agents with narrow spectrum, guided by the microbiological results (n = 19), bacterial pneumonia (n = 2), or DAP-related adverse effects (i.e., myalgia [n = 1], increased CPK levels [n = 4]). No dosage of DAP was performed.
Table 1.
Characteristics of patients of the study
| Patients (n = 26) | n (%)a |
|---|---|
| Gender: male | 21 (80.8) |
| Mean age (years ± SD) | 62 ± 10.7 |
| Comorbidities | |
| Diabetes mellitus | 4 (15.4) |
| Immunosuppression | 5 (19.2) |
| COPD | 8 (30.8) |
| Hypertension | 21 (80.8) |
| Arterial coronary disease | 14 (53.9) |
| Severe renal chronic failure (<30 mL/min) | 1 (3.9) |
| Moderate renal chronic failure (30–60 mL/min) | 7 (26.9) |
| Clinical presentation at entry | |
| Intracavitary PVGI | 18 (69.2) |
| Extracavitary PVGI | 8 (30.8) |
| Early PVGI | 14 (53.9) |
| Late PVGI | 12 (46.2) |
| Fever | 21 (80.8) |
| Local erythema | 15 (57.7) |
| Productive fistula | 14 (53.9) |
| Abdominal pain | 8 (30.8) |
| Septic shock | 6 (23.1) |
| Weight (mean ± SD; kg) | 76.2 ± 11.7 |
| Biological data at entry | |
| Creatinine clearance (mean ± SD; mL/min) | 82.9 ± 33 |
| WBC (mean ± SD; /mm3) | 12,445 ± 5,389 |
| C-reactive protein (mean ± SD; mg/L) | 102 ± 96 |
| Microbiological data | |
| Positive blood sample | 9 (34.6) |
| Positive intraoperative sample | 21 (80.8) |
| No bacterial growth | 5 (19.2) |
| Polymicrobial sample | 5 (19.2) |
| MSSA | 11 (42.3) |
| MRSA | 5 (19.2) |
| CNS | 2 (7.7) |
| Streptococcus sp. | 5 (19.2) |
| Enterococcus faecalis | 2 (19.2) |
| Gram-negative bacilli | 8 (30.8) |
| Fungi | 1 (3.9) |
| Initial treatment option of PGVI | |
| PVGI removed | 17 (65.4) |
| Debridement in situ without prosthetic removal | 6 (23.1) |
| Medical treatment without surgery | 3 (11.5) |
| Outcome | |
| New surgery | 12 (46.2) |
| Previous or concomitant treatment | |
| Previous antibiotic treatment | 16 (61.5) |
| Concomitant treatment with statins | 9 (34.6) |
CNS coagulase-negative staphylococci, COPD chronic obstructive pulmonary disease, MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-sensitive Staphylococcus aureus, PVGI prosthetic vascular graft infection, WBC white blood cells
aVaules are presented as n (%) unless otherwise stated
Fig. 1.
Creatine phosphokinase (CPK) and creatinine level rate during daptomycin (DAP) regimen
Discussion
Results of the present study suggest that DAP >8 mg/kg/day, when used to treat a variety of PVGI due to Gram-positive cocci in severely ill patients with multiple comorbidities, shows a favorable safety profile, in agreement with previous studies, as well as a satisfactory clinical success rate [19–22].
Most of our patients were over 65 and severely ill, with high risk of mortality, sepsis, renal disorders due to the sepsis, vasopressor drug use, occlusive arterial disease, and “clampage of the aorta”. Despite these recognized risk factors in renal failure, nephrotoxicity was not detected in our population of patients, in contrast to results of vancomycin therapy reported in recent clinical studies [26, 27].
Several patients in our study experienced increased CPK blood levels, some with concomitant statins. With or without statins, clinical and biological abnormalities disappeared within a week. In pre-clinical studies [28, 29], DAP has been linked to fully reversible skeletal muscle toxicity, with no effect on smooth or cardiac muscle. A significant rise in the CPK level was noted, from 2.5% to 8.3%. However, in the present study, it was not clear whether the rise in CPK levels was due to the higher dosage regimen and concomitant use of statins, or whether it was the effect of peri-operative status (i.e., time of clampage of the aorta, septic shock, concomitant use of vasopressive agents) [30, 31]. No eosinophilic pneumonia was observed in our study.
Unlike vancomycin, DAP has shown similar efficacy against both MRSA and MSSA, making it an attractive option for empirical therapy of suspected severe infections due to Gram-positive cocci [5–7, 13–17, 19–22].
DAP exhibits bactericidal activity against both methicillin-susceptible and -resistant staphylococci, including those with MIC >1 mg/L, and, in vitro, it kills bacteria faster than comparable drugs [7, 8]. Experimental studies [5, 6, 13–17] suggest that it may prevent bacterial adherence, penetrate into the biofilm and prevent further biofilm formation.
