Abstract
The integrase strand transfer inhibitors (INSTIs) are the newest antiretroviral class in the HIV treatment armamentarium. Dolutegravir (DTG) is the only second-generation INSTI with FDA approval (2013). It has potential advantages in comparison to first-generation INSTI’s, including unboosted daily dosing, limited cross resistance with raltegravir and elvitegravir, and a high barrier to resistance. Clinical trials have evaluated DTG as a 50-mg daily dose in both treatment-naïve and treatment-experienced, INSTI-naïve participants. In those treatment-naïve participants with baseline viral load <100,000 copies/mL, DTG combined with abacavir and lamivudine was non-inferior and superior to fixed-dose combination emtricitabine/tenofovir/efavirenz. DTG was also superior to the protease inhibitor regimen darunavir/ritonavir in treatment-naïve participants regardless of baseline viral load. Among treatment-experienced patients naïve to INSTI, DTG (50 mg daily) demonstrated both non-inferiority and superiority when compared to the first-generation INSTI raltegravir (400 mg twice daily) regardless of the background regimen. No phenotypically significant DTG resistance has been demonstrated in INSTI-naïve participant trials. The VIKING trials evaluated DTG’s ability to treat persons with HIV with prior INSTI exposure. VIKING demonstrated twice-daily DTG was more efficacious than daily dosing when treating participants receiving and failing first-generation INSTI regimens. DTG maintained potency against single mutations from any of the three major INSTI pathways (Y143, H155, Q148); however, the Q148 mutation with two or more additional mutations significantly reduced its potency. The long-acting formulation of DTG, GSK1265744LA, is the next innovation in this second-generation INSTI class, holding promise for the future of HIV prevention and treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-014-0029-7) contains supplementary material, which is available to authorized users.
Keywords: Antiretroviral therapy (ART), Dolutegravir (DTG), GSK1265744LA, HIV, Integrase strand transfer inhibitor (INSTI), Nanoparticle formulation
Introduction
The ability of HIV to rapidly mutate and develop resistance to standard antiretroviral therapy (ART) necessitates the ongoing drug development of new and efficacious therapeutic options that are well tolerated and evade prior resistance pathways. In the last decade, development of new antiretroviral drugs has rapidly expanded, resulting in six ART drug classes: nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs/NtRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and newer drug classes including fusion inhibitors (FIs), CCR5 co-receptor antagonists (CRAs), and integrase strand transfer inhibitors (INSTIs). These newer drug classes provide novel mechanisms of action, but with certain limitations. For example, enfuvirtide, a FI, requires twice-daily injections and maraviroc, a CRA requires an expensive tropism assay to determine effectiveness. The INSTIs provide advantages in newer HIV treatment options by offering minimal toxicity, daily dosing, and antiviral activity against viruses resistant to other drug classes. The objective of this review is to provide an update and overview of DTG, the newest generation INSTI.
Overview of Available FDA-Approved INSTI Drugs
Three drugs in the INSTI class are currently approved by the FDA: raltegravir (RAL) (2007 treatment experienced only, 2009 treatment naïve approval), elvitegravir (EVG) (2012 as a combination pill), and most recently dolutegravir (DTG) (2013 approval for use in treatment-naïve and treatment-experienced adolescents and adults, including those who have been treated with other integrase strand transfer inhibitors) (Table 1). The INSTI mechanism of action is to prevent HIV integrase from incorporating proviral DNA into the human host cell, thus inhibiting the HIV-catalyzed strand transfer step. This step has no human homolog, making it a specific and effective HIV drug target with excellent tolerability and minimal toxicity.
Table 1.
Comparison of currently available and future integrase inhibitors
| Drug | Raltegravir | Elvitegravir | Dolutegravir | GSK1265744LA |
|---|---|---|---|---|
| Manufacturer | Merck & Co | Gilead Sciences | ViiV & GSK | ViiV |
| Generation | First | First | Second | Second |
| FDA approved | 2007 | 2012 | 2013 | N/A |
| Tolerability | Good | Good | Good | N/A |
| Food requirement | No | Yes | No | No |
| Metabolism | UGT1A1 | Cytochrome P450 CYP3A4 major; UGT1A1/3 minor |
Glucuronidation UGT1A1 major; CYP3A4 minor |
|
| Half-life: t 1/2 | 9 h | 3 h alone; 9 h with cobicistat boost | 12–15 h (average 14 h) | 21–50 days (nanosuspension) |
| Renal/biliary | Competitive use of UGT1A1a to metabolize drug and unconjugated bilirubin | Inhibitor of OCT2b, inhibiting creatinine secretion, no effect on GFR; small rise in bilirubina | ||
| Adult dosing | 400 mg twice daily | Daily combination pill | 50 mg daily in INSTI-naïve; 50 mg twice daily in INSTI-experienced |
Treatment: 600-mg bimonthly injection (under investigation) PrEP: 800-mg quarterly injection (under investigation) |
| Pediatric dosing | Chewable tabs 100 and 25 mg | Not available | 50 mg once daily in 12 years and older and weighing at least 40 kg; pediatric granule formulation in process | Not available |
| Co-treatment for tuberculosis | Rifampin: decreases AUC, induces UGT1A1, increase dose by 100%; rifabutin: no dose adjustment | Avoid with rifabutin due to drug/drug interactions | Rifampin: dose adjust 50 mg twice daily; rifabutin: no need to dose adjust | |
| Cross-resistant | Yes | Yes | No; exception may be Q148 + ≥2 additional mutations | No |
AUC area under curve, GFR glomerular filtration rate, GSK GlaxoSmithKline, INSTI integrase strand transfer inhibitor, PrEP pre-exposure prophylaxis, t 1/2 half-life
aUGT1A1 is the same metabolic enzyme that processes unconjugated bilirubin setting up a competitive use
bHuman organic cation transporter
The INSTI’s are generally metabolized by glucuronidation by the hepatic enzyme UGT1A1. EVG is unique among this drug class as it is primarily metabolized by the potent hepatic and intestinal cytochrome P450 (CYP3A4); for this reason, EVG must be pharmacokinetically boosted with a CYP3A4 inhibitor. Cobicistat (COBI) is currently FDA approved for this purpose in a combination “quad” pill: EVG/COBI/tenofovir (TDF)/emtricitabine (FTC).
INSTI: The First Generation
Numerous clinical trials have investigated optimal dosing and efficacy of the integrase inhibitors. RAL 800 mg daily dosing is statistically inferior (P = 0.04) to 400-mg twice-daily dosing when combined with the daily fixed-dose combination of FTC/TDF [1]. The STARTMRK study (NCT00369941) of treatment-naïve participants demonstrates that those who received daily FTC/TDF plus RAL 400 mg twice daily have non-inferior virologic outcomes as compared to the daily fixed-dose combination of FTC/TDF/efavirenz (EFV) at 48 weeks [2], 96 weeks [3], and sustained to 156 weeks [4]. The RAL regimen has fewer adverse events and significantly less elevation of fasting lipids from baseline to week 144 when compared to EFV [4]. The maker of RAL, Merck, funded these studies.
The daily fixed-dose combination EVG/COBI/TDF/FTC is also non-inferior to FTC/TDF/EFV at 48 weeks [5], 96 weeks [6] and sustained to 144 weeks [7]. When tested against the combination FTC/TDF plus a daily protease inhibitor backbone regimen atazanavir 300 mg/ritonavir 100 mg (ATZ/r), EVG/COBI/TDF/FTC is non-inferior at 48 weeks [8] and 96 weeks [9]. These studies support the durable efficacy and safety profile of this INSTI daily formulation. Gilead, the maker of EVG and the “quad” pill, funded these studies.
