Abstract
Introduction
Lower respiratory tract infection (LRTI) is the leading cause of infant mortality globally in post-neonatal infants (i.e., 28–364 days of age). Respiratory syncytial virus (RSV) is the most commonly identified pathogen for infant LRTI and is the second most important cause of death in post-neonatal infants. Despite 50 years of RSV vaccine research, there is still no approved vaccine. Therefore, passive immunity with the monoclonal antibody palivizumab is the sole regulatory-approved option for the prevention of serious LRTI caused by RSV in pediatric patients at high risk of RSV disease.
Methods
We conducted a comprehensive systematic literature review of randomized controlled trials (RCTs), open-label non-comparative clinical trials, and prospective observational studies/registries, and summarized the evidence related to the safety, efficacy, and effectiveness of palivizumab.
Results
The efficacy of palivizumab, as measured by the relative reduction in RSV-related hospitalization rate compared with placebo ranged from 39% to 78% (P < 0.05) in the 2 pivotal RCTs. A meta-analysis of the RSV-related hospitalization rate from 5 randomized placebo-controlled trials yielded an overall odds ratio of 0.41 (95% CI, 0.31–0.55) in favor of palivizumab prophylaxis over placebo (P < 0.00001). Low rates of RSV-related hospitalizations were observed in palivizumab recipients consistently over time in more than 42,000 pediatric subjects across 7 RCTs, 4 open-label non-comparative trials, and 8 observational studies/registries conducted in 34 countries. In addition, among palivizumab-prophylaxed subjects with breakthrough RSV LRTI, rates of intensive care unit admission and mechanical ventilation from RSV hospitalization also were low and consistent across studies. With respect to safety, no differences were observed between palivizumab and placebo in the blinded RCTs.
Conclusion
Rates of RSV hospitalizations and RSV hospitalization-related endpoints in pediatric subjects who received prophylaxis with palivizumab were low and constant over time and across RCTs, open-label non-comparative trials, and observational studies/registries.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-014-0046-6) contains supplementary material, which is available to authorized users.
Keywords: Efficacy, Palivizumab, Respiratory syncytial virus, Safety, Serious lower respiratory tract infection, Systematic review
Introduction
Respiratory syncytial virus (RSV) is one of the most common causes of viral lower respiratory tract infection (LRTI) in children worldwide and is associated with considerable morbidity and mortality [1]. LRTI was the leading cause of infectious disease hospitalizations among infants according to a recent analysis of hospital admissions in the United States (US) [2]. Globally, LRTI is the leading cause of death among post-neonatal infants (i.e., 28–364 days of age). In 2010, 20.1% of the 2 million global deaths among post-neonatal infants were caused by LRTI; malaria was the second most common cause, accounting for 11.8% of post-neonatal mortality [3]. The most commonly identified pathogen of LRTI in post-neonatal infants is RSV [3]. These findings highlight the significance of the malaria parasite Plasmodium falciparum and RSV as the two most important pathogen-specific causes of global mortality in this age group [3]. In addition to severe acute disease, evidence also suggests that children who had severe RSV infection early in life are more likely to develop subsequent wheezing during early childhood [4] and hyperreactive airways and asthma later in life [5].
RSV is a typically seasonal virus with outbreaks spanning from late autumn through early spring in temperate climates and throughout the rainy season in tropical climates. RSV is an extremely infectious virus, such that almost all children have contracted RSV by the age of 2 years [6]. Furthermore, re-infections are frequent because previous infection with RSV does not confer long-term immunity [7]. RSV disease manifestations in infants range from mild upper respiratory tract infection to respiratory failure. Certain high-risk groups, including premature infants; infants with underlying medical conditions such as chronic lung disease of prematurity (CLDP), also known as bronchopulmonary dysplasia (BPD); hemodynamically significant congenital heart disease (CHD); immunocompromised conditions; and severe neuromuscular disease, are more prone to serious disease due to RSV with higher hospitalization and mortality rates than those without these conditions [8, 9].
RSV is classified in the Pneumovirus genus of the Paramyxoviridae family of RNA viruses. RSV is an enveloped virus, containing a negative-sense, single-stranded RNA genome comprising ~15,000 nucleotides that encode 11 viral proteins [10]. The antigenicity of RSV is determined by two transmembrane glycoproteins. The RSV G glycoprotein is responsible for viral attachment to cells, and the RSV F glycoprotein promotes fusion of viral and cell membranes [10, 11]. Both the G and F glycoproteins are targets for RSV-neutralizing antibodies. RSV is classified in A and B subgroups, based on antigenic differences in the G protein [12].
The current goal of RSV vaccine development is the prevention of serious RSV disease in the population at highest risk, i.e., young infants [13]. In the absence of an effective, curative treatment for RSV infection [13], disease management is guided by the severity of respiratory distress and primarily involves supportive strategies such as hydration and oxygenation [6, 14]. An effective vaccine against RSV would be expected to decrease the global health burden and is urgently needed; however, one does not currently exist owing to the complexity and inherent challenges of RSV vaccine development. For example, neonates at risk for developing RSV disease have not been able to mount a strong immune response following administration of vaccines in development. Strategies for vaccinating pregnant women have been proposed with the intent of maternal transfer of anti-RSV antibodies to the fetus [13]. However, this approach may not provide adequate protection for very premature infants, who may not benefit from immunity afforded by maternal immunization because transplacental transport of antibodies occurs during the third trimester [15]. Efficacy was not demonstrated in a series of trials in the 1960s that evaluated a formalin-inactivated whole virus RSV vaccine. Ironically, respiratory disease from natural RSV infection was more severe in children who received the experimental vaccine compared with children who did not receive the vaccine [16–18]. Moreover, in 1 trial in which 31 infants 2–7 months of age received ≥1 injection of an experimental antigenic inactivated RSV vaccine, 2 deaths were attributed to the exaggerated clinical course of disease during RSV infection and were deemed to be associated with the vaccine [17]. Although the biologic cause leading to this phenomenon was never definitively determined, these discouraging results led to a more measured and conservative approach in the development of an RSV vaccine. There are currently a number of vaccine candidates in preclinical or phase I or II clinical development [13]; however, for the time being, passive immunity is the only means available to help reduce hospitalizations due to severe RSV disease.
The current prophylactic approach is passive immunity with antibodies. The first of such products was a polyclonal immunoglobulin formulation enriched for RSV-neutralizing antibody (RespiGam®; MedImmune, LLC, Gaithersburg, MD, USA) that reduced moderate or severe LRTI disease caused by RSV by 72% [19] and, in 1996, was the first prophylactic agent approved by the US Food and Drug Administration (FDA) for use in high-risk children. However, its use was hampered by the requirement that large volumes of immunoglobulin be administered by intravenous (IV) infusion over several hours on a monthly basis throughout the RSV season. While RespiGam was effective in reducing the incidence of RSV-related hospitalization in both preterm infants and infants with BPD [20], RespiGam did not significantly reduce the RSV-related hospitalization rate in children with CHD and was associated with increased cyanotic episodes and cardiac-related deaths in children with cyanotic CHD [21].
Research efforts were then directed toward the development of alternative agents with less complex methods of administration and potentially increased efficacy. The use of RespiGam was discontinued in 2003 following the 1998 approval of palivizumab (Synagis®; MedImmune, LLC, Gaithersburg, MD, USA) and the demonstration that a monoclonal antibody could be efficacious against an infectious agent. Compared with the IV administration of RespiGam, palivizumab is administered via an intramuscular (IM) injection. The evolution from polyclonal IV to monoclonal IM injection eliminated concerns for transmission of bloodborne pathogens as well as potential fluid overload. Palivizumab is a humanized monoclonal antibody specific for the antigenic site A on the highly conserved F protein on the surface of RSV. Palivizumab has potent neutralizing and fusion-inhibiting activity against RSV subgroups A and B [22]. Depending on the patient population, the efficacy of palivizumab ranged from a 39% to 78% reduction in RSV-related hospitalization compared with placebo in the 2 pivotal randomized controlled trials (RCTs) [23, 24]. Palivizumab was approved by the FDA in 1998 for use in reducing the risk of serious LRTI disease caused by RSV in children at high risk of RSV disease. Populations where efficacy has been established are children with BPD, CHD, and premature infants (≤35 weeks gestational age [GA]) [23, 24]. Prophylaxis with palivizumab begins before the expected start of the RSV season, with additional doses given monthly throughout the season. In its original formulation, palivizumab was supplied as a lyophilized product that required reconstitution with sterile water for IM injection. This process took ~20 min. A liquid formulation of palivizumab was subsequently developed, precluding the need for reconstitution. Bioequivalence between the lyophilized and liquid formulations was demonstrated in children ≤6 months of age with a history of prematurity [25]. The liquid formulation was first approved in 2004 in the US and is also currently available in Japan; broad global filings and approvals of the liquid preparation are ongoing.
We conducted a timely and comprehensive systematic review of RCTs, open-label non-comparative clinical trials, and prospective observational studies/registries to summarize and describe the existing evidence related to the safety, efficacy, and effectiveness of palivizumab for reducing the risk of serious RSV LRTI disease in high-risk infants and children.
