Abstract
The elevation of protein kinase A (PKA) activity activates the large-conductance voltage- and Ca2+-activated K+ (BK) channels in urinary bladder smooth muscle (UBSM) cells and consequently attenuates spontaneous phasic contractions of UBSM. However, the role of constitutive PKA activity in UBSM function has not been studied. Here, we tested the hypothesis that constitutive PKA activity is essential for controlling the excitability and contractility of UBSM. We used patch clamp electrophysiology, line-scanning confocal and ratiometric fluorescence microscopy on freshly isolated guinea pig UBSM cells, and isometric tension recordings on freshly isolated UBSM strips. Pharmacological inhibition of the constitutive PKA activity with H-89 or PKI 14–22 significantly reduced the frequency and amplitude of spontaneous transient BK channel currents (TBKCs) in UBSM cells. Confocal and ratiometric fluorescence microscopy studies revealed that inhibition of constitutive PKA activity with H-89 reduced the frequency and amplitude of the localized Ca2+ sparks but increased global Ca2+ levels and the magnitude of Ca2+ oscillations in UBSM cells. H-89 abolished the spontaneous transient membrane hyperpolarizations and depolarized the membrane potential in UBSM cells. Inhibition of PKA with H-89 or KT-5720 also increased the amplitude and muscle force of UBSM spontaneous phasic contractions. This study reveals the novel concept that constitutive PKA activity is essential for controlling localized Ca2+ signals generated by intracellular Ca2+ stores and cytosolic Ca2+ levels. Furthermore, constitutive PKA activity is critical for mediating the spontaneous TBKCs in UBSM cells, where it plays a key role in regulating spontaneous phasic contractions in UBSM.
Keywords: protein kinase A, large-conductance voltage- and Ca2+-activated K+ channels, Ca2+ sparks, H-89, PKI 14–22
the protein kinase a (PKA) signaling pathway has been implicated in the regulation of urinary bladder function (1, 4, 32, 39, 44). PKA activation attenuates the excitability and contractility of urinary bladder smooth muscle (UBSM) by activating K+ channels, particularly the large-conductance voltage- and Ca2+-activated K+ (BK) channels, which are key regulators of spontaneous action potentials in UBSM (8, 13, 15, 30–32). Furthermore, elevated PKA activity in response to extracellular stimulation, such as the activation of β-adrenergic receptors, potentiates Ca2+ uptake from the cytosol into the sarcoplasmic reticulum (SR) by SR Ca2+-ATPase. This provides a Ca2+ source needed for intracellular Ca2+ release signaling (2, 22, 32, 41). Notably, the elevation of PKA activity leads to an increase in the rapid and localized Ca2+ releases from the SR via ryanodine receptors (RyRs) known as “Ca2+ sparks” and consequently increases transient BK channel currents (TBKCs) in UBSM cells (4, 19, 32, 39, 44, 45). However, prior to the current study, the physiological role of constitutive PKA activity in the regulation of UBSM excitability and contractility was not clearly understood.
It has been demonstrated that elevated PKA activity plays an important role in β-adrenergic receptor activation as well phosphodiesterase (PDE) inhibition-mediated suppression of UBSM excitability and contractility (4, 13, 31, 32, 39, 44). cAMP/PKA activity depends on the balance between the activity of adenylyl cyclases, which convert ATP to cAMP, and of PDEs that hydrolyze cAMP to AMP (9). The activation of adenylyl cyclases via β-adrenergic receptors or pharmacological inhibition of PDEs shifts the balance, leading to a rapid increase in cellular cAMP levels and PKA activity (9). The elevation of PKA activity can effectively increase TBKCs in UBSM cells and attenuate both the excitability and spontaneous phasic contractions of UBSM (4, 19, 44). Specifically, the elevation of PKA activity as a result of β-adrenergic receptor activation or PDE4 inhibition increases Ca2+ spark activity and the functionally coupled TBKCs, resulting in suppression of the excitability and spontaneous phasic contractions of guinea pig UBSM (32, 44). In addition, the increase in PKA expression compensates for the loss of BK channels in BK channel knockout mice and enhances the β-adrenergic receptor-mediated attenuation of UBSM contractions (4, 39).