Based on these clinical trials data, RAL in combination with FTC/TDF is a recommended first-line therapy for starting ART [10–12]. EVG in the form of the “quad” pill is also an acceptable starting regimen for ART-naïve patients with pre-treatment creatinine clearance >70 mL/min [10]. Monitoring creatinine is necessary as COBI blocks the renal tubular secretion, though with no appreciable affect on glomerular filtration rate (GFR).
INSTI: The Next Generation
Dolutegravir is the latest ART agent to be FDA approved. It is a second-generation integrase inhibitor, named for its unique properties: unboosted daily dosing, a high barrier to resistance, low cross-resistance to the first-generation INSTI’s, and is now a preferred ART regimen to initiate treatment among HIV-infected adolescents and adults [13].
In Vitro and In Vivo Studies (Table 2)
Table 2.
Important clinical trials for dolutegravir
| Study design and funding | Setting and demographics | Results | Conclusion | |
|---|---|---|---|---|
| Phase 1 |
Dose-finding [15] R, DB, PC Funding: GSK |
S: USA D: single dose: 75% Caucasian; 83% male (n = 10; 8 = drug, 2 = placebo) Multiple dose: 85% Caucasian; 90% male |
IC: healthy adults R: single dose study: Cohort 1: received 2 mg, 10 mg, 50 mg; Cohort 2: received 5, 25, 100 mg. Multiple dose study: Cohort 1: 10-mg QD; Cohort 2: 25a mg QD; Cohort 3: 50-mg QD × 10 days Results: daily dose of 50 mg maintained levels 25-fold higher than the IC90; t 1/2 15 h; minimal to no CYP3A4 activity based on midazolam experiment |
Daily dose of 50 mg will achieve therapeutic levels |
|
IMPAACT P1093 I/II OL Cohort 1 [38] Cohort 2 [40] Funding: IMPAACT as funded by NIH, NIAID, NICHD, NIMH and ViiV Healthcare |
S: USA D: Cohort 1 (12–18 years old): 22% male, x = 15 years old (IQR 12, 16) n = 23 participants Cohort 2 (>6 and <12 years old): 64% male, 36% African American, x = 9.5 years old, n = 11 participants |
IC: meeting the cohort age designation; failing ART regimen (HIV-1 RNA >1,000 c/mL) OL: DTG ~1 mg/kg daily was added to the failing regimen for intensive PK evaluation on days 5–10. Then OBR with at least one fully active drug (30% received FTC/TDF/DRV/r) 1°EP: HIV-1 RNA <400 c/mL or >1 log10 decline at 24 weeks; 2°EP HIV-1 RNA <400 c/mL or >1 log10 decline at 48 weeks Results: Cohort 1: baseline HIV-1 RNA was 4.3 log10 c/mL, and 83% ≥40 kg receiving 50 mg daily dose. At 24 weeks, 83% demonstrated virologic suppression <400 c/mL (70% <50 c/mL at 24 weeks); at 48 weeks this fell to 74% remaining virologically suppressed (61% <50 c/mL) due to incomplete adherence. Cohort 2: baseline HIV-1 RNA was 5.0 log10 c/mL. At 24 weeks, virologic suppression was achieved in 82% (64% <50 c/mL); 48 weeks data not available Pending Cohort 3: 2 to <6 years; Cohort 4: 6 months to <2 years; Cohort 5: >6 weeks to <6 months |
Weight band of 1 mg/kg appears a safe and efficacious dose among children and adolescents in this clinical study | |
| Phase 2 |
PK/PD (2a) R, DB, dose-finding [16] Funding: ViiV Healthcare |
S: USA D: 80% Caucasian; 100% males; n = 35 participants |
IC: ≥18 years old, HIV-infected, INSTI naïve; not receiving ART R: placebo (n = 7), 2 mg (n = 9), 10 mg (n = 9), and 50 mg (n = 10) for 10 days monotherapy; baseline characteristics were similar across all groups Results: significant reduction in HIV-1 RNA in all treatment groups when compared to placebo (P < 0.001); decrease of HIV RNA 1.51–2.46 log10 copies on monotherapy depending on dose received (day 11) |
DTG demonstrated potency, tolerability, and predictable PK/PD relationships |
|
SPRING-1 (2b) R, PB (dose-masked) OL 48 weeks [27] 96 weeks [28] Funding: ViiV Healthcare |
S: USA and Europe (Spain, France, Germany, Italy, Russia) D: 80% Caucasian; 86% male; x = 37 years old |
IC: ≥18 years, naïve to ART, VL >1,000 c/mL; CD4+ >200 c/μL R (1:1:1:1): DTG 10, 25, 50 mg versus EFV 600 mg with investigator-selected NRTI backbone ABC/3TC or TDF/FTC 1°EP: VL <50 c/mL at week 16 2°EP: VL <50 c/mL at 24 and 48 weeks Results: at 16 weeks, rate of viral decay was robust such that 96, 92, and 90% of 50, 25, 10 mg doses respectively with <50 c/mL compared to 60% for those receiving EFV (1°EP); at 48 weeks results were 91, 88, 90%, versus 82% EFV, respectively (2°EP), DTG sustained efficacy and tolerability through week 96: 88% maintained viral response <50 c/mL for the 50 mg DTG arm versus 72% EFV arm. In the EFV arm, 10% withdrew due to adverse events versus 3% in the DTG arm influencing this difference |
DTG demonstrated rapid viral decay as compared to EFV 50 mg daily dose was chosen for phase 3 (maximum tolerated; all doses efficacious) No emerging resistance on DTG |
|
|
VIKING (2b) dosing study OL [22] Funding: ViiV Healthcare |
S: France, Italy, Spain, Canada, US D: 84% Caucasian; 84% male, x = 48 years old |
IC: ≥18 years. Treatment experienced with RAL, VL >1,000 c/mL, genotypic INSTI resistance, and ≥1 compound with genotypic/phenotypic resistance in ≥2 classes NRTI, NNRTI, or PI classes R: Cohort 1 (n = 27) daily dosing; Cohort 2 (n = 24) twice daily dosing. DTG was substituted for RAL continuing the failing background regimen to day 10. On day 11, an OBR with at least 1 active drug was substituted 1°EP: HIV RNA ≥0.7 log decrease from baseline or <400 c/mL at day 10. 2°EP: change from baseline HIV-1 RNA after day 11 on OBR, proportion of those suppressed (<400 or <50 c/mL), change in CD4+ cell count Results: 96% in cohort 2 versus 78% in cohort 1 reached 1°EP. At week 24 with an OBR, 75% (cohort 2) versus 41% (cohort 1) had VL <50 c/mL at 24 weeks. A higher IC50 fold change was noted in daily dosing, especially when Q148 + 2 additional mutations were present |
In treatment-experienced participants, twice-daily DTG was better than daily dosing Mutation combination Q148 + ≥2 additional mutations was most likely to confer DTG resistance |
|
| Phase 3 ART naive |
SPRING-2 R, DB NI 48 weeks [29] 96 weeks [30] Funding: ViiV Healthcare |
S: Canada, USA, Australia, Europe D: 85% Caucasian; 85% male, x = 36 years old |
IC: ≥18 years, naïve to ART, VL >1,000 c/mL; CD4+ >200 c/μL R (1:1): RAL BD compared to DTG QD with investigator-selected NRTI backbone ABC/3TC or TDF/FTC 1°EP: VL <50 c/mL at week 48 2°EP: CD4, severity of AE, lab parameters, evidence of resistance. VF defined as confirmed VL >50 c/mL Results: DTG was NI to RAL at 48 weeks regardless of NRTI background regimen (88% versus 85%) and NI was sustained to 96 weeks (81% versus 76%, respectively). None in DTG had genotypic or phenotypic emerging resistance |
DTG is NI to RAL The potential advantage of DTG (QD) versus RAL (BD) could not be assessed due to placebo-dosed randomization No emerging resistance on DTG |
|
SINGLE R, DB, NI → Superiority 48 weeks [32] 96 weeks [33] (OL after 96 weeks) Funding: ViiV Healthcare |
S: Canada, USA, Australia, Europe D: black (24%); non-white (32%); males (84%); x = 35 years |