Methods
Literature Search
We performed a literature search in MEDLINE (via PubMed), Embase, BIOSIS Previews, and Derwent Drug File using the following general terms and limits: “respiratory syncytial virus” AND “palivizumab OR Synagis” AND “premature” AND “congenital heart disease OR bronchopulmonary dysplasia OR chronic lung disease” AND “efficacy OR effect” AND “limits: human, premature/preterm (up to 35 weeks), English, clinical trial OR prospective observational study”. Separate literature searches were performed for RCTs and prospective observational studies/registries (see the Appendix in the electronic supplementary material). Published articles and congress abstracts indexed from January 1996 through July 2013 in MEDLINE and from January 1996 through August 2013 in the other databases were searched. References contained in any systematic reviews and meta-analyses found through the literature search were also reviewed to identify additional relevant studies not already captured.
Study Selection
All results from the literature search were reviewed at the abstract level by three authors (LKT, GN, CW). Inclusion criteria for the systematic review were studies reporting the primary outcome of the RSV-related hospitalization rate in children at high risk of severe RSV disease who received ≥1 injection of palivizumab at 15 mg/kg. High risk was defined as children with a history of prematurity (≤35 weeks GA) or children with BPD or hemodynamically significant CHD. Retrospective studies, epidemiologic studies, case reports, letters, comments, editorials, reviews, and meta-analyses were excluded from the systematic review. Results from the literature search and cross-referenced publications found from systematic reviews or meta-analyses that appeared to meet these selection criteria were independently reviewed at the full-text level by the same three authors; any disagreements over whether to include a study in the systematic review were resolved by discussion among the authors.
Data Analyses
A meta-analysis of the primary outcome of RSV-related hospitalization was conducted for the randomized placebo-controlled trials included in this systematic review. A fixed-effects meta-analysis was used (Mantel–Haenszel method) to assess the odds ratio (OR) of palivizumab compared with placebo using Review Manager Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Because of the heterogeneity of the study populations and methodologic variability among the studies, no formal combined statistical analyses were performed across the other types of studies or for any of the other major outcomes (i.e., RSV hospitalization-related outcomes, drug-related adverse events [AEs] and serious adverse events [SAEs]). Therefore, we describe the results of the included studies separately.
Statement of Ethics Compliance
The analysis in this article is based on previously published studies and does not involve any new studies of human subjects performed by any of the authors.
Results
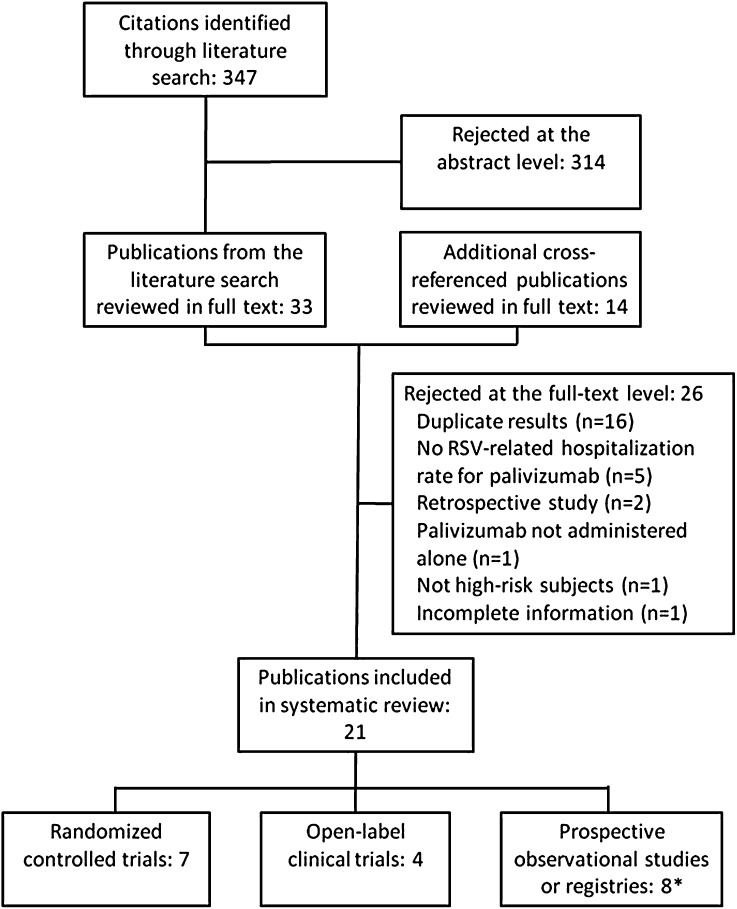
A total of 347 publications were retrieved from the literature search and reviewed at the abstract level (Fig. 1); 314 abstracts were rejected for not meeting the selection criteria or for duplicative reporting. Thirty-three publications from the literature search and an additional 14 cross-referenced publications were reviewed at the full-text level, with 26 being rejected after author review for the reasons shown in Fig. 1. The final 21 publications included in this review originated from 7 RCTs, 4 open-label non-comparative trials, and 8 prospective observational studies or registries, with 3 publications [26–28] reporting results from different time periods or populations of the same registry. The analysis comprises more than 42,000 high-risk infants/children from 34 countries. Characteristics of the studies included in this systematic review are described in Appendix Table A1.
Fig. 1.
Study selection diagram. Asterisk indicates three articles reported results on different time periods and subject populations from the same registry. RSV Respiratory syncytial virus
Efficacy: Reduction of RSV-Related Hospitalization
Efficacy of Palivizumab in Randomized, Placebo-Controlled Clinical Trials
Palivizumab administered at 15 mg/kg monthly during the RSV season was initially evaluated as a pre-approval IV formulation in a phase I/II, randomized, double-blind, placebo-controlled trial conducted from 1995 to 1996 in the US [29]. In this trial, the incidence of RSV-related hospitalization in children ≤24 months of age with BPD or infants ≤6 months of age who were born at ≤35 weeks GA was 10.0% (2/20) with placebo and 0% (0/22) with palivizumab (Table 1).
Table 1.
RSV-associated hospitalizations and related efficacy endpoints in the randomized controlled trials
| Population | Study | Study drug | RSV-associated hospitalization | RSV ICU admission | Mechanical ventilation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence, n/N (%) | Relative reduction rate vs. placebo, % (95% CI) | P value vs. placebo | Total days/100 subjects | Days with increased supplemental oxygen | % Subjects | Total days | % Subjects | Total days | |||
| BPD/CLDP or prematurity | Subramanian et al. [29] | Placebo | 2/20 (10.0) | NA | NA | NA | NA | NA | NA | ||
| Palivizumab | 0/22 (0) | 100 | NA | NA | NA | NA | NA | NA | NA | ||
| IMpact-RSV [24] | Placebo | 53/500 (10.6) | 62.6 | 50.6 | 3.0 | 12.7 | 0.2 | 1.7 | |||
| Palivizumab | 48/1,002 (4.8) | 55 (38, 72) | <0.001 | 36.4 (P < 0.001) | 30.3 (P < 0.001) | 1.3 (P = 0.026) | 13.3 (P = 0.023) | 0.7 | 8.4 | ||
| Carbonell-Estrany et al. [70] | Palivizumab | 62/3,306 (1.9) | 18.1 | 9.5/100 subjects | 0.6 | 6.3/100 subjects | 0.3 | 3.8/100 subjects | |||
| Motavizumab | 46/3,329 (1.4) | 9.1 | 4.1/100 subjects | 0.3 | 2.0/100 subjects | 0.1 | 0.5/100 subjects | ||||
| BPD/CLDP | IMpact-RSV [24] | Placebo | 34/266 (12.8) | NA | NA | NA | NA | NA | NA | ||
| Palivizumab | 39/496 (7.9) | 39 (20, 58) | 0.038 | NA | NA | NA | NA | NA | NA | ||
| Carbonell-Estrany et al. [70] | Palivizumab | 28/723 (3.9) | NA | NA | NA | NA | NA | NA | |||
| Motavizumab | 22/722 (3.0) | NA | NA | NA | NA | NA | NA | ||||
| Prematurity (all) | Carbonell-Estrany et al. [70] | Palivizumab | 34/2,583 (1.3) | NA | NA | NA | NA | NA | NA | ||
| Motavizumab | 24/2,607 (0.9) | NA | NA | NA | NA | NA | NA | ||||
| ≤32 weeks GA | Carbonell-Estrany et al. [70] | Palivizumab | 19/1,265 (1.5) | NA | NA | NA | NA | NA | NA | ||
| Motavizumab | 13/1,306 (1.0) | NA | NA | NA | NA | NA | NA | ||||
| Tavsu et al. [31] | No prophylaxis |
Prophylaxis year: 10/41 (24.4) Following year: 10/41 (24.4) |
NA | NA | NA | NA | NA | NA | |||
| Palivizumab |
Prophylaxis year: 0/39 (0) Following year: 0/39 (0) |
100 | 0.001 | NA | NA | NA | NA | NA | NA | ||
| 33–35 weeks GA | Carbonell-Estrany et al. [70] | Palivizumab | 15/1,318 (1.1) | NA | NA | NA | NA | NA | NA | ||
| Motavizumab | 11/1,301 (0.8) | NA | NA | NA | NA | NA | NA | ||||