In the present study, >80% of PVGI were microbiologically documented. Once the results of bacterial culture were available, empiric antimicrobial therapy was adapted, using, if possible, a step-down strategy. All patients underwent adaptation of antimicrobial drugs during the post-operative period, which we feel is crucial to prevent recolonization of the newly implanted graft. During their hospital stay, five patients died; deaths were related to graft disruption, possibly explained by problems in anastomosis between the graft and the infected aorta tissues. However, in our study, the efficacy of DAP in PVGI cannot be determined. Indeed, the overwhelming majority of patients received beta-lactam antibiotics with or without aminoglycosides in addition to DAP. A secondary surgery procedure was required for 10 patients with persistent infection. For these last patients, we considered that the first surgery was not considered to be optimal. Randomized studies would be more appropriate to study the efficacy of DAP in patients treated for PVGI. The main limitation would be the homogenous patients treated with homogenous surgical and medical procedure.
Conclusion
In conclusion, results of the present study suggest that high-dose DAP (i.e., >8 mg/kg) for patients with PVGI due to Gram-positive cocci represents a potentially interesting option for treatment of such infections. High-dose DAP therapy has a satisfactory toxicity profile even in severely ill patients with multiple comorbidities, and might favorably compete with vancomycin, especially in terms of risk of induced nephrotoxicity. However, monitoring of CPK levels is recommended in these patients, especially if they are concomitantly treated with statins.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. We thank Jerri Bram for her English language assistance. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. A part of this work has been submitted for the next ESCMID in Berlin, Germany.
Conflict of interest
Laurence Legout has received travel Grants from Pfizer and has been a speaker for Novartis. Olivier Leroy has received travel Grants from Pfizer and has been a speaker for Novartis. Eric Senneville has received travel Grants from Sanofi-Aventis and participated in data monitoring boards for Merck Sharp and Dohme-Chibret and has been a speaker for Novartis and Pfizer. Piervito D’Elia, Beatrice Sarraz-Bournet, Nicolas Ettahar, and Stephan Haulon have no conflict of interest to declare. No authors received any Grants for this work.
Compliance with ethics guidelines
This study was approved by the institutional review boards of Dron Hospital and the University Hospital of Lille. All patients included in this study were informed and gave their consent.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother. 2005;56(6):996–999. doi: 10.1093/jac/dki382. [DOI] [PubMed] [Google Scholar]
- 2.Yeager RA, McConnell DB, Sasaki TM, Vetto RM. Aortic and peripheral prosthetic graft infection: differential management and causes of mortality. Am J Surg. 1985;150(1):36–43. doi: 10.1016/0002-9610(85)90007-8. [DOI] [PubMed] [Google Scholar]
- 3.Legout L, Sarraz-Bournet B, D'Elia PV, et al. Characteristics and prognosis in patients with prosthetic vascular graft infection: a prospective observational cohort study. Clin Microbiol Infect. 2012;18(4):352–358. doi: 10.1111/j.1469-0691.2011.03618.x. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor S, Andrew P, Batt M, Becquemin JP. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg. 2006;44(1):38–45. doi: 10.1016/j.jvs.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Smith K, Perez A, Ramage G, Gemmell CG, Lang S. Comparison of biofilm-associated cell survival following in vitro exposure of meticillin-resistant Staphylococcus aureus biofilms to the antibiotics clindamycin, daptomycin, linezolid, tigecycline and vancomycin. Int J Antimicrob Agents. 2009;33(4):374–378. doi: 10.1016/j.ijantimicag.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Edmiston CE, Jr, Goheen MP, Seabrook GR, et al. Impact of selective antimicrobial agents on staphylococcal adherence to biomedical devices. Am J Surg. 2006;192(3):344–354. doi: 10.1016/j.amjsurg.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 8.New MRSA guidelines highlight a dearth of evidence. Lancet Infect Dis. 2011;11(3):153. [DOI] [PubMed]
- 9.Green MR, Anasetti C, Sandin RL, Rolfe NE, Greene JN. Development of daptomycin resistance in a bone marrow transplant patient with vancomycin-resistant Enterococcus durans. J Oncol Pharm Pract. 2006;12(3):179–181. doi: 10.1177/1078155206069165. [DOI] [PubMed] [Google Scholar]