IC: ≥18 years, naïve to ART, VL >1,000 c/mL, screening for HLA-B*5701, a contraindication to ABC use R: DTG + ABC/3TC versus FTC/TDF/EFV stratified by VL ≤ or >100,000 c/mL and CD4 ≤ or >200 cells/mL 1°EP: VL <50 c/mL at week 48 Results: DTG demonstrated rapid viral suppression at 28 versus 84 days in the EFV arm (P < 0.0001). 1°EP: 88% DTG + ABC/3TC versus 81% FTC/TDF/EFV meeting NI, and also superiority (P = 0.003, ITT) at 48 weeks and persisted to 96 weeks, 80% versus 72%, respectively (P = 0.006; 95% CI 2.3%, 13.8%). When stratified by VL >100,000 this difference was lost |
ABC/3TC/DTG is superior to FTC/TDF/EFV DTG statistically significant more rapid virologic decay compared to EFV No primary emerging resistance on DTG |
|
|
Flamingo [34] R, OL NI → Superiority Funding: ViiV Healthcare |
S: well-resourced countries D: non-white (28%); males 85%; x = 34 years; n = 484 |
IC: ≥18 years, naïve to ART, VL >1,000 c/mL OL: DTG 50 mg QD versus DRV/r 800 mg/100 mg QD with background either TDF/FTC or ABC/3TC. Stratified by VL ≤ or >100,000 c/mL (25% >100,000 c/mL) 1°EP: VL <50 c/mL at week 48 (NI margin −12%) Results: 48-week snapshot analysis showed 90 versus 83% had VL <50 c/mL. This demonstrated not only NI, but also S (P = 0.025; adjusted difference 7.1%; 95% CI 0.9–13.2). When stratified by those with VL >100,000 DTG superior to DRV/r 93% versus 70%, respectively. Fewer AE with DTG contributed to superiority. DTG had lower LDL values (2% versus 7%, P < 0.001) and less diarrhea (17% versus 29%) |
DTG is superior to DRV/r in treatment-naïve participants | |
| Phase 3 ART experienced |
SAILING [35] R, DB, NI Funding: ViiV Healthcare |
S: 1st to include RLSb Australia, Canada, Europe D: 68% male; 48% from RLS. 68% subtype B; 14% subtype C; 6% complex subtype. x = 43 years n = 715 participants |
IC: ART-experienced, INSTI-naïve; VL >400 c/mL × 2 consecutive or >1,000 c/mL at screening; resistance to ≥2 classes of ARV with 1–2 fully active drugs for OBR stratified by VL ≤ or >50,000 c/mL and DRV/r R: DTG 50 mg QD versus 400 mg RAL BD and investigator-selected OBR. 1°EP: HIV-1 RNA <50 c/mL at week 48. 2°EP: proportion of patients with tx-emergent INSTI resistance Results: 71% in DTG and 64% in RAL met 1°EP. Pre-specified statistical criteria revealed NI of DTG to RAL (adjusted treatment difference greater than −12%) and superiority (P = 0.03 mITT-E analysis; 95% CI >0). Results were stratified by DRV/r use in the presence of primary PI mutations or no DRV/r use versus DRV/r use in the absence of primary mutations and by VL ≤ or >50,000 c/mL. Significantly fewer patients receiving DTG had treatment-emergent INSTI resistance 4/354 (1%) versus 17/361 (5%) RAL arm (P = 0.003). One patient in each arm had baseline pre-existing RAL primary resistance (DTG, Q148H/G140S pathway; RAL, Y143 pathway) and associated elevated fold changes. The genotypic resistance developed in 1% in the DTG arm, though this did not translate to de novo phenotypic resistance |
DTG both NI and superior to RAL among ART-experienced but INSTI-naïve participants The benefit of daily dosing could not be assessed given the blinded nature of the trial No phenotypic resistance to DTG occurred, although 1% did have INSTI genotypic substitutions |
|
VIKING-3 Single arm, OL Week 24 [23] Week 48 [36] Funding ViiV Healthcare |
S: US, Canada, Europe D: 77% male; 71% white; n = 183 participants |
IC: >18 years, VF ≥500 c/mL, documented resistance to RAL and/or EVG + ≥2 other classes with 1 active option remaining for OBR OL: DTG 50-mg BD to evaluate a monotherapy phase of DTG in INSTI-resistant patients for 7 days; OBR from day 8 to 24 weeks 1°EP was changed in baseline HIV-1 RNA at 8 days; proportion achieving <50 c/mL at week 24 Results: on day 8, mean change from baseline RNA was −1.43 log10 c/mL (95% CI −1.52, −1.34). Major resistance mutations included 79% with ≥2 NRTI, 70% with ≥2 PI, 75% had ≥1 NNRTI, 62% with CXCR4 virus detected. Because of this variety, OBR changes on day 8 were diverse. At week 24, 69% achieved <50 c/mL; 56% at week 48. Presence of Q148 plus ≥2 additional mutations were associated with reduction of 0.69 log10 c/mL in day 8 response as compared to those with no Q148 mutation at baseline (P < 0.001) |
DTG 50-mg BD was potent as functional monotherapy in highly resistant participants Presence of mutation Q148 plus 2 or more additional mutations reduced effectiveness |
|
|
VIKING 4 [37] R, DB, PC OL on day 8 Funding: ViiV Healthcare |
Not published |
IC: ≥18 years; HIV-1 RNA >1,000 c/mL, ART experienced with INSTI resistance; >2 additional class resistance R: DTG 50 mg BID + OBR versus placebo + OBR; day 8 becomes OL: DTG 50 mg BD + OBR versus placebo + OBR 1°EP: mean change from baseline in HIV RNA at day 8; 2°EP: proportion of patients with HIV RNA <400 c/mL, <50 c/mL Results: not yet published nor presented |
Different from VIKING 3 in that includes a randomized placebo |
1°EP primary endpoint, 2°EP secondary endpoint, ABC/3TC abacavir/lamivudine, AE adverse events, ART antiretroviral therapy, BD twice daily dose, c/mL copies/mL, CI confidence interval, D demographics, DB double-blind, DRV/r darunavir/ritonavir, DTG dolutegravir, EFV efavirenz, F funding, FTC emtricitabine, GSK GlaxoSmithKline, IC inclusion criteria, IC 50 half-maximal inhibitory concentration, IC 90 ninety percent inhibitor concentration, INSTI integrase strand transfer inhibitors, IQR interquartile range, ITT intention to treat, LDL low-density lipoprotein, mITT-E modified intent-to-treat-exposed, NI non-inferior, NIAID National Institute of Allergy and Infectious Diseases, NICHD National Institute of Child Health and Human Development, NIH National Institutes of Health, NIMH National Institute of Mental Health, NNRTI non-nucleoside reverse transcriptase inhibitors, NRTI nucleoside reverse transcriptase inhibitors, OBR optimized background regimen, OL open label, PB partially blind, PC placebo-controlled, PD pharmacodynamics, PI protease inhibitor, PK pharmacokinetics, QD daily dose, R randomized, RAL raltegravir, RLS resource-limited setting, S setting, t 1/2 half-life, TDF tenofovir, VF virologic failure, VL viral load, x average age
aThose receiving 25 mg had a sub-study with midazolam to test CYP3A4 activity
bLatin America, Taiwan, South Africa and USA
Selecting an appropriate drug dose and predicting the dose response requires evaluation of both pharmacokinetics (PK) and pharmacodynamics (PD). The in vitro protein-adjusted half-maximal effective concentration (PA-EC50) of DTG is 75 nM or 31.4 ng/mL [14]. The in vitro protein-adjusted half-maximal inhibitory concentration (PA-IC50), against HIV in peripheral blood mononuclear cells was 0.5 nM [15]. PD characteristics in vitro estimate the protein-adjusted ninety percent inhibitor concentration (PA-IC90) to be 0.064 μg/mL [15, 16]. In a phase 1 trial, drug concentrations reached steady state in plasma by approximately 5 days and half-life (t 1/2) between 13 and 15 h [15]. DTG demonstrated excellent oral bioavailability, a moderate elimination of half-life, and this study maintained the drug trough concentration well exceeding the PA-IC90 0.064 μg/mL by 5- to 26-fold, predicting its potency as a new antiretroviral therapeutic agent.