| MAKI [30] | Placebo | 11/215 (5.1) | NA | NA | NA | NA | NA | NA | |||
| Palivizumab | 2/214 (0.9) | 82 (18, 157) | 0.01 | NA | NA | NA | NA | NA | NA | ||
| ≤35 weeks GA | IMpact-RSV [24] | Placebo | 19/234 (8.1) | NA | NA | NA | NA | NA | NA | ||
| Palivizumab | 9/506 (1.8) | 78 (66, 90) | <0.001 | NA | NA | NA | NA | NA | NA | ||
| CHD (all) | Cardiac [23] | Placebo | 63/648 (9.7) | 129.0 | 658 | 3.7 | 461 | 2.2 | 354 | ||
| Palivizumab | 34/639 (5.3) | 45 (23, 67) | 0.003 | 57.4 (P = 0.003) | 178 (P = 0.014) | 2.0 | 101 | 1.3 | 42 | ||
| Feltes et al. [71] | Palivizumab | 16/612 (2.6) | 18.9 | 19.8 | 1.0 | 27.5 | 0.7 | 28.5 | |||
| Motavizumab | 12/623 (1.9) | 12.7 | 8.5 | 0.8 | 7.0 | 0.3 | 8.0 | ||||
| Cyanotic | Cardiac [23] | Placebo | 7.9 | NA | NA | NA | NA | NA | NA | ||
| Palivizumab | 5.6 | 29 | 0.285 | NA | NA | NA | NA | NA | NA | ||
| Feltes et al. [71] | Palivizumab | 7/319 (2.2) | NA | NA | NA | NA | NA | NA | |||
| Motavizumab | 7/346 (2.0) | NA | NA | NA | NA | NA | NA | ||||
| Acyanotic | Cardiac [23] | Placebo | 11.8 | NA | NA | NA | NA | NA | NA | ||
| Palivizumab | 5.0 | 58 | 0.003 | NA | NA | NA | NA | NA | NA | ||
| Feltes et al. [71] | Palivizumab | 9/293 (3.1) | NA | NA | NA | NA | NA | NA | |||
| Motavizumab | 5/277 (1.8) | NA | NA | NA | NA | NA | NA | ||||
| Age <6 months | Cardiac [23] | Placebo | 12.2 | NA | NA | NA | NA | NA | NA | ||
| Palivizumab | 6.0 | 51 | NA | NA | NA | NA | NA | NA | NA | ||
| 6–12 months | Placebo | 7.3 | NA | NA | NA | NA | NA | NA | |||
| Palivizumab | 6.1 | 16 | NA | NA | NA | NA | NA | NA | NA | ||
| 1–2 years | Placebo | 4.3 | NA | NA | NA | NA | NA | NA | |||
| Palivizumab | 1.8 | 58 | NA | NA | NA | NA | NA | NA | NA | ||
Detailed characteristics of these studies are presented in Appendix Table A1
BPD Bronchopulmonary dysplasia, CHD hemodynamically significant congenital heart disease, CLDP chronic lung disease of prematurity, GA gestational age, ICU intensive care unit, NA not available, RSV respiratory syncytial virus
Subsequent studies of palivizumab included in this review were performed using the IM injection formulation. The efficacy of palivizumab as measured by the reduction in the RSV-related hospitalization rate was subsequently assessed in two large, randomized, double-blind, placebo-controlled trials [23, 24].
The IMpact-RSV trial was conducted during a single RSV season from 1996 to 1997 and studied a total of 1,502 children ≤24 months of age with BPD or infants ≤6 months of age who were born prematurely (≤35 weeks GA) in the US, Canada, and the United Kingdom (UK) [24]. In children who were premature or who had BPD, palivizumab reduced RSV-associated hospitalization by 55%, from an incidence of 10.6% (53/500) in children receiving placebo versus 4.8% (48/1,002) in children receiving palivizumab (Table 1; Fig. 2). In addition, the reduction of RSV-related hospitalization was observed both in children with BPD (34/266 [12.8%] with placebo versus 39/496 [7.9%] with palivizumab; 39% relative reduction) and in premature infants without BPD (19/234 [8.1%] with placebo versus 9/506 [1.8%] with palivizumab; 78% relative reduction; Table 1).
Fig. 2.
RSV-related hospitalization rates in the large randomized controlled trials: a IMpact-RSV [24], b Carbonell-Estrany et al. [70], c MAKI [30], d Tavsu et al. [31], e Cardiac [23], and f Feltes et al. [71] studies. Relative reduction rate compared with placebo is shown as ↓%. Asterisk indicates without BPD/CLDP. BPD Bronchopulmonary dysplasia, CHD hemodynamically significant congenital heart disease, CLDP chronic lung disease of prematurity, GA gestational age, mo months, RSV respiratory syncytial virus, wk weeks, y years
The Cardiac trial was conducted over 4 consecutive seasons from 1998 to 2002 in a total of 1,287 children ≤24 months of age with hemodynamically significant CHD in the US, Canada, Sweden, Germany, France, the UK, and Poland [23]. In this trial, palivizumab reduced RSV-associated hospitalization by 45% in children with hemodynamically significant CHD from an incidence of 9.7% (63/648) in children receiving placebo versus 5.3% (34/639) in children receiving palivizumab (Table 1; Fig. 2) [23]. Although the Cardiac study was not powered for subgroup analyses, there were reductions in RSV-related hospitalization rates in both cyanotic and acyanotic children (Table 1; Fig. 2).
A recent prospective RCT (ClinicalTrials.gov #ISRCTN73641710) conducted from 2008 to 2010 in the Netherlands further defined the efficacy of palivizumab in premature infants by GA [30]. In 429 preterm infants born at 33–35 weeks GA without underlying health conditions, the incidence of RSV-associated hospitalization was reduced by 82%, from an incidence of 5.1% in those receiving placebo (11/215) versus 0.9% in those receiving palivizumab (2/214; P = 0.01; Table 1) [30]. In addition, RSV infection requiring medical attention but not hospitalization was also significantly reduced by 81%, from an incidence of 4.7% in recipients of placebo (10/215) versus 0.9% in recipients of palivizumab (2/214; P = 0.02) [30].
More recently, Tavsu et al. [31] conducted a study from 2009 to 2011 in 80 infants with a GA of <32 weeks in Turkey. These infants were randomized to receive prophylaxis with palivizumab (study group, n = 39) or no prophylaxis (control group, n = 41). The incidence of RSV-related hospitalization was significantly lower in the study group than the control group both in the year of prophylaxis and the following year (0% vs. 24.4% in both years; P = 0.001; OR 1.32 [95% CI, 1.11–1.57]; Table 1).
Combining the results from these 5 randomized placebo-controlled trials in a meta-analysis of the RSV-related hospitalization rate yielded an overall OR of 0.41 (95% CI, 0.31–0.55) in favor of palivizumab prophylaxis over placebo (P < 0.00001; Fig. 3).
Fig. 3.
Meta-analysis of RSV-related hospitalization in the randomized, placebo-controlled trials. M–H Mantel–Haenszel method, RSV respiratory syncytial virus
Efficacy of Palivizumab in Prospective, Open-Label, Non-Comparative Clinical Trials
During the 1998–1999 RSV season, Abbott Laboratories (now AbbVie) conducted an Expanded Access Trial, which was a phase III and IV, multicenter, single-arm, open-label study to collect additional safety data on palivizumab-prophylaxed infants in countries where palivizumab was not yet available [32]. The study included preterm children born at ≤35 weeks GA who were ≤6 months old at enrollment, and children with BPD. A total of 565 children were enrolled in 16 countries in Europe, North America, and the Middle East, with 530 completing the study. Fifty-one (65%) of 78 hospitalizations during the study were due to respiratory causes. Among 29 cases tested for RSV, 7 were positive and 22 were negative. When the RSV test positivity rate (24%) was applied to the 22 untested respiratory cases, the estimated RSV hospitalization rate was 2.1% (12/565; Table 2).
Table 2.
