Abstract
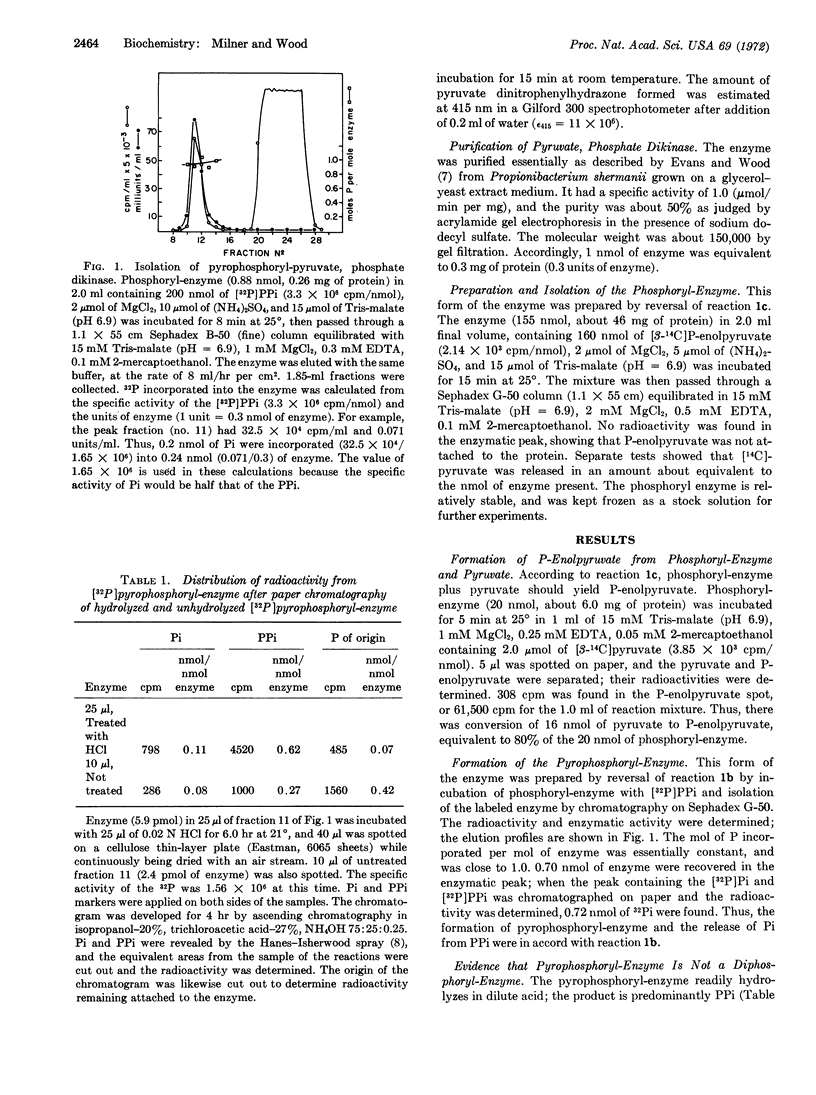
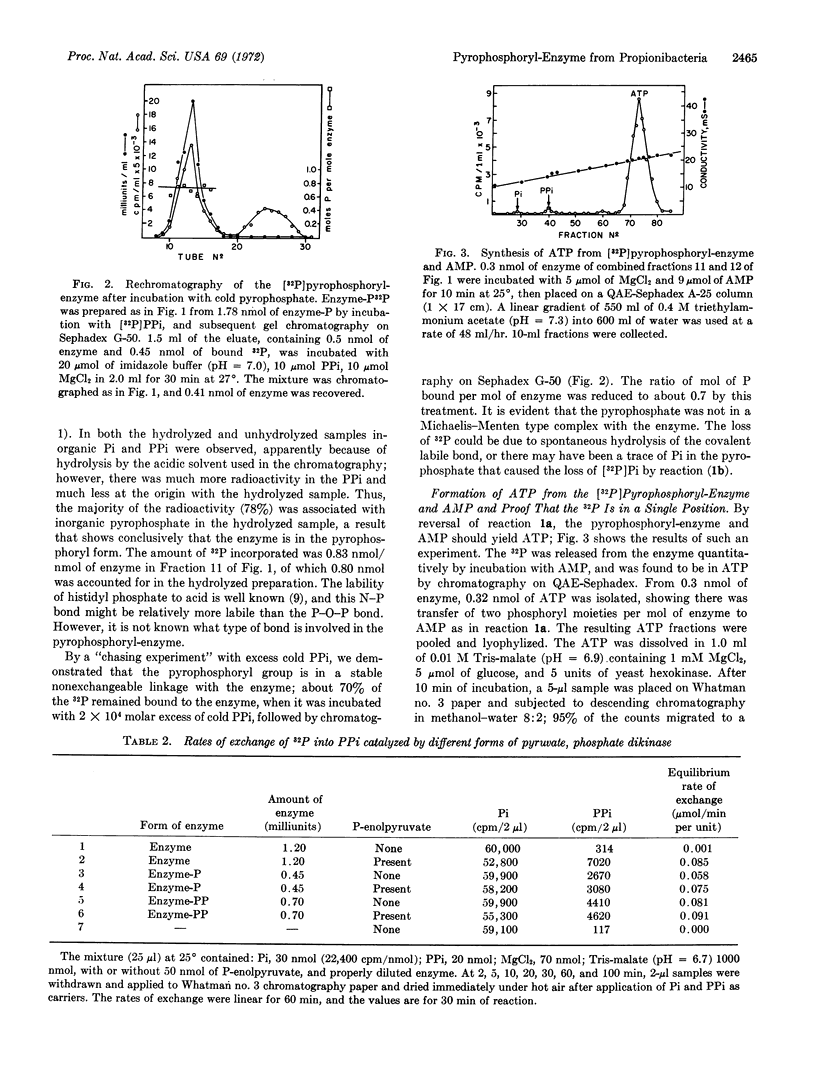
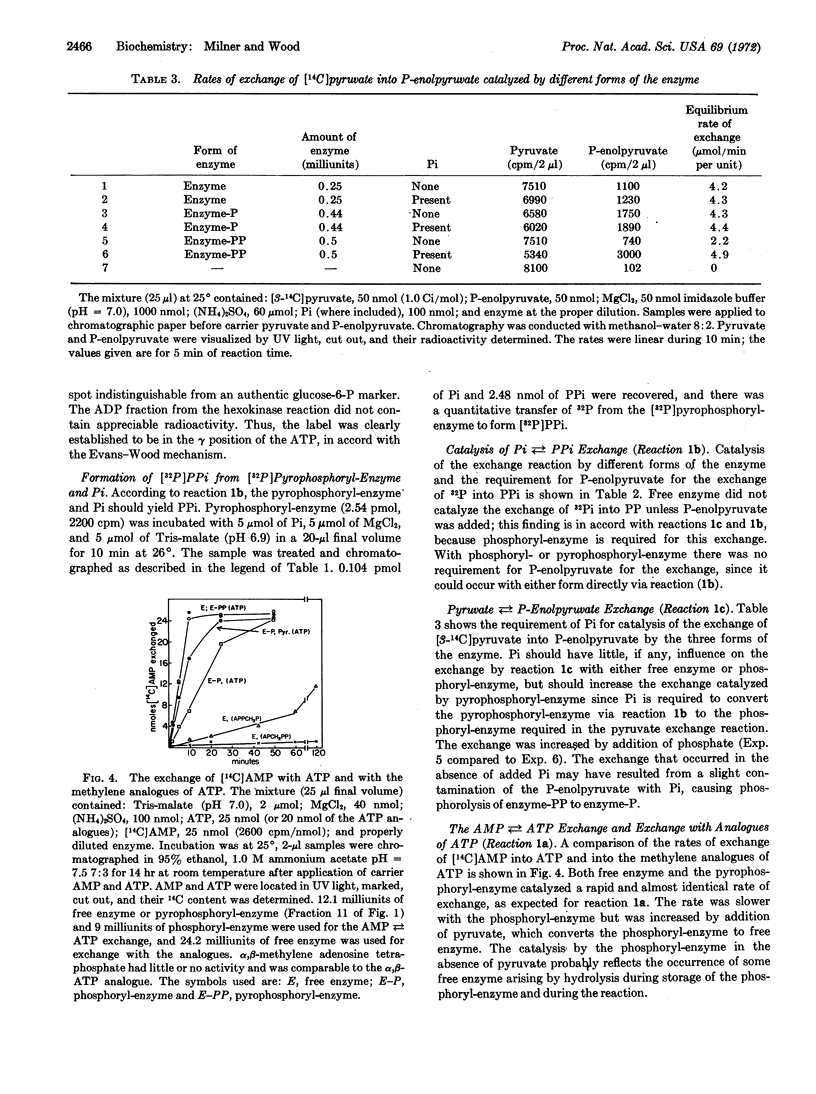
Pyruvate, phosphate dikinase from Propionibacterium shermanii catalyzes the formation of P-enolpyruvate, AMP, and inorganic pyrophosphate from pyruvate, ATP, and orthophosphate; the mechanism involves three partial reactions and three forms of the enzyme: pyrophosphoryl-enzyme, phosphoryl-enzyme, and free enzyme. The phosphoryl-enzyme was prepared by incubation with P-enolpyruvate and isolated by gelchromatography. The phosphoryl-enzyme was converted to 32P31P-enzyme and [32P]Pi by incubation with [32P]PPi; 1 mol of pyrophosphoryl-enzyme was formed per mol of enzyme of molecular weight 150,000. The labeled enzyme released its radioactivity upon incubation with Pi or AMP to produce the expected [33P]PPi or [γ-32P]ATP, respectively. Hydrolysis of the pyrophosphoryl-enzyme with dilute acid yielded PPi. The β,γ-methylene analogue of ATP was reactive in exchange reactions with [14C]AMP. To our knowledge, this is the first proven example of a pyrophosphoryl-enzyme.
Keywords: phosphoenolpyruvate, ATP, enzyme