Understanding the correlation of dose–response and change in HIV-1 RNA informs optimal dosing, especially if higher doses carry safety concerns. The PD of DTG was evaluated in a phase 2a placebo-controlled trial among HIV-infected, INSTI-naïve participants. DTG 2, 10, or 50 mg versus placebo were randomly assigned, and the plasma drug level and rate of HIV RNA decline were measured [16]. The PA-IC90 was maintained with daily dosing of 50 mg DTG throughout a 10-day dosing interval with a well-described exposure–response relationship, low inter-patient variability, and predictable PK-PD properties [16]. Potency was demonstrated with a 2.5 log10 copies/mL mean decline in viral load after receiving 10 consecutive days of 50 mg daily DTG as monotherapy; 70% (7/10) had viral load <50 copies/mL by day 10 of monotherapy which was sustained to day 14 [16].
Given these promising data, PK studies evaluated DTG for interactions with potential ART combinations. ATZ is a PI that is a UGT1A1 inhibitor. Concurrent dosing of ATZ 300 mg daily or ATZ/r (300/100 mg, respectively) with DTG was found to be safe, not requiring dose adjustment when combined with DTG [17]. Similar studies evaluated the combination of etravirine (200 mg twice daily), a NNRTI and a CYP34A inducer, as well as etravirine plus the PI combination lopinavir/ritonavir (200/400/100 mg twice daily, respectively). Though the CYP34A metabolic pathway of DTG is minor, etravirine did significantly reduce the exposure of DTG such that this combination without a boosted PI should be avoided [18]. When co-administered with a PI, however, the inhibitory effect of UGT1A1 presumably counteracts the inducement of CYP34A and no dose adjustment is necessary with this triple ART regimen [18].
Resistance
Genotypic assays identify specific mutations that may be associated with phenotypic resistance. DTG was specifically engineered to deliver a novel resistance profile to avoid cross resistance with the first-generation INSTI class and to maintain a high barrier to resistance [19].
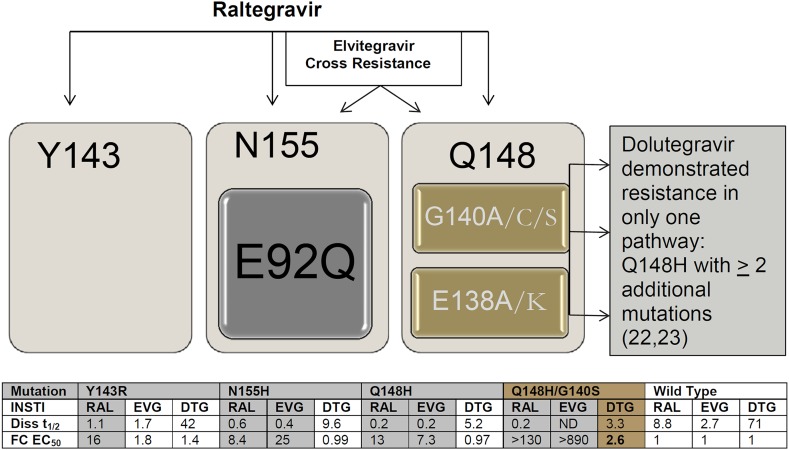
In vitro passaging experiments identify resistance mutations by passaging virus in cell culture in the presence of drug to select resistance. Biochemical studies suggest INSTI’s bind to HIV integrase in a two-step mechanism. Mutations may alter the second step and lead to fast INSTI dissociation kinetics that contribute to the development of integrase resistance. In biochemical analyses with wild-type integrase DNA complexes, DTG demonstrated a dissociative t 1/2 of 71 h as compared to 8.8 h for RAL and 2.7 h for EVG; thus, DTG exhibited an off-rate 5–40 times slower than RAL and EVG (P < 0.0001) (Fig. 1) [20]. This slow dissociation may contribute to DTG’s high barrier to resistance and suggests that prolonged binding plays a role in its unique resistance profile [20, 21]. Single mutations of the major RAL pathway Y143, N155, and Q148 do not increase DTG-fold change, and have variable effect on the off-rate of DTG with half-lives of dissociation from 42 to 60 h for Y143 mutants, 9.6 h for N155H, and 5.2 to 11 h for Q148 mutants. Q148 plus additional mutations do increase the dissociative kinetics and impart a fold change. A fold change ≥3 as measured by change in half-maximal effective concentration (EC50) of mutant versus wild-type HIV-1 was considered resistant for in vitro studies [19, 21]. When mutations Q148H and G140S are present, the dissociative t 1/2 of DTG is reduced to 3.3 h [20] with a 2.6-fold change in EC50 [19]. The VIKING studies (discussed below; NCT01328041, NCT00950859) demonstrate that DTG maintains activity against RAL- and EVG-resistant virus [22]; however, treatment-experienced participants with Q148 + ≥2 associated mutations had reduced potency when compared to no Q148 mutations at baseline (P < 0.0001) [23]. The current FDA label cautions that poor virologic response has been observed in subjects with a Q148 substitution plus two or more additional INSTI-resistance substitutions [24] (Fig. 1). These data underpin the danger in maintaining a failing regimen that may lead to further accumulation of resistance mutations that can impact the efficacy of newer drug options.
Fig. 1.