RSV-associated hospitalizations and related efficacy endpoints in the open-label non-comparative studies
| Population | Study | RSV-associated hospitalization | RSV ICU admission | Mechanical ventilation | ||
|---|---|---|---|---|---|---|
| Incidence, n/N(%) | Total days/100 subjects | Days with increased supplemental oxygen | % Subjects | % Subjects | ||
| BPD/CLDP, prematurity, or CHD | Turti et al. [40] | 0/100 (0) | NA | NA | NA | NA |
| BPD/CLDP or prematurity | Expanded Access [32] | 12/565 (2.1) | NA | NA | NA | NA |
| BPD/CLDP | Lacaze-Masmonteil et al. [38] | First-season exposure: 1/71 (1.4) | NA | NA | NA | NA |
| Second-season exposure: 4/63 (6.3) | ||||||
| Prematurity | ||||||
| 29–32 weeks GA | PROTECT [39] | 5/285 (1.8) | 17.6 | Median 7.5 (range 6.0–9.0) | 0.7 | 0.7 |
| ≤35 weeks GA | Lacaze-Masmonteil et al. [38] | 5/134 (3.7) | NA | NA | NA | NA |
Detailed characteristics of these studies are presented in Appendix Table A1
BPD Bronchopulmonary dysplasia, CLDP chronic lung disease of prematurity, GA gestational age, ICU intensive care unit, NA not available, RSV respiratory syncytial virus
Many guidelines are aligned with the licensed indications of palivizumab and recommend that children with BPD requiring medical intervention at the onset of the RSV season receive prophylaxis up to the age of 24 months at the start of the RSV season (US, UK, Canada, Spain, and Germany) [33–37]. Therefore, some children with severe BPD will receive prophylaxis for more than one RSV season. A multicenter, open-label study was conducted in 7 European countries and Canada in 134 children <2 years old at risk for serious RSV infection, primarily because of BPD [38]. Seventy-one subjects without previous palivizumab exposure (mean age 8 months) and 63 subjects exposed to palivizumab during the previous season (mean age 16 months) received prophylaxis with palivizumab during the 1999–2000 RSV season. Five subjects (3.7%) were hospitalized for RSV-related respiratory illness overall, with no differences in incidence observed between the first-season exposure (1 [1.4%]) and second-season exposure subjects (4 [6.3%]; P = 0.187; Table 2) [38]. This study demonstrated a risk of serious RSV disease persisting beyond the first year of life in children with BPD.
Premature infants, regardless of their GA, are at high risk for serious RSV disease. As the subset of infants born at 29–32 weeks GA without CLDP was not specifically evaluated in the IMpact-RSV study [24], the PROTECT (Palivizumab RSV Open-label Trial of Effectiveness and Clinical Tolerability) study [39] was conducted to gather additional data in this patient population. During the 2000–2001 season, 285 subjects were enrolled from 16 European countries and Saudi Arabia. Of 20 (7%) hospitalizations for respiratory-related infections, 5 (1.8%) subjects were hospitalized for RSV-positive LRTI (Table 2). This RSV hospitalization rate is comparable to the incidence observed in the IMpact-RSV study [24].
Prior to palivizumab approval in the Russian Federation, a multicenter, prospective, open-label, non-comparative clinical study (ClinicalTrials.gov #NCT01006629) was conducted in high-risk children [40]. The study included children at high risk of serious RSV disease, defined as infants born at ≤35 weeks GA who were ≤6 months old at enrollment or children ≤24 months old with a clinical diagnosis of BPD and/or hemodynamically significant CHD. One hundred subjects received ≥1 injection of palivizumab during the 2009–2010 RSV season, and 94 completed their dosing schedule. There were no RSV hospitalizations (Table 2) or deaths. Of the seven subjects hospitalized for respiratory/cardiac conditions, six were tested for RSV and all test results were negative.
RSV-Related Hospitalizations in Prospective Observational Studies/Registries
Prospective observational studies and registries provide valuable information regarding the use of palivizumab in routine clinical practice and have accumulated a wealth of real-world information on the clinical effectiveness of RSV immunoprophylaxis with palivizumab. Over 13 years (1999–2011) and across 8 observational studies/registries in Spain, France, Germany, Canada, and the US, RSV-related hospitalization rates for prophylaxed infants/children ranged from 0.8% to 7.6% [26, 41–46] (Table 3). The highest rate (7.6%) was observed in a French registry [42], whereas the rate ranged from 0.8% to 3.95% in the other observational studies/registries [26, 41, 43–46]. The higher RSV hospitalization rate observed in the French registry may have been driven by enrollment of a very-high-risk population where 88% of subjects in the cohort had a GA ≤32 weeks, 52% were children born before 28 weeks GA, and the rate of BPD was 81%. In comparison, the rate of BPD among children enrolled in the IMpact-RSV study was 50% [24, 42]. In general, RSV-related hospitalization rates were similar for palivizumab recipients in the observational studies/registries and in the randomized placebo-controlled trials.
Table 3.
RSV-associated hospitalizations and related efficacy endpoints in the prospective observational studies/registries
| Population | Study | RSV-associated hospitalization | RSV ICU admission | Mechanical ventilation | ||||
|---|---|---|---|---|---|---|---|---|
| Incidence, % of N | Total days, mean ± SD | Days with increased supplemental oxygen, mean ± SD | % Subjects | Total days, mean ± SD | % Subjects | Total days, mean ± SD | ||
| BPD/CLDP or prematurity | Pedraz et al. [45] | Nonprophylaxed: 13.25 of 1,583 | Median (IQR), 8 (5, 11) | NA | 2.1 | NA | 0.8 | NA |
| Palivizumab: 3.95 of 1,919 | Median (IQR), 6 (4, 9) (P < 0.01) | 0.5 | 0.4 | |||||
| BPD/CLDP | Pedraz et al. [45] | Nonprophylaxed: 19.7 of 71 | NA | NA | NA | NA | NA | NA |
| Palivizumab: 5.5 of 217 (P < 0.007) | ||||||||
| French ATU Program [42] | 9.0 of 400 | NA | NA | NA | NA | NA | NA | |
| Palivizumab Outcomes Registry [41] | 2.40 of 4,329 | NA | NA | NA | NA | NA | NA | |
| CARESS (2005–2009)a [26] | 1.31b of 449 | NA | NA | NA | NA | NA | NA | |
| Prematurity | ||||||||
| ≤28 weeks GA | Pedraz et al. [45] | Nonprophylaxed: 13.0 of 246 | NA | NA | NA | NA | NA | NA |
| Palivizumab: 5.4 of 739 (P < 0.0001) | ||||||||
| French ATU Program [42] | 5.8 of 258 | NA | NA | NA | NA | NA | NA | |
| CARESS (2005–2009)c [26] | 1.34b of 1,704 | NA | NA | NA | NA | NA | NA | |
| 29–32 weeks GA | Pedraz et al. [45] | Nonprophylaxed: 9.9 of 1,297 | NA | NA | NA | NA | NA | NA |
| Palivizumab: 2.5 of 1,170 (P < 0.0000) | ||||||||
| French ATU Program [42] | 10.4 of 182 | NA | NA | NA | NA | NA | NA | |
| CARESS (2005–2009)a [26] | 1.25b of 1,449 | NA | NA | NA | NA | NA | NA | |
| <32 weeks GA | Palivizumab Outcomes Registry [41] | 1.84 of 7,786 | NA | NA | NA | NA | NA | NA |
| ≤32 weeks GA | Canadian Special Access Programme [44] | 1.6b of 324 | NA | NA | NA | NA | NA | NA |
| CARESS (2006–2011)a [28] | 1.5b of 5,183 | 6.7 ± 5.4 | 0.5 ± 2.2 | 0.3 | 1.2 ± 2.7 | 0.3 | 1.3 ± 2.9 | |
| 32–35 weeks GA | Palivizumab Outcomes Registry [41] | 0.83 of 9,294 | NA | NA | NA | NA | NA | NA |
| 33–35 weeks GA | CARESS (2005–2009)a [26] | 0.2b of 588 | NA | NA | NA | NA | NA | NA |
| CARESS (2006–2011)a [28] | 1.4b of 1,471 | 5.2 ± 5.0 | 0.2 ± 0.9 | 0.3 | 1.3 ± 2.4 | 0.3 | 0.3 ± 1.8 | |
| <35 weeks GA | Lesnick et al. [43] | 1.0 of 2,838 | NA | NA | NA | NA | NA | NA |
| ≤35 weeks GA | CARESS (2005–2009)a [26] | 1.12b of 3,741 | NA | NA | NA | NA | NA | NA |
| CARESS (2006–2010)a [27] | 1.3b of 4,880 | 7.3 ± 6.9 | 0.7 ± 2.9 | 0.3 | 1.7 ± 3.7 | 0.2 | 1.0 ± 3.1 | |
| CHD | Palivizumab Outcomes Registry [41] | 1.88 of 1,490 | NA | NA | NA | NA | NA | NA |
| CARESS (2005–2009)a [26] | 1.99b of 508 | NA | NA | NA | NA | NA | NA | |
| All prescribed | French ATU Program [42] | 7.6c of 516 | NA | NA | 1.9 | NA | 0.8 | NA |
| Canadian Special Access Programme [44] | 2.4b of 444 evaluable | 5.5 ± 3.8 | NA | NA | NA | NA | NA | |
| Lesnick et al. [43] | 0.8 of 3,520 | NA | NA | NA | NA | NA | NA | |
| Golombek et al. [72] | 1.17 of 1,446 overall (3.57 of 224 physician’s office setting; 0.93 of 969 home setting) | NA | NA | NA | NA | NA | NA | |
| Palivizumab Outcomes Registry [41] | 1.3 of 19,474d evaluable | NA | NA | NA | NA | NA | NA | |
| German Palivizumab Registry [46] | 1.6c of 9,833 evaluable | NA | NAc | 0.5 | NA | 0.1 | NA | |
| CARESS (2005–2009)d [26] | 1.38b of 5,286 | 8.0 ± 6.8 | 0.9 ± 3.1 | NA | 2.4 ± 4.5 | NA | 2.0 ± 4.1 | |
| Congenital airway abnormality/severe neuromuscular disease | Palivizumab Outcomes Registry [41] | 2.14 of 1,122 | NA | NA | NA | NA | NA | NA |
| >35 weeks GA and non-BPD/CHD medical disorders | CARESS (2006–2010)a [27] | 2.4b of 952 | 7.3 ± 5.8 | 1.6 ± 3.6 | 0.8 | 3.3 ± 5.4 | 1.3 | 3.6 ± 5.0 |
| >35 weeks GA | Palivizumab Outcomes Registry [41] | 1.13 of 2,390 | NA | NA | NA | NA | NA | NA |
| Other | CARESS (2005–2009)a [26] | 2.78b of 592 | NA | NA | NA | NA | NA | NA |
Detailed characteristics of these studies are presented in Appendix Table A1. Pedraz et al. 2003 [45] was a prospective, observational, case-controlled study; all other studies were prospective observational studies/registries. For all studies, results for subgroups with <100 palivizumab-prophylaxed patients were not reported here because of the small numbers
BPD bronchopulmonary dysplasia, CHD hemodynamically significant congenital heart disease, CLDP chronic lung disease of prematurity, ICU intensive care unit, IQR interquartile range, GA gestational age, NA not available, RSV respiratory syncytial virus
aSome reported patient populations may overlap
bRSV hospitalization rate = hospitalization rate × (subjects RSV positive/subjects tested)
cRSV hospitalization rate = hospitalization rate × (subjects RSV positive/subjects tested) was 8.1% for the French ATU Program [42] and 2.5% for the German Palivizumab Registry [46]
d1,047 subjects were enrolled for >1 season and were counted as separate enrollments per season enrolled
Secondary Outcomes: RSV Hospitalization-Related Endpoints
Although prophylaxis with palivizumab reduces the incidence of RSV-related hospitalizations, some children still develop breakthrough disease and require hospitalization. Tables 1, 2 and 3 illustrate the effect of palivizumab prophylaxis on RSV hospitalization-related endpoints. These are important surrogate markers of disease severity and include duration of RSV-related hospitalization, intensive care unit (ICU) admission, oxygen supplementation, and mechanical ventilation.