- 10.Hidron AI, Schuetz AN, Nolte FS, Gould CV, Osborn MK. Daptomycin resistance in Enterococcus faecalis prosthetic valve endocarditis. J Antimicrob Chemother. 2008;61(6):1394–1396. doi: 10.1093/jac/dkn105. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JS, 2nd, Owens A, Cadena J, Sabol K, Patterson JE, Jorgensen JH. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother. 2005;49(4):1664–1665. doi: 10.1128/AAC.49.4.1664-1665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden MK, Rezai K, Hayes RA, Lolans K, Quinn JP, Weinstein RA. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5285–5287. doi: 10.1128/JCM.43.10.5285-5287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirioni O, Mocchegiani F, Ghiselli R, et al. Daptomycin and rifampin alone and in combination prevent vascular graft biofilm formation and emergence of antibiotic resistance in a subcutaneous rat pouch model of staphylococcal infection. Eur J Vasc Endovasc Surg. 2010;40(6):817–822. doi: 10.1016/j.ejvs.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 14.LaPlante KL, Woodmansee S. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob Agents Chemother. 2009;53(9):3880–3886. doi: 10.1128/AAC.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrigos C, Murillo O, Lora-Tamayo J, et al. Fosfomycin-daptomycin and other fosfomycin combinations as alternative therapies in experimental foreign body infection by methicillin resistant Staphylococcus aureus (MRSA) Antimicrob Agents Chemother. 2013;57(1):606–610. doi: 10.1128/AAC.01570-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John AK, Baldoni D, Haschke M, et al. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob Agents Chemother. 2009;53(7):2719–2724. doi: 10.1128/AAC.00047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose WE, Leonard SN, Rybak MJ. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2008;52(9):3061–3067. doi: 10.1128/AAC.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui L, Tominaga E, Neoh HM, Hiramatsu K. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(3):1079–1082. doi: 10.1128/AAC.50.3.1079-1082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durante-Mangoni E, Casillo R, Bernardo M, et al. High-dose daptomycin for cardiac implantable electronic device-related infective endocarditis. Clin Infect Dis. 2012;54(3):347–354. doi: 10.1093/cid/cir805. [DOI] [PubMed] [Google Scholar]
- 20.Kullar R, Davis SL, Levine DP, et al. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicentre, retrospective study. Pharmacotherapy. 2011;31(6):527–536. doi: 10.1592/phco.31.6.527. [DOI] [PubMed] [Google Scholar]
- 21.Parra-Ruiz J, Pena-Monje A, Tomas-Jimenez C, Pomares-Mora J, Hernandez-Quero J. Efficacy and safety of high dose (≥8 mg/kg/day) daptomycin. Enferm Infecc Microbiol Clin. 2011;29(6):425–427. doi: 10.1016/j.eimc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa DA, Mangini E, Amodio-Groton M, et al. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin Infect Dis. 2009;49(2):177–180. doi: 10.1086/600039. [DOI] [PubMed] [Google Scholar]
- 23.Roon AJ, Malone JM, Moore WS, Bean B, Campagna G. Bacteremic infectability: a function of vascular graft material and design. J Surg Res. 1977;22(5):489–498. doi: 10.1016/0022-4804(77)90031-2. [DOI] [PubMed] [Google Scholar]
- 24.Malone JM, Moore WS, Campagna G, Bean B. Bacteremic infectability of vascular grafts: the influence of pseudointimal integrity and duration of graft function. Surgery. 1975;78(2):211–216. [PubMed] [Google Scholar]
- 25.Van Hal SJ, Paterson DL, Lodise TP. Vancomycin-induced nephrotoxicity in troughs of 15–20 mg/L era: a systematic analysis review and meta-analysis. Antimicrob Agents Chemother. 2012 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 26.Comité de l’Antibiogramme de la Société Française de Microbiologie. http://www.sfm.asso.fr. Accessed August 2014.
- 27.Horey A, Mergenhagen KA, Mattappallil A. The relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran's population: a retrospective analysis. Ann Pharmacother. 2012;46:1477–1483. doi: 10.1345/aph.1R158. [DOI] [PubMed] [Google Scholar]
- 28.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob Agents Chemother. 2006;50:3245–3249. doi: 10.1128/AAC.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dvorchik B, Brazier D, De Bruin M, Arbeit R. Daptomycin phramacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother. 2003;47:1318–1323. doi: 10.1128/AAC.47.4.1318-1323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papadopoulos S, Ball AM, Liewer SE, Martin CA, Winstead PS, Murphy BS. Rhabdomyolysis during therapy with daptomycin. Clin Infect Dis. 2006;42:e108–e110. doi: 10.1086/504379. [DOI] [PubMed] [Google Scholar]
- 31.Kazory A, Dibadj K, Weiner D. Rhabdomyolysis and acute renal failure in a patient treated with daptomycin. J Antimicrob Chemother. 2006;57:578–579. doi: 10.1093/jac/dki476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.