INSTI pathways of HIV-1 resistance with associated dissociative t 1/2 and fold change in EC50 [19] compared to wild-type virus. Diss t 1/2 dissociative values previously reported [20, 21]. Major integrase mutations are denoted in black bold: E92Q/V; Y143C/H/R; Q148H/K/R; N155H. Accessory mutations are denoted in gray: E138A/K; G140A/C/S [25]. DTG dolutegravir, EC 50 half-maximal effective concentration, EVG elvitegravir, FC fold change, INSTI integrase strand transfer inhibitor, ND not determined, RAL raltegravir, t 1/2 half-life
Evaluation of 3,294 genotypic resistance tests ordered for clinical decision making from 2009 to 2012 at a United States national referral lab revealed that integrase resistance mutations were often paired with PI resistance [25]. Although the treatment regimen was not available, presumably subjects included in the database were receiving RAL based on the timing of FDA approvals. Three major resistance pathways reportedly lead to RAL resistance: Y143, N155, and Q148 all of which are in close proximity to the integrase active site and may reduce viral fitness [25]. DTG remains active against those with single mutations, but accumulation of resistance mutations in the Q148 pathway can compromise DTG activity. Those with serial genotypic tests (n = 224) and wild-type virus at baseline (n = 22) accumulated INSTI mutations on average by 224 days, with equal distribution of the three major pathways. Overall, high-level DTG resistance was predicted in 12% of patients with RAL- or EVG-resistant virus (Q148 + ≥2 additional integrase mutations; the majority with Q148 + G140 + E138). Thus, those failing treatment regimens containing first-generation INSTI should be changed early to preserve the second-generation INSTI with high barrier to resistance.
Clinical Trials of Dolutegravir (Table 2)
Clinical trials of DTG have been conducted in both treatment-naïve and treatment-experienced patients. Most clinical trials are statistically powered for non-inferiority to demonstrate that the new treatment is no less effective than standard therapy. In certain circumstances, superiority may be demonstrated. Clinical equivalence (Δ) is the largest difference that is clinically acceptable such that a larger difference would alter clinical practice [26]. In a non-inferiority trial, clinical equivalence should be clearly defined such that non-inferiority is demonstrated when the 95% confidence interval (CI) falls entirely to the right of the lower limit (−Δ). If the 95% CI of the tested treatment effect lies both above the lower limit of the pre-specified difference (−Δ) and above zero, the trial was properly designed and carried out in accordance with requirements of a non-inferiority trial, and the two-sided P value for superiority is presented according to the intention to treat (ITT) principle remains significant (P < 0.05), then superiority may also be claimed [26].
Trials Among ART-Naïve Participants
SPRING-1 (NCT00951015) is a dose-finding study comparing the increasing daily doses of DTG 10, 25, or 50 mg to efavirenz 600 mg with a dual-NRTI background regimen (FTC/TDF or abacavir (ABC)/lamivudine (3TC) in a randomized, open-label (dose-masked) trial [27]. Participants and investigators were not blinded to the study drug, but were blind to the DTG dose. Across the dosing spectrum of DTG, the rate of viral decay was robust and 50 mg daily dosing of DTG remained efficacious and well tolerated to 48 and 96 weeks [27, 28]. No treatment-emergent mutations were detected [28]. Creatinine clearance rose in week 1, gradually returning to baseline by week 48. Lipid profile was more favorable than with EFV with little to no increase from baseline [27, 28].
SPRING-2 (NCT01227824) followed as the first trial to compare the efficacy of two INSTI’s head to head: 400-mg twice-daily RAL versus 50-mg once-daily DTG in ART-naïve patients [29]. DTG was found to be non-inferior to RAL at 48 weeks, regardless of NRTI background regimen, with 88% versus 85% participants with HIV-1 RNA <50 copies/mL. Non-inferiority was sustained to 96 weeks (81% versus 76%, respectively) [30]. Fewer participants in the DTG group had protocol-defined virologic failure (8 versus 18), and no treatment-emergent resistance mutations were noted in the DTG arm. Of note, virologic failure was conservatively defined as two consecutive viral load measures >50 copies/mL. If participants were followed to a higher viral load, perhaps increased levels of resistance would have been detected; therefore lack of emergent resistance should be interpreted with caution [31]. Though safety in both arms was excellent, an increase in alanine aminotransferase (ALT) with possible drug-induced liver injury (DILI) was noted, one case in each study arm.
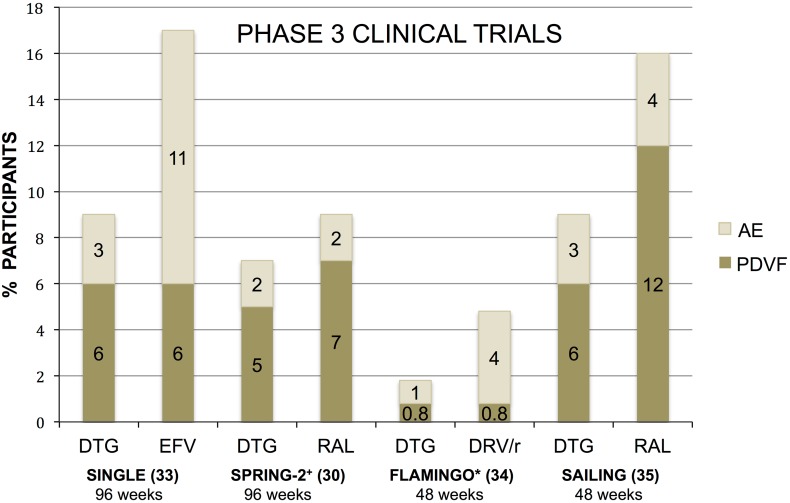
SINGLE (NCT01263015) is a randomized, double-blinded trial, comparing DTG plus ABC/3TC to the fixed-dose combination FTC/TDF/EFV in a non-inferiority statistical design [32]. The DTG arm had a rapid viral decay, with 28 days to viral suppression (<50 copies/mL) versus 84 days in the EFV arm (P < 0.0001). In the DTG arm, 88% had HIV-1 RNA <50 copies/mL at 48 weeks compared to 81% receiving EFV. This result met non-inferiority criteria, and also superiority (P = 0.003) in the ITT analysis with the 95% CI not crossing zero. The superior responses were primarily driven by less discontinuation of the DTG + ABC/3TC regimen as compared to FTC/TDF/EFV due to adverse events, (primarily neuropsychiatric with EFV and insomnia with DTG) (Fig. 2). Through 96 weeks, one individual receiving DTG and three individuals receiving TDF/FTC/EFV withdrew for insomnia. At week 96, 80% remained suppressed (<50 copies/mL) in the DTG + ABC/3TC arm compared to 72% in the TDF/FTC/EFV arm (P = 0.006; 95% CI 2.3%, 13.8%) [33]. This difference was less pronounced for those with baseline virologic failure >100,000 copies/mL due to withdrawals for reasons unrelated to treatment (DTG + ABC/3TC = 14, TDF/FTC/EFV = 8) (e.g., lost to follow-up, withdrawn consent, protocol deviation) [33]. No major resistance emerged on DTG, although a single polymorphism of E157Q/P was noted of uncertain significance and with no change in phenotypic susceptibility. The lack of resistance may reflect low-level viremia, with 20/25 (80%) participants having <200 copies/mL at the time of virologic failure at 96 weeks [33]. The study is continuing open label as of week 96.
Fig. 2.
Phase 3 clinical trials of DTG and comparator antiretroviral therapy evaluating PDVF criteria versus discontinuation due to adverse events. PDVF defined by study endpoint (>50 copies/mL) including those who never suppressed or those who rebounded; *FLAMINGO study endpoint (>200 copies/mL); +SPRING-2 study endpoint (>50 copies/mL × 2 from week 24–48; then up to 200 copies/mL after week 48). DRV/r darunavir/ritonavir, DTG dolutegravir, EFV efavirenz, PDVF protocol-derived virologic failure, RAL raltegravir
FLAMINGO (NCT01449929) is a randomized, open-label trial comparing DTG 50 mg daily versus darunavir/ritonavir (DRV/r) 800 mg/100 mg daily [34]. At 48 weeks, 90% receiving DTG versus 83% receiving DRV/r was virologically suppressed. The adjusted difference of 7.1% (95% CI 0.9–13.2%) and P = 0.025 in ITT analysis establishes DTG as both non-inferior and statistically superior to DRV/r. Virologic failure (>200 copies/mL) occurred in two participants in each study arm, and no primary mutations were captured. When stratified by baseline viral load, those with HIV RNA >100,000 copies/mL (~25%) revealed an even greater distinction with 93% of those in the DTG arm suppressed versus 70% in the DRV/r arm. Fewer adverse events and withdrawals occurred in the DTG group, and likely contributed to statistical superiority [34] (Fig. 2).