In the IMpact-RSV study, children with BPD or a history of prematurity randomized to receive prophylaxis with palivizumab spent significantly fewer days in the hospital and required fewer days of supplemental oxygen during RSV hospitalization compared with children randomized to receive placebo (all P < 0.001; Table 1) [24]. The overall requirement for ICU admission and/or mechanical ventilation was low and was influenced by a small number of children with complex underlying disease. Differences in the incidence of and days on mechanical ventilation did not differ significantly between groups [24].
Children with hemodynamically significant CHD receiving palivizumab prophylaxis also had significantly fewer days of RSV-related hospitalization (P = 0.003) and significantly fewer days with increased oxygen requirement compared with children receiving placebo (P = 0.014; Table 1) [23]. Other secondary efficacy endpoints, including incidence and days in the ICU and days on mechanical ventilation were not significant but did exhibit trends favoring palivizumab over placebo.
The results of the RSV hospitalization-related outcomes from the non-comparative trials and observational studies are similar to the results from the RCTs. For example, the percentage of subjects with BPD or prematurity who required mechanical ventilation was 0.7% in the open-label PROTECT trial (Table 2) conducted in 16 European countries and Saudi Arabia [39] and 0.1–0.8% in 2 observational studies (Table 3) conducted in Spain and the US [27, 28, 45] compared with 0.3–1.3% in the RCTs (Table 1). In the only observational study to include case controls, there was a statistically significant reduction in RSV hospitalization duration and numerical decreases in the percentage of subjects requiring ICU admission or mechanical ventilation in Spanish subjects who received prophylaxis with palivizumab compared with those who did not receive prophylaxis [45].
Safety and Immunogenicity
The safety data for palivizumab was first established in 2,789 infants enrolled in the two registrational randomized placebo-controlled trials (Table 4) [23, 24]. In the IMpact-RSV trial, there was no difference in the placebo and palivizumab groups in the number of children who had AEs that were judged to be related to the study drug by the blinded investigator (10% vs. 11%) [24]. Study subjects rarely discontinued prophylaxis due to palivizumab-related AEs (0.3%), and the incidence of related AEs did not differ significantly between the placebo and palivizumab groups. Similar safety findings were demonstrated in the Cardiac study [23]. The proportion of children with AEs judged by the blinded investigator to be related to the study drug was similar between the placebo and palivizumab groups (6.9% vs. 7.2%). No child had the study drug discontinued for a related AE, and the incidence of related SAEs was low and similar in the placebo and palivizumab groups (0.5% vs. 0%).
Table 4.
Summary of safety in the randomized clinical trials
| Population | Study | Study drug dose | Subjects with ≥1 at least possibly related, % | Most commonly reported AEs, % | Commonly reported SAEs, % | Injection site reaction, % | AEs leading to D/C, % | Deaths, % | |
|---|---|---|---|---|---|---|---|---|---|
| AE | SAE | ||||||||
| BPD/CLDP, prematurity, or CHD | Expanded Access [32] | Palivizumab (n = 565) | 6.9 | NA | Fever 1.5%, diarrhea 0.8%, nervousness/irritability 0.8%a | NA | 2.3a | 1.9 | 0.4 |
| Lacaze-Masmonteil et al. [38] | Palivizumab, season 1 (n = 71) | NA | 0 | Infection, injection site reaction, diarrhea 1.4% eacha | Bronchiolitis 5.6%, BPD aggravation 2.8%, vomiting 2.8% | 1.4a | 0 | 0 | |
| Palivizumab, season 2 (n = 63) | NA | 0 | Fever 3.2%; anorexia, epistaxis, ataxia 1.6% eacha | Bronchiolitis 4.8%, fever 3.2% | 0a | 0 | 0 | ||
| Turti et al. [40] | Palivizumab (n = 100) | 2.0 | 0 | Rhinitis, dermatitis 1.0% each | NA | NA | 1.0 | 0 | |
| BPD/CLDP or prematurity | Subramanian et al. [29] | Placebo (n = 20) | 15.0 | NA | NA | NA | NA | 0 | 5.0 |
| Palivizumab 15 mg/kg (n = 22) | 23.0 | NA | NA | NA | NA | 0 | 0 | ||
| IMpact-RSV [24] | Placebo (n = 500) | 10.0 | NA | Fever 3.0%, nervousness 2.6%, injection site reaction 1.6%a | NA | 1.8 | NA | 1.0 | |
| Palivizumab 15 mg/kg (n = 1,002) | 11.0 | NA | Fever 2.8%, nervousness 2.5%, injection site reaction 2.3%a | NA | 2.7 | 0.3 | 0.4 | ||
| Carbonell-Estrany et al. [70] | Palivizumab 15 mg/kg (n = 3,298) | NA | NA | URTI 30.1%, pyrexia 16.9%, rhinitis 13.5%, otitis media 12.8%, bronchiolitis 9.9%, teething 8.6%, diarrhea 8.5%, respiratory disorder 8.4%, nasal congestion 8.0%, conjunctivitis 7.6%, gastroenteritis 7.1%, bronchitis 6.9%, constipation 6.9%, cough 6.5%, gastroesophageal reflux disease 6.3%, diaper dermatitis 5.8%, vomiting 5.2%, irritability 5.0% | NA | 2.7 | 0.3 | 0.1 | |
| Prematurity | MAKI [30] | Placebo (n = 215) | NA | NA | NA | NA | NA | NA | 0 |
| Palivizumab 15 mg/kg (n = 214) | NA | NA | NA | NA | NA | NA | 0 | ||
| CHD | Cardiac [23] | Placebo (n = 648) | 6.9 | 0.5 | URTI 46.1%, fever 23.9%, conjunctivitis 9.3%, cyanosis 6.9%, infection 2.9%, arrhythmia 1.7% | Arrhythmia 0.3%, cyanosis 2.2% | 2.2 | 0a | 4.2 |
| Palivizumab 15 mg/kg (n = 639) | 7.2 | 0 | URTI 47.4%, fever 27.1%, conjunctivitis 11.3%, cyanosis 9.1%, infection 5.6%, arrhythmia 3.1% | Arrhythmia 0.2%, cyanosis 3.6% | 3.4 | 0a | 3.3 | ||
| Feltes et al. [71] | Palivizumab 15 mg/kg (n = 612) | 8.8 | 1.0 | Pyrexia 29.2%, URTI 28.1%, rhinitis 12.6%, cough 11.6%, otitis media 11.4%, gastroenteritis 10.8%, diarrhea 10.6%, nasopharyngitis 9.5%, vomiting 8.3%, Tetralogy of Fallot 8.2%, bronchitis 7.8%, rhinorrhea 7.4%, ventricular septal defect 6.2%, teething 5.9%, pneumonia 5.9%, blood urea increased 5.6%, nasal congestion 5.4%, constipation 5.1%, viral infection 5.1%, dermatitis diaper 5.1% | Tetralogy of Fallot 8.0%, ventricular septal defect 5.9%, pneumonia 3.8%, atrioventricular septal defect 3.6% | NA | 0.2 | 1.6 | |
Detailed characteristics of these studies are presented in Appendix Table A1. The Tavsu et al. 2014 [31] trial did not report safety events and was therefore not included in this table
AE adverse event, BPD bronchopulmonary dysplasia, CHD congenital heart disease, CLDP chronic lung disease of prematurity, D/C discontinuation of study drug, NA not available, SAE serious adverse event, URTI upper respiratory tract infection
aConsidered at least possibly related to study drug
Given that palivizumab has been in clinical use for 16 years, follow-up clinical trials, outcome data from several international registries and post-marketing experience are consistent with the initial safety profile. Uncontrolled trial (Table 5) and observational/registry data (Appendix Table A2) presented in this systematic review are consistent with respect to safety findings. Commonly reported AEs from these data include injection site reactions and fever. Across RCTs and open-label non-comparative trials, <2% of AEs led to study drug discontinuation. In addition, injection site reactions and severe thrombocytopenia (platelet count <50,000 per microliter) have been voluntarily reported during post-approval use of palivizumab in over 3.2 million seasonal courses of therapy (data on file, AbbVie). As these events are voluntarily reported, their frequency and causal relationship to palivizumab cannot always be reliably estimated [22].