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Hatch M. D. Properties and mechanism of action of pyruvate, phosphate dikinase from leaves. Biochem J. 1969 Aug;114(1):117–125. doi: 10.1042/bj1140117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER P. D., DELUCA M., EBNER K. E., HULTQUIST D. E., PETER J. B. Identification of phosphohistidine in digests from a probable intermediate of oxidative phosphorylation. J Biol Chem. 1962 Oct;237:PC3306–PC3308. [PubMed] [Google Scholar]
- Bell R. M., Koshland D. E., Jr Covalent enzyme-substrate intermediates. Science. 1971 Jun 18;172(3989):1253–1256. doi: 10.1126/science.172.3989.1253. [DOI] [PubMed] [Google Scholar]
- Berman K. M., Cohn M. Phosphoenolpyruvate synthetase of Escherichia coli. Purification, some properties, and the role of divalent metal ions. J Biol Chem. 1970 Oct 25;245(20):5309–5318. [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The mechanism of the phosphoenolpyruvate synthase reaction. Biochim Biophys Acta. 1967 Jun 13;141(1):211–213. doi: 10.1016/0304-4165(67)90269-3. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Wood H. G. Purification and properties of pyruvate phosphate dikinase from propionic acid bacteria. Biochemistry. 1971 Mar 2;10(5):721–729. doi: 10.1021/bi00781a001. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Wood H. G. The mechanism of the pyruvate, phosphate dikinase reaction. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1448–1453. doi: 10.1073/pnas.61.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Kinetic mechanism and end product inhibition. J Biol Chem. 1972 Apr 10;247(7):2126–2131. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., Menzies R. A., Hsu D. S. The pyruvate-phosphate dikinase reaction. The fate of phosphate and the equilibrium. J Biol Chem. 1968 Oct 25;243(20):5486–5491. [PubMed] [Google Scholar]
- Switzer R. L. Mechanism of phosphoribosylpyrophosphate synthetase: evidence for an enzyme-pyrophosphate intermediate. Biochem Biophys Res Commun. 1968 Jul 26;32(2):320–325. doi: 10.1016/0006-291x(68)90388-4. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. II. Excchange reactions catalyzed by the enzyme. J Biol Chem. 1970 Feb 10;245(3):483–495. [PubMed] [Google Scholar]
- Yagil G., Hoberman H. D. Rate of isotope exchange in enzyme-catalyzed reactions. Biochemistry. 1969 Jan;8(1):352–360. doi: 10.1021/bi00829a049. [DOI] [PubMed] [Google Scholar]