Clinical Trials of Dolutegravir in Treatment of ART-Experienced Patients
In SAILING (NCT01231516), ART-experienced, INSTI-naïve participants were randomized (1:1) to 50-mg daily DTG or 400-mg twice-daily RAL plus investigator-selected background therapy. SAILING was the first and thus far only DTG study to include resource-limited settings. Treatment was double-blinded, active-controlled, and designed as a non-inferiority study with statistical superiority analysis [35]. At week 48, 71% receiving DTG versus 64% receiving RAL demonstrated virologic suppression <50 copies/mL, meeting non-inferiority as well as superiority criteria [35]. Treatment-emergent resistance to the background regimen, 3% RAL and <1% DTG, and to INSTI, 5% RAL and 1% DTG. No phenotypic resistance to DTG was reported.
VIKING (NCT00950859) was the first study to evaluate DTG activity among participants with genotypic RAL resistance in a standard 50-mg daily dose (Cohort 1) [22]. During this study, a protocol amendment to include a cohort receiving twice-daily 50-mg DTG was created to compare efficacy (VIKING Cohort 2). Twice-daily dosing was found to be more efficacious both at day 10 (96% versus 78% for the primary endpoint of ≥0.7 log10 copies/mL change from baseline in HIV-1 RNA or <400 copies/mL) and at week 24 after optimizing the background regimen (OBR) (75% versus 41% with HIV-1 RNA <50 copies/mL). Those with viral mutations including Q148H/K/R plus G140S plus additional RAL mutations had a reduced response to DTG.
VIKING-3 (NCT01328041) further investigated the use of DTG in treating INSTI-experienced participants failing their current regimen (viral load >500 copies/mL). DTG was substituted for the first-generation INSTI, acting essentially as functional monotherapy until day 8 when OBR occurred [23]. On day 8 of DTG 50 mg twice daily, the average change of HIV-1 RNA from baseline was −1.43 log10 copies/mL (95% CI −1.52, −1.34). DTG was continued with OBR with at least one active drug on day 8, with 69% achieving <50 copies/mL at week 24, and 63% at week 48 [36]. The presence of Q148 plus ≥2 additional mutations was associated with reduced viral decay as compared to those with no Q148 mutation at baseline (P < 0.001). Baseline INSTI resistance (genotypic and phenotypic) and baseline viral load were highly significant predictors of response at week 24. For every twofold increase in DTG change, the odds of virologic suppression to <50 copies/mL were 63% lower, and were 96% lower if the virus contained Q148 +≥2 mutations.
VIKING 4 (unpublished; NCT01568892) is designed similar to VIKING 3, but with a randomized (1:1), double-blind placebo study design to quantitatively evaluate antiviral activity specifically attributed to DTG [37]. Results of this study are not yet published.
Pediatric Formulations
IMPAACT study P1093 (NCT01302847) is an ongoing Phase I/II safety and dose-finding study for treatment-experienced, INSTI-naïve infants, children and adolescents. Similar to the VIKING studies, DTG is first added to a failing regimen for 5–10 days, then OBR for further follow-up. This study is composed of five age-related cohorts ranging from >6 weeks up to 18 years. Data for the oldest two cohorts have been presented at scientific meetings [38–40]. The first cohort >12 and <18 years provided data contributing to the FDA label approving DTG down to 12 years of age with a weight minimum of 40 kg [24]. These pediatric studies measure virologic suppression <400 copies/mL at 24 weeks (83%) [38] and 48 weeks (74%) with an additional secondary endpoint as <50 copies/mL (70% and 61%, respectively) [39]. Virologic failure (<400 copies/mL) at week 48 in all 6 of 23 adolescents was attributed to incomplete adherence based on a 3-day pill recall questionnaire. There were no DTG drug-related adverse events and no discontinuations. The target area under the curve at 24 h (AUC24) and concentration at 24 h (C24) were achieved with ~1 mg/kg dosing [39, 40]. A pediatric granule formulation has been developed and tested, demonstrating that drug exposure exceeds that of the tablet form, is palatable, and can be given without food or liquid restrictions [41].
Adverse Events and Side Effects
Creatinine typically rises in the first 2 weeks after starting DTG, returning to baseline by 48 weeks [27, 29]. This rise in creatinine is attributed to DTG’s potent inhibition of human organic cation transporter (OCT2) that inhibits proximal renal tubular creatinine secretion without affecting GFR, similar to other drugs including trimethoprim and cimetidine [42]. Approximately 1.7% of aggregate participants in cited clinical trials experienced increased ALT levels (>5× the normal limit) with approximately three participants (~0.2%) with evidence of DILI, possibly attributed to DTG [23, 28, 29, 32, 35]. These findings have mostly been explained by the inclusion of participants co-infected with hepatitis B and/or hepatitis C, who experience immune reconstitution inflammatory syndrome (IRIS) attributed to the potency of DTG. Overall, the most common side effects of DTG include diarrhea, nausea, fatigue, headaches, and insomnia.
Unique Populations
Treatment of pregnant women, and persons with co-infections including tuberculosis, hepatitis, or renal insufficiency can alter treatment recommendations. While a PK study evaluating DTG in pregnant women is underway, to date no clinical trials have evaluated DTG use in pregnant women, though animal studies demonstrate that DTG can cross the placenta [24]. The FDA label states that DTG should be prescribed in pregnancy only if potential benefit justifies the potential risk, category B [24]. DTG should be given twice daily when co-administered with rifampin (600 mg daily) as rifampin decreases DTG exposure by approximately 50% due to minor metabolism via CYP3A4 [43]. Rifabutin also reduces DTG trough concentration by about 30%, but this reduction maintains concentrations above the PA-IC50 (0.016 μg/mL) and does not require dose adjustment [24, 43, 44]. Transaminase monitoring for hepatotoxicity is recommended when treating patients with hepatitis B and/or hepatitis C co-infection. Those with mild-to-moderate hepatic impairment (Child–Pugh Score A or B) do not require dose adjustments, but treatment in severe hepatic impairment (Child–Pugh Score C) is not recommended. DTG has not been studied in patients on dialysis, and those with severe renal impairment may have decreased drug concentrations that could dampen therapeutic effect and lead to resistance [24, 44, 45].
The Future
Dolutegravir is now a recommended first-line agent in the United States for both treatment-naïve or treatment-experienced INSTI-naïve (once-daily dosing) and treatment-experienced with suspected INI-resistance (twice-daily dosing) adults and adolescents at least 12 years old weighing a minimum of 40 kg [13]. In resource-limited settings, ART is typically limited to combination NRTI/NNRTI as first-line regimens, and NRTI/boosted PI regimens as second line. Third-line regimens containing integrase inhibitors are rare, and it is unclear if they will become available in a resource-limited context. A fixed-dose combination of ABC/3TC/DTG has shown bioequivalence to individual formulations [46] and could hold promise, especially for resource-limited settings such as sub-Saharan Africa where the HIV burden is high, the HLA-B*5701 mutation is rare, and renal monitoring for regimens that include tenofovir are limited. In 2010, ViiV Healthcare announced the intention to make their patents, including DTG, available to generic manufacturers under a royalty-free agreement. Whether these negotiations will result in the ability of resource-limited settings to access DTG is uncertain [47, 48]. To date, clinical trials of DTG have primarily included white males from developed countries. Future studies that include more women and children, non-subtype B virus, HIV-2 (primarily West Africa), and non-white ethnicity are encouraged.