Table 5.
Summary of safety in the open-label non-comparative studies
| Population | Study | Study drug dose | Subjects with ≥1 at least possibly related, % | Most commonly reported AEs, % | Commonly reported SAEs, % | Injection site reaction, % | AEs leading to D/C, % | Deaths, % | |
|---|---|---|---|---|---|---|---|---|---|
| AE | SAE | ||||||||
| BPD/CLDP, prematurity, or CHD | Expanded Access [32] | Palivizumab (n = 565) | 6.9 | NA | Fever 1.5%, diarrhea 0.8%, nervousness/irritability 0.8%a | NA | 2.3a | 1.9 | 0.4 |
| Lacaze-Masmonteil et al. [38] | Palivizumab, season 1 (n = 71) | NA | 0 | Infection, injection site reaction, diarrhea 1.4% eacha | Bronchiolitis 5.6%, BPD aggravation 2.8%, vomiting 2.8% | 1.4a | 0 | 0 | |
| Palivizumab, season 2 (n = 63) | NA | 0 | Fever 3.2%; anorexia, epistaxis, ataxia 1.6% eacha | Bronchiolitis 4.8%, fever 3.2% | 0a | 0 | 0 | ||
| Turti et al. [40] | Palivizumab (n = 100) | 2.0 | 0 | Rhinitis, dermatitis 1.0% each | NA | NA | 1.0 | 0 | |
| Prematurity | PROTECT [39] | Palivizumab (n = 285) | NA | NA | Fever, infection, enteritis, bronchiolitis, bronchitis, increased cough, pneumonia, rhinitis, conjunctivitis 0.4% eacha | RSV infection, RSV bronchiolitis, pneumonia, conjunctivitis 0.4% eacha | NA | 0.7 | 0 |
Detailed characteristics of these studies are presented in Appendix Table A1
AE Adverse event, BPD bronchopulmonary dysplasia, CHD congenital heart disease, CLDP chronic lung disease of prematurity, D/C discontinuation of study drug, NA not available, RSV respiratory syncytial virus, SAE serious adverse event, URTI upper respiratory tract infection
aConsidered at least possibly related to study drug
SAE reporting was limited in the registries. In the German Palivizumab Registry [46] conducted from 2002 to 2007, 10 (0.09%) of 10,686 patients had ≥1 SAE considered possibly or probably related to palivizumab administration: dyspnea/cyanosis with or without fever (n = 4); skin rash; thrombocytopenia with petechiae; osteomyelitis of the distal femur epiphysis; seizure; transient unresponsiveness; and fever, restlessness, and feeding difficulties (n = 1 each). From 2005 to 2009, in the total population of 5,286 subjects enrolled in CARESS [26], 61 SAEs were reported overall, of which 56 were hospitalizations due to respiratory infection (including 14 from breakthrough RSV infection).
Allergic reactions, including very rare cases of anaphylaxis and anaphylactic shock, have been reported following palivizumab administration in post-marketing settings. In some cases, fatalities have been reported. However, because the AEs identified via post-marketing surveillance are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to palivizumab exposure.
With repeated exposure, there is a theoretical concern that recipients of palivizumab could develop an immune response to the monoclonal antibody. In the IMpact-RSV trial, anti-palivizumab antibodies were assessed before the first and last palivizumab injections and in a randomized manner before the second, third, or fourth injection [24]. In the placebo and palivizumab groups, titers >1:40 were detected in 2.8% and 1.2% of subjects, respectively. These elevations generally occurred at single time points and were not associated with low palivizumab concentrations or increased AEs. In a French study conducted to assess the incidence of anti-palivizumab antibodies and clinical AEs in children prophylaxed with palivizumab for a first (no previous palivizumab exposure) versus a second RSV season, similar serum concentrations of palivizumab were observed for both first-season and second-season subjects, and none had a significant anti-palivizumab antibody response, defined as a titer of ≥1:80 occurring at any time during the study [38].
Discussion
This systematic review, which assessed results from 7 RCTs, 4 open-label non-comparative trials, and 8 prospective observational studies or registries comprising over 42,000 high-risk infants/children from 34 countries, found that palivizumab has shown consistent efficacy or effectiveness in the reduction of RSV-related hospitalizations in high-risk populations. Prophylactic administration of palivizumab demonstrated an acceptable safety profile in the identified studies. The data presented in this systematic review are important for understanding how palivizumab is currently used in clinical practice as well as the issues that remain of interest to the medical community.
Current Issues in RSV Immunoprophylaxis
Strategies for diminishing the health care burden from RSV infections include appropriate and targeted prophylaxis in children at high risk of severe RSV disease. Palivizumab is widely approved across Europe, US, Canada, Asia, and Latin America; however, in an effort to ensure optimal balance of benefit and cost from this intervention, clinical guidelines are country specific and vary with regard to their recommendations for prophylaxis in premature infants [33–37, 47–49]. Compared with term infants, preterm infants have increased susceptibility to severe RSV disease, irrespective of the degree of prematurity, due to interrupted lung development and an immature immune system. Alveolar development is not universally present until 36 weeks GA and continues following birth through 2 years of age [50]. While the immune systems of term infants are aided by maternal transfer of antibodies across the placenta in the third trimester, preterm infants are born prior to completion of this transfer [51]. There is general agreement that because of the cost of palivizumab prophylaxis, guidelines should be put in place to ensure cost-effective use in well-defined high-risk populations rather than to arbitrarily restrict RSV prophylaxis from a portion of this vulnerable population. Late-preterm infants account for approximately three quarters of the preterm birth population [52], and there is evidence that they experience increased morbidity and even higher neonatal mortality compared with near-term or full-term infants [52–55]; however, the cost-effectiveness of passive immunoprophylaxis for all late-preterm infants is questioned. Using risk factors in this population, cost-effectiveness can be improved. Robust, validated, evidence-based risk score models based on defined variables have been developed in Canada and Europe [56–58] to identify the late-preterm infants at the highest risk for RSV-related hospitalization. These RSV risk score models have demonstrated cost-effectiveness; thus, these recommendations have been adopted by international pediatric advisory committees [35, 59–62].
Although immunoprophylaxis with palivizumab has been demonstrated to be effective in decreasing RSV hospitalization in high-risk children, hospitalization is only one of the possible consequences of RSV infection. More research is needed to examine the impact of RSV infection on subsequent morbidity and mortality, to understand the full impact and burden of RSV infections, and to clarify additional health benefits and the value of prophylaxis. There is evidence suggesting that palivizumab is associated with reduced infant all-cause mortality [63, 64]. In addition, prophylaxis with palivizumab has been associated with a reduction in long-term respiratory morbidity of severe RSV infection, such as recurrent wheeze [65, 66]. In a placebo-controlled trial in otherwise healthy preterm infants of 33–35 weeks GA, prophylaxis with palivizumab led to a 61% reduction in wheezing days that was maintained beyond the end of therapy and throughout the infants’ first year of life (P < 0.05) [30]. A prospective analysis of clinical trial data in preterm children ≤35 weeks GA reported that palivizumab exposure may decrease the incidence of subsequent asthma-like symptoms [65] and provide a protective effect on recurrent wheezing in prophylaxed children without a family history of atopy through 24 months compared with matched controls [67]. Finally, a prospective Japanese study in preterm children born at 33–35 weeks GA found a lower incidence of recurrent wheezing during the first 3 years of life after palivizumab exposure [66]. When considering the impact of palivizumab on long-term respiratory effects, it is important to note that the majority of the evidence is from non-randomized studies and more research with well-designed RCTs is needed before definitive conclusions can be made.
Data concerning the long-term safety of palivizumab are limited. Considering that early human life is an important period of development, research is needed to evaluate the long-term effects after exposure to palivizumab in early childhood.
The emergence of viral escape mutants in response to administration of a monoclonal antibody such as palivizumab must be considered. Palivizumab binds to antigenic site A, a highly conserved region on the extracellular domain of RSV F, which encompasses amino acids 262–275. Only changes in antigenic site A of the F protein have been demonstrated to confer resistance to palivizumab [68]. The rate of palivizumab-resistant mutations is low in both immunoprophylaxis-naïve patients (<1%) [68] and in patients experiencing breakthrough RSV disease while taking palivizumab (6.3%) [22]. Palivizumab-resistant variants exhibited a growth disadvantage compared with parental viruses and are therefore unlikely to propagate in the community in the absence of selective pressure from palivizumab exposure. In addition, a review of clinical findings among the children who experienced breakthrough RSV disease while taking palivizumab did not reveal an association between mutations in the RSV F protein gene and RSV disease severity [69]. However, the clinical impact of transfer or distribution of palivizumab-resistant mutants is not clearly understood.
Limitations
There are several limitations of this systematic review that must be considered. Although multiple studies and post-marketing experience involving thousands of high-risk infants indicate that palivizumab reduces overall hospitalization rates due to RSV, it is important to note there are few RCTs and the majority of the evidence is based on prospective observational studies/registries. As the observational studies/registries do not involve a control arm, they cannot definitively evaluate the true impact of RSV prophylaxis as documented in the placebo-controlled randomized trials [23, 24]. In addition, the populations in these cohorts may be variable, as enrollment is based on country-specific pediatric prophylaxis guidelines and the data reflect the way in which health care providers use palivizumab in the real world. Finally, RSV hospitalization detection rates are influenced by the hospitalization/ICU threshold changes over time, changes in bronchiolitis diagnosis over time, the type of samples collected, and the type of tests conducted.
Conclusion
We conducted a systematic review of published palivizumab RCT results, prospective, non-comparative clinical trial results, and published observational data to describe the safety, efficacy, and effectiveness of palivizumab for reducing the risk of serious RSV LRTI disease in high-risk infants and children. Since approval in 1998, palivizumab has been used in more than 80 countries for the passive prevention of serious RSV disease in high-risk children, and the patient exposure to palivizumab has been over 3.2 million seasonal courses of therapy (data on file, AbbVie). Compared with placebo, the efficacy of palivizumab, as measured by a reduction in the rate of RSV-related hospitalization, depends on the high-risk groups assessed and varied from 39% to 82% in subjects with BPD and CHD and premature infants (≤35 weeks GA) [23, 24, 30]. A meta-analysis of the RSV-related hospitalization rate from 5 randomized, placebo-controlled trials yielded an overall OR of 0.41 (95% CI, 0.31–0.55) in favor of palivizumab prophylaxis over placebo (P < 0.00001). Palivizumab has shown an acceptable safety profile, including a low incidence of anti-palivizumab antibodies in children with BPD, infants with a history of prematurity, and children with hemodynamically significant CHD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Medical writing support was provided by Jennifer Han, MS, and Daniel E. McCallus, PhD, of Complete Publication Solutions, LLC; this support was funded by AbbVie Inc. This work was supported by AbbVie Inc., who sponsored the study; contributed to its design; and participated in the collection, analysis, and interpretation of the data; and in the writing, review, and approval of the final version; and is funding the article processing charges. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Colleen Wegzyn is an employee of AbbVie and may own stock or stock options. Lim Kai Toh is an employee of AbbVie and may own stock or stock options. Gerard Notario is an employee of AbbVie and may own stock or stock options. Sophie Biguenet is an employee of AbbVie and may own stock or stock options. Kristina Unnebrink is an employee of AbbVie and may own stock or stock options. Caroline Park is an employee of AbbVie and may own stock or stock options. Doris Makari is an employee of MedImmune and may own stock or stock options in AstraZeneca, the parent company of MedImmune. Michael Norton is an employee of AbbVie and may own stock or stock options.
Compliance with ethics guidelines
The analysis in this article is based on previously published studies and does not involve any new studies of human subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics. 2008;121:244–252. doi: 10.1542/peds.2007-1392. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kneyber MCJ, Steyerberg EW, de Groot R, Moll HA. Long-term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Paediatr. 2000;89:654–660. doi: 10.1111/j.1651-2227.2000.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohapatra SS, Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev. 2008;21:495–504. doi: 10.1128/CMR.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics . Summaries of infectious diseases: respiratory syncytial virus. In: Pickering LK, editor. Red book: 2012 report of the committee on infectious diseases. 29. Elk Grove Village: American Academy of Pediatrics; 2012. pp. 609–618. [Google Scholar]
- 7.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Simoes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:39–57. doi: 10.1007/978-3-642-38919-1_2. [DOI] [PubMed] [Google Scholar]
- 9.Sommer C, Resch B, Simoes EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J. 2011;5:144–154. doi: 10.2174/1874285801105010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev. 1999;12:298–309. doi: 10.1128/cmr.12.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudraraju R, Jones BG, Sealy R, Surman SL, Hurwitz JL. Respiratory syncytial virus: current progress in vaccine development. Viruses. 2013;5:577–594. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PR, Jr, Olmsted RA, Prince GA, et al. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987;61:3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaaijk P, Luytjes W, Rots NY. Vaccination against RSV: is maternal vaccination a good alternative to other approaches? Hum Vaccin Immunother. 2013;9:1263–1267. doi: 10.4161/hv.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson-Caswell M, Muncie HL., Jr Respiratory syncytial virus infection in children. Am Fam Physician. 2011;83:141–146. [PubMed] [Google Scholar]
- 15.Graham BS, Anderson LJ. Challenges and opportunities for respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:391–404. doi: 10.1007/978-3-642-38919-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 17.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 18.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 19.Groothuis JR, Simoes EA, Levin MJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 20.Johnson S, Oliver C, Prince GA, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 21.Simoes EA, Sondheimer HM, Top FH, Jr, et al. Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. The Cardiac Study Group. J Pediatr. 1998;133:492–499. doi: 10.1016/S0022-3476(98)70056-3. [DOI] [PubMed] [Google Scholar]
- 22.Synagis® (palivizumab). Full prescribing information, MedImmune, LLC, Gaithersburg, MD, 2014. Available from: http://www.medimmune.com/docs/default-source/pdfs/prescribing-information-for-synagis.pdf. Accessed September 15, 2014.