Long-Acting Agent GSK1265744LA
GSK1265744, a distinct integrase inhibitor from the same chemical series as DTG, can be formulated as a crystalline nanoparticle suspension and is thereby amenable to delivery as a long-acting injectable (744LA). The 744LA formulation has unique properties including high potency (PA-IC90 166 ng/mL), poor water solubility (<10 μg/mL), slow metabolism, and high melting point, allowing it to be formulated as a nanoparticle solution [49, 50]. The t 1/2 ranges from 21 to 50 days. Phase I studies demonstrate that this compound is safe and well tolerated with plasma concentrations above the PA-IC90 for 24 weeks or longer with doses 200 mg or greater [51].
The 744LA formulation in combination with the long-acting rilpivirine formulation (TMC278 LA) is being developed for use in treatment of HIV-infected patients. This combination holds potential promise to expand HIV treatment options by providing an innovative mechanism to improve adherence, eliminate NRTI- and/or ritonavir-related drug toxicities, and potentially enhance drug delivery to reservoirs such as lymphoid tissue and the central nervous system based on preliminary data of a macrophage–carriage system for nanoformulated crystalline ART in experimental animal models [49, 52, 53].
The 744LA formulation is also being developed as a single agent for pre-exposure prophylaxis (PrEP). An animal study challenging rhesus macaques with Simian/Human Immunodeficiency Virus (SHIV) recently demonstrated proof of concept of 744LA as PrEP [50]. Macaques receiving placebo became SHIV-infected by the second SHIV challenge on average (range 1–7); in contrast, those receiving 744LA had no systemic viremia for 10 weeks after the last SHIV challenge, demonstrating a 28-fold lower risk of infection (hazard ratio 95% CI 5.8, 136.8; P < 0.0001) [50]. A drug level three times greater than the PA-IC90 offered 100% protection; one to three times the PA-IC90 conferred 97% protection, suggesting that a quarterly dose of 800 mg of 744LA might be appropriate in humans for PrEP [50]. Phase I trials evaluating penetration of a 400-mg dose in rectal and cervicovaginal tissue in healthy volunteers revealed detectable, but relatively low levels and were slightly higher in cervicovaginal tissue as compared with rectal tissue [54]. The amount of drug penetration into genital tract tissues and fluids needed to prevent infection is unknown.
Summary
Dolutegravir is the latest FDA-approved compound of the INSTI class. Its unique properties of once-daily dosing for ART-naïve patients, lack of cross resistance to first-generation INSTI, high genetic barrier to resistance, and favorable safety profile welcome DTG as the newest addition to the HIV armamentarium in the developed world. The clinical trials that brought DTG to market are funded by the drug manufacturer, ViiV Healthcare and took place primarily in well-resourced countries. Efforts are being made to share this costly drug with less-resourced countries, although DTG is not yet available and the timeline and procedures to obtain access are not finalized. The long-acting formulation of GSK1265744LA holds great promise for the future of HIV prevention and treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This review was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) training grant to the Division of Infectious Diseases, Department of Pediatrics, Duke University Medical Center (T32 HD060558 to Dorothy E Dow) and by the US National Institutes of Health awards P30AI64518, U01AI067854, D43CA153722, and D43TW06732, and Health Resources and Services Administration T84HA21123 to John A Bartlett. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Conflict of interest
Dorothy E. Dow declares recent inheritance of stock in GlaxoSmithKline. John A Bartlett declares he has no conflict of interest.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors. The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Eron JJ, Jr, Rockstroh JK, Reynes J, Andrade-Villanueva J, Ramalho-Madruga JV, Bekker LG, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11(12):907–915. doi: 10.1016/S1473-3099(11)70196-7. [DOI] [PubMed] [Google Scholar]
- 2.Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 3.Lennox JL, Dejesus E, Berger DS, Lazzarin A, Pollard RB, Ramalho Madruga JV, et al. Raltegravir versus Efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39–48. doi: 10.1097/QAI.0b013e3181da1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockstroh JK, Lennox JL, Dejesus E, Saag MS, Lazzarin A, Wan H, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53(8):807–816. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 5.Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 6.Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;63(1):96–100. doi: 10.1097/QAI.0b013e318289545c. [DOI] [PubMed] [Google Scholar]
- 7.Wohl DA, Cohen C, Gallant JE, Mills A, Sax PE, Dejesus E, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65(3):e118–e120. doi: 10.1097/QAI.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 8.DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–2438. doi: 10.1016/S0140-6736(12)60918-0. [DOI] [PubMed] [Google Scholar]
- 9.Rockstroh JK, DeJesus E, Henry K, Molina JM, Gathe J, Ramanathan S, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62(5):483–486. doi: 10.1097/QAI.0b013e318286415c. [DOI] [PubMed] [Google Scholar]
- 10.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Section Accessed March 5, 2014.
- 11.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 12.European AIDS Clinical Society (EACS). Guidelines for treatment of HIV-infected adults in Europe Version 7.0; 2013. http://www.eacsociety.org/Guidelines.aspx. Section Accessed May 6, 2014.
- 13.AIDSinfo. Recommendation on Integrase Inhibitor Use in Antiretroviral Treatment-Naive HIV-Infected Individuals from the HHS Panel on Antiretroviral Guidelines for Adults and Adolescents; 2013. http://aidsinfo.nih.gov/contentfiles/upload/AdultARV_INSTIRecommendations.pdf. Accessed March 5, 2014.
- 14.Sato A, Kokayashi M, Seki T, Morimoto CW, Yoshinaga T, Fujiwara T, Johns FA, Underwood MR (2010) S/GSK1349572: a next generation integrase inhibitor (INI) with lmited or no-cross resistance to first generation INIs or other classes of anti-virals. In: 8th European HIV drug resistance workshop, Sorrento.
- 15.Min S, Song I, Borland J, Chen S, Lou Y, Fujiwara T, et al. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54(1):254–258. doi: 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. Aids. 2011;25(14):1737–1745. doi: 10.1097/QAD.0b013e32834a1dd9. [DOI] [PubMed] [Google Scholar]
- 17.Song I, Borland J, Chen S, Lou Y, Peppercorn A, Wajima T, et al. Effect of atazanavir and atazanavir/ritonavir on the pharmacokinetics of the next-generation HIV integrase inhibitor, S/GSK1349572. Br J Clin Pharmacol. 2011;72(1):103–108. doi: 10.1111/j.1365-2125.2011.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song I, Borland J, Min S, Lou Y, Chen S, Patel P, et al. Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir. Antimicrob Agents Chemother. 2011;55(7):3517–3521. doi: 10.1128/AAC.00073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, et al. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemotherapy. 2011;55(2):813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hightower KE, Wang R, Deanda F, Johns BA, Weaver K, Shen Y, et al. Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase–DNA complexes. Antimicrob Agents Chemother. 2011;55(10):4552–4559. doi: 10.1128/AAC.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeAnda F, Hightower KE, Nolte RT, Hattori K, Yoshinaga T, Kawasuji T, et al. Dolutegravir interactions with HIV-1 integrase–DNA: structural rationale for drug resistance and dissociation kinetics. PLoS ONE. 2013;8(10):e77448. doi: 10.1371/journal.pone.0077448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–748. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014. [DOI] [PMC free article] [PubMed]
- 24.Tivicay (dolutegravir) tablet [product label]. Research Triangle Park, NC: Manufactured for ViiV Healthcare Company by GlaxoSmithKline. Initial U.S. approval 2013. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922. Last Accessed March 26, 2014.