- 23.Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540. doi: 10.1067/S0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 24.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 25.Robbie GJ, Makari D, Harris B, Losonsky GA, Jafri HS. Randomized, double-blind study of the pharmacokinetics and safety of palivizumab liquid formulation compared with lyophilized formulation. Infect Dis Ther. 2014 (in press). [DOI] [PMC free article] [PubMed]
- 26.Mitchell I, Paes BA, Li A, Lanctot KL. CARESS: the Canadian registry of palivizumab. Pediatr Infect Dis J. 2011;30:651–655. doi: 10.1097/INF.0b013e31821146f7. [DOI] [PubMed] [Google Scholar]
- 27.Paes B, Mitchell I, Li A, Lanctot KL. Respiratory hospitalizations and respiratory syncytial virus prophylaxis in special populations. Eur J Pediatr. 2012;171:833–841. doi: 10.1007/s00431-011-1654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paes B, Mitchell I, Li A, Lanctot KL. A comparative study of respiratory syncytial virus (RSV) prophylaxis in premature infants within the Canadian Registry of Palivizumab (CARESS) Eur J Clin Microbiol Infect Dis. 2012;31:2703–2711. doi: 10.1007/s10096-012-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian KN, Weisman LE, Rhodes T, et al. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group. Pediatr Infect Dis J. 1998;17:110–115. doi: 10.1097/00006454-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 31.Tavsu I, Gursoy T, Dirman S, Erbil N, Ovali F. Palivizumab prophylaxis: does it have any influence on the growth and development of the infants? Am J Perinatol. 2014;31:667–672. doi: 10.1055/s-0033-1356485. [DOI] [PubMed] [Google Scholar]
- 32.Groothuis JR. Safety and tolerance of palivizumab administration in a large Northern Hemisphere trial. Northern Hemisphere Expanded Access Study Group. Pediatr Infect Dis J. 2001;20:628–630. doi: 10.1097/00006454-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Robinson JL, Canadian Paediatric Society. Infectious Diseases and Immunization Committee Preventing respiratory syncytial virus infections. Paediatr Child Health. 2011;16:488–490. doi: 10.1093/pch/16.8.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.German Society for Paediatric Infectious Diseases (DGPI) German Society of Pediatric Cardiology (DGPK) Society of Pediatric Pneumology (GPP) Society of Neonatology and Pediatric Intensive Care Medicine (GNPI) RSV prophylaxis with palivizumab in high-risk children. Updated German national guidelines 2008. Monatsschr Kinderheilkd. 2009;157:61–64. doi: 10.1007/s00112-008-1926-1. [DOI] [Google Scholar]
- 35.Figueras Aloy J, Carbonell Estrany X, Comite de Estandares de la Sociedad Espanola de Neonatologia Recommendations for the use of palivizumab in the prevention of respiratory syncytial virus infection in late preterm infants (32(1) to 35(0) weeks of gestation) An Pediatr (Barc). 2010;73:98.e1-4. doi: 10.1016/j.anpedi.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Figueras Aloy J, Quero J, Domenech E, et al. Recommendations for the prevention of respiratory syncytial virus infection. An Pediatr (Barc). 2005;63:357–362. doi: 10.1157/13079818. [DOI] [PubMed] [Google Scholar]
- 37.American Academy of Pediatrics Committee on Infectious Diseases. American Academy of Pediatrics Bronchiolitis Guidelines Committee Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 38.Lacaze-Masmonteil T, Seidenberg J, Mitchell I, et al. Evaluation of the safety of palivizumab in the second season of exposure in young children at risk for severe respiratory syncytial virus infection. Drug Saf. 2003;26:283–291. doi: 10.2165/00002018-200326040-00005. [DOI] [PubMed] [Google Scholar]
- 39.Groothuis JR. Safety of palivizumab in preterm infants 29–32 weeks’ gestational age without chronic lung disease to prevent serious respiratory syncytial virus infection. Eur J Clin Microbiol Infect Dis. 2003;22:414–417. doi: 10.1007/s10096-003-0961-z. [DOI] [PubMed] [Google Scholar]
- 40.Turti TV, Baibarina EN, Degtiareva EA, et al. A prospective, open-label, non-comparative study of palivizumab prophylaxis in children at high risk of serious respiratory syncytial virus disease in the Russian Federation. BMC Res Notes. 2012;5:484. doi: 10.1186/1756-0500-5-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frogel M, Nerwen C, Cohen A, et al. Prevention of hospitalization due to respiratory syncytial virus: results from the Palivizumab Outcomes Registry. J Perinatol. 2008;28:511–517. doi: 10.1038/jp.2008.28. [DOI] [PubMed] [Google Scholar]
- 42.Lacaze-Masmonteil T, Roze JC, Fauroux B. Incidence of respiratory syncytial virus-related hospitalizations in high-risk children: follow-up of a national cohort of infants treated with Palivizumab as RSV prophylaxis. Pediatr Pulmonol. 2002;34:181–188. doi: 10.1002/ppul.10175. [DOI] [PubMed] [Google Scholar]
- 43.Lesnick BL, Huddle ME, Shi S, Bomar R. Efficacy of palivizumab use in high-risk infants, 1999–2003. Chest. 2003;124:73S. doi: 10.1378/chest.124.4_MeetingAbstracts.73S. [DOI] [Google Scholar]
- 44.Oh PI, Lanctot KL, Yoon A, et al. Palivizumab prophylaxis for respiratory syncytial virus in Canada: utilization and outcomes. Pediatr Infect Dis J. 2002;21:512–518. doi: 10.1097/00006454-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J. 2003;22:823–827. doi: 10.1097/01.inf.0000086403.50417.7c. [DOI] [PubMed] [Google Scholar]
- 46.Simon A, Nowak H, Sterz R. Use of palivizumab in Germany: data from 2002–2007. Klin Padiatr. 2011;223:292–298. doi: 10.1055/s-0030-1270515. [DOI] [PubMed] [Google Scholar]
- 47.Chi H, Hsu CH, Chang JH, et al. A novel six consecutive monthly doses of palivizumab prophylaxis protocol for the prevention of respiratory syncytial virus infection in high-risk preterm infants in Taiwan. PLoS One. 2014;9:e100981. doi: 10.1371/journal.pone.0100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakazawa M, Saji T, Ichida F, et al. Guidelines for the use of palivizumab in infants and young children with congenital heart disease. Pediatr Int. 2006;48:190–193. doi: 10.1111/j.1442-200X.2006.02179.x. [DOI] [PubMed] [Google Scholar]
- 49.Nishida H, Fujimura M, Takeuchi Y, et al. Prevention of RS virus infection (the guidelines for the use of palivizumab in Japan) J Jpn Pediatr Soc. 2002;106:1288–1292. [Google Scholar]
- 50.Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32–36 weeks’ gestational age. Pediatrics. 2010;126:115–128. doi: 10.1542/peds.2009-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res. 1986;20:899–904. doi: 10.1203/00006450-198609000-00019. [DOI] [PubMed] [Google Scholar]
- 52.Bird TM, Bronstein JM, Hall RW, et al. Late preterm infants: birth outcomes and health care utilization in the first year. Pediatrics. 2010;126:e311–e319. doi: 10.1542/peds.2009-2869. [DOI] [PubMed] [Google Scholar]
- 53.Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419–425. doi: 10.1001/jama.2010.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitsommart R, Janes M, Mahajan V, et al. Outcomes of late-preterm infants: a retrospective, single-center, Canadian study. Clin Pediatr (Phila). 2009;48:844–850. doi: 10.1177/0009922809340432. [DOI] [PubMed] [Google Scholar]
- 55.Pulver LS, Denney JM, Silver RM, Young PC. Morbidity and discharge timing of late preterm newborns. Clin Pediatr (Phila). 2010;49:1061–1067. doi: 10.1177/0009922810376821. [DOI] [PubMed] [Google Scholar]
- 56.Sampalis JS, Langley J, Carbonell-Estrany X, et al. Development and validation of a risk scoring tool to predict respiratory syncytial virus hospitalization in premature infants born at 33 through 35 completed weeks of gestation. Med Decis Making. 2008;28:471–480. doi: 10.1177/0272989X08315238. [DOI] [PubMed] [Google Scholar]
- 57.Blanken MO, Koffijberg H, Nibbelke EE, Rovers MM, Bont L. Prospective validation of a prognostic model for respiratory syncytial virus bronchiolitis in late preterm infants: a multicenter birth cohort study. PLoS One. 2013;8:e59161. doi: 10.1371/journal.pone.0059161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simoes EA, Carbonell-Estrany X, Fullarton JR, et al. A predictive model for respiratory syncytial virus (RSV) hospitalisation of premature infants born at 33–35 weeks of gestational age, based on data from the Spanish FLIP Study. Respir Res. 2008;9:78. doi: 10.1186/1465-9921-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanctot KL, Masoud ST, Paes BA, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32–35 weeks: a Canadian-based analysis. Curr Med Res Opin. 2008;24:3223–3237. doi: 10.1185/03007990802484234. [DOI] [PubMed] [Google Scholar]
- 60.Paes B, Steele S, Janes M, Pinelli J. Risk-Scoring Tool for respiratory syncytial virus prophylaxis in premature infants born at 33–35 completed weeks’ gestational age in Canada. Curr Med Res Opin. 2009;25:1585–1591. doi: 10.1185/03007990902929112. [DOI] [PubMed] [Google Scholar]
- 61.Samson L. Prevention of respiratory syncytial virus infection. Paediatr Child Health. 2009;14:521–532. doi: 10.1093/pch/14.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carbonell-Estrany X, de Lazaro y Mercado P. Health economics and RSV. Paediatr Respir Rev. 2009;10(Suppl 1):12–13. doi: 10.1016/S1526-0542(09)70006-5. [DOI] [PubMed] [Google Scholar]
- 63.Checchia PA, Nalysnyk L, Fernandes AW, et al. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: a systematic literature review and meta-analysis. Pediatr Crit Care Med. 2011;12:580–588. doi: 10.1097/PCC.0b013e3182070990. [DOI] [PubMed] [Google Scholar]
- 64.Andabaka T, Nickerson JW, Rojas-Reyes MX, et al. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013;4:CD006602. doi: 10.1002/14651858.CD006602.pub4. [DOI] [PubMed] [Google Scholar]
- 65.Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 66.Yoshihara S, Kusuda S, Mochizuki H, et al. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132:811–818. doi: 10.1542/peds.2013-0982. [DOI] [PubMed] [Google Scholar]
- 67.Simoes EA, Carbonell-Estrany X, Rieger CH, et al. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126:256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Q, Patel NK, McAuliffe JM, et al. Natural polymorphisms and resistance-associated mutations in the fusion protein of respiratory syncytial virus (RSV): effects on RSV susceptibility to palivizumab. J Infect Dis. 2012;205:635–638. doi: 10.1093/infdis/jir790. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Q, McAuliffe JM, Patel NK, et al. Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J Infect Dis. 2011;203:674–682. doi: 10.1093/infdis/jiq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carbonell-Estrany X, Simoes EA, Dagan R, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 71.Feltes TF, Sondheimer HM, Tulloh RM, et al. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr Res. 2011;70:186–191. doi: 10.1203/PDR.0b013e318220a553. [DOI] [PubMed] [Google Scholar]
- 72.Golombek SG, Berning F, Lagamma EF. Compliance with prophylaxis for respiratory syncytial virus infection in a home setting. Pediatr Infect Dis J. 2004;23:318–322. doi: 10.1097/00006454-200404000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.