- 25.Hurt CB, Sebastian J, Hicks CB, Eron JJ. Resistance to HIV integrase strand transfer inhibitors among clinical specimens in the United States, 2009–2012. Clin Infect Dis. 2014;58(3):423–431. doi: 10.1093/cid/cit697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee for Proprietary Medicinal Products Points to consider on switching between superiority and non-inferiority. Br J Clin Pharmacol. 2001;52(3):223–228. doi: 10.1046/j.1365-2125.2001.01397-3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Lunzen J, Maggiolo F, Arribas JR, Rakhmanova A, Yeni P, Young B, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–118. doi: 10.1016/S1473-3099(11)70290-0. [DOI] [PubMed] [Google Scholar]
- 28.Stellbrink HJ, Reynes J, Lazzarin A, Voronin E, Pulido F, Felizarta F, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. Aids. 2013;27(11):1771–1778. doi: 10.1097/QAD.0b013e3283612419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 30.Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–935. doi: 10.1016/S1473-3099(13)70257-3. [DOI] [PubMed] [Google Scholar]
- 31.Arribas JR, Eron J. Advances in antiretroviral therapy. Curr Opin HIV AIDS. 2013;8(4):341–349. doi: 10.1097/COH.0b013e328361fabd. [DOI] [PubMed] [Google Scholar]
- 32.Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutierrez F, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
- 33.Walmsley S, Berenguer J, Khuong-Josses M, Kilby JM, Lutz T, Podzamczer D, Roth N, Granier C, Wynne B, Pappa K. Dolutegravir regimen statistically superior to efavirenz/tenofovir/embricitabine: 96-week results from the single study (ING114467) [Abstract 543]. Presented at conference on retroviruses and opportunistic infections (CROI), Boston; 2014.
- 34.Feinberg J, Clotet B, Khuong MA, et al. Once-daily dolutegravir is superior to darunavir/ritonavir in antiretroviral naive adults: 48 week results from FLAMINGO (ING114915) [Abstract H1464a]. Presented at the 53rd interscience conference on antimicrobial agents and chemotherapy (ICAAC), Denver; 2013. http://www.icaaconline.com/php/icaac2013abstracts/start.htm. Accessed March 24, 2014.
- 35.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 36.Vavro CL, Huang J, Avatapally C, Min S, Ait-Khaled M. Durable efficacy and limited integrase resistance evolution in subjects receiving dolutegravir after failing a prior integrase inhibitor (INI) regimen: week 48 results from VIKING-3. Published at 12th European meeting on HIV and hepatitis-treatment strategies and antiviral drug resistance, Barcelona; 2014.
- 37.ViiV Healthcare. Study assessing dolutegravir in HIV-1 infected subjects with virus resistant to raltegravir and/or elivitegravir (VIKING-4). http://www.clinicaltrials.gov/ct2/results?term=01568892&Search=Search. Accessed March 28, 2014.
- 38.Viani RM, Zheng N, Alvero C, Hazra R, O’Gara E, Petzoid E, Heckman B, Steimers D, Min S, Wizina A; the P1093 Team. Safety and efficacy of dolutegravir (DTG; GSK1349572) in treatment-experienced HIV-1 infected adolescents: 24-week results from IMPAACT P1093 [Abstract 172]. Presented at IDWeek, San Francisco; 2013.
- 39.Viani RM, Alvero C, Fenton T, Acosta E, Hazra R, O’Gara E, Heckman B, Steimers, D, Min, S, Wizina, A; the P1093 Team. Safety and efficacy of dolutegravir in HIV treatment-experienced adolescents: 48-week results [Abstract LB-2788]. Presented at conference on retroviruses and opportunistic infections (CROI), Boston; 2014.
- 40.Viani RM, Alvero C, Fenton T, Acosta E, Hazra R, O’Gara E, Heckman B, Steimers D, Min S, Wizina A; the P1093 Team. Safety pharmacokinetics and efficacy of dolutegravir in treatment experienced HIV + children [Abstract 119]. Presented at conference on retroviruses and opportunistic infections (CROI), Boston; 2014.
- 41.Patel P, Song I, Borland J, Chen S, Peppercorn A, Wajima T, et al. Relative bioavailability of a paediatric granule formulation of the HIV integrase inhibitor, dolutegravir, in healthy adult subjects. Antiviral Ther. 2013. [DOI] [PubMed]
- 42.Koteff J, Borland J, Chen S, Song I, Peppercorn A, Koshiba T, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol. 2013;75(4):990–996. doi: 10.1111/j.1365-2125.2012.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21–27. doi: 10.1097/QAI.0b013e318276cda9. [DOI] [PubMed] [Google Scholar]
- 44.Rathbun RC, Lockhart SM, Miller MM, Liedtke MD. Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection. Ann Pharmacother. 2014;48:395–403. doi: 10.1177/1060028013513558. [DOI] [PubMed] [Google Scholar]
- 45.Weller S, Borland J, Chen S, Johnson M, Savina P, Wynne B, Piscitelli S. Pharmacokinetics (PK) and safety of dolutegravir (DTG) in subjects with severe renal impairment and healthy controls [Abstract: A-1571]. Presented at the 53rd annual interscience conference on antimicrobial agents and chemotherapy (ICAAC), Denver; 2013.
- 46.Weller S, Chen S, Borland J, Savina P, Wynne B, Piscitelli S. Bioequivalence of a dolutegravir, abacavir and lamivudine fixed dose combination tablet and the effect of food [Abstract: A-1572]. Presented at the 53rd annual interscience conference on antimicrobial agents and chemotherapy (ICAAC), Denver; 2013.
- 47.National AIDS Treatment Advocacy Project. 2010. ViiV Healthcare announces further initiatives to improve access to HIV medications for people living in the least developed countries; generics voluntary licensing. http://www.natap.org/2010/newsUpdates/071910_05.htm. Accessed March 27, 2014.
- 48.Simon Collins. ViiV goes for gold: U.S. premium pricing may make dolutegravir redundant in the UK. HIV i-Base; 2013. http://www.thebodypro.com/content/72987/viiv-goes-for-gold-us-premium-pricing-may-make-dol.html. Accessed March 27, 2014.
- 49.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8(6):565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343(6175):1151–1154. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013;14(5):192–203. doi: 10.1310/hct1405-192. [DOI] [PubMed] [Google Scholar]
- 52.Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, et al. Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int J Nanomed. 2012;7:2373–2388. doi: 10.2147/IJN.S29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gautam N, Roy U, Balkundi S, Puligujja P, Guo D, Smith N, et al. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrob Agents Chemother. 2013;57(7):3110–3120. doi: 10.1128/AAC.00267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ford S, Margolis D, Chen S, et al. Plasma and tissue GSK1265744 pharmacokinetics following long-acting parenteral administration in healthy male and female subjects [Abstract O_02]; 14th international workshop on clinical pharmacology of HIV therapy, Amsterdam; 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.