Specifically, the elevation of cellular cAMP levels increases PKA phosphorylation of phospholamban (PLB), a regulatory protein of the Ca2+-ATPase. The phosphorylated PLB has a reduced inhibitory effect on Ca2+-ATPase compared with the dephosphorylated PLB (22). Consequently, the elevation of PKA activity increases SR Ca2+-ATPase activity and SR Ca2+ content (22). The elevated SR Ca2+ load increases the open probability of RyRs and the activity of Ca2+ sparks (12). However, whether constitutive PKA activity is necessary for the spontaneous Ca2+ sparks and TBKCs has yet to be investigated. The present study determined the physiological function of constitutive PKA activity in UBSM by investigating its regulation of basal Ca2+ sparks, TBKCs, spontaneous transient hyperpolarizations in UBSM cells, and the spontaneous phasic contractions of the UBSM.
MATERIALS AND METHODS
UBSM tissue collection and single-cell isolation.
A total of 31 male Hartley Albino guinea pigs (Charles River Laboratory, Raleigh, NC) with an average weight of 437 ± 11 g were used in this study. Guinea pigs were euthanized with CO2 inhalation, followed by thoracotomy, following animal use protocol no. 1747 that was reviewed and approved by the Institutional Animal Care and Use Committee of the University of South Carolina. UBSM strips (∼2–3 mm wide and ∼6–7 mm long) were prepared by removing the mucosa and used for single UBSM cell isolation and isometric UBSM tension recordings. UBSM single cells were freshly isolated by enzymatic digestion of UBSM strips in solutions containing papain and then in solutions containing type II collagenase, following the procedures as described previously (43–45).
Electrophysiological recordings.
The amphotericin B-perforated whole cell patch clamp technique was used for electrophysiological recordings in freshly isolated UBSM single cells, as described previously (43–45). The TBKCs were recorded at a holding potential of −40 mV, using a pipette with a resistance between 4 and 6 MΩ. The membrane potential of UBSM cells was recorded in current clamp mode of the patch clamp technique (Ih = 0). All patch clamp experiments were conducted at room temperature (∼22–23°C).
Ca2+ spark recordings with high-speed line-scanning confocal microscopy in freshly isolated UBSM cells.
Ca2+ sparks were recorded in cells loaded with the fluorescent Ca2+ probe fluo 4-AM using a high-speed line-scanning LSM700-Meta confocal microscope and analyzed using ImageJ with the SparkMaster plug-in, as reported previously (33, 44). The threshold for the detection of events was 3.8 times the standard deviation of the background noise over the mean value of the background intensity. The Ca2+ spark frequency and amplitude were averaged for the last five scanning cycles under control or test compound-treated conditions, respectively.
Recording of global intracellular Ca2+ levels in freshly isolated UBSM cells.
The global intracellular Ca2+ levels were monitored using a ratiometric fluorescent Ca2+ probe fura 2-AM, as described previously (45). Cells were imaged with an Olympus IX81 inverted microscope equipped with a ×40 oil objective and MetaFluor 7.7.2.0 software (Molecular Devices, Sunnyvale, CA). Fura 2 was excited for 20 ms at 340 and 380 nm of light with an interval of 0.6 s. The relative intracellular Ca2+ level was expressed as the ratio of F340/F380 emission intensities at 510 nm. All Ca2+ imaging experiments were carried out at room temperature (22–23°C).
Isometric UBSM tension recordings.
Isometric contractions of UBSM isolated strips were measured using a Myomed myograph system (MED Associates, St. Albans, VT), as described previously (43–45). To minimize the potential effects of neurotransmitter release from neurons in the UBSM tissue, the spontaneous phasic contractions were recorded in the presence of 1 μM tetrodotoxin, a selective blocker of the neuronal Na+ channels.
Data analysis and statistics.
The amplitude and frequency of TBKCs, in addition to the UBSM phasic contraction parameters, were analyzed using MiniAnalysis (Synaptosoft, Decatur, GA). The cell membrane potential was analyzed using Clampfit 10.2 (Molecular Devices, Union City, CA). The last 5 min of recording under control or treated conditions were analyzed. Data were further analyzed with GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA). For the statistical analysis, the parameters of Ca2+ sparks, TBKCs, and spontaneous contractions were normalized to their controls from the same cell or UBSM strip, respectively. Data are expressed as means ± SE; n = the number of cells or UBSM strips, and N = the number of guinea pigs. Statistical significance was performed using paired Student's t-test, and P < 0.05 was considered significant.
Solutions and drugs.
The nominally Ca2+-free dissection solution contained (in mM) 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2; pH was adjusted to 7.3 with NaOH. The extracellular solution for whole cell patch clamp and Ca2+ imaging experiments contained (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES; pH was adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA; pH was adjusted to 7.2 with NaOH and supplemented with freshly dissolved (every 1–2 h) 200 μg/ml amphotericin B. The Ca2+-containing physiological saline solution was prepared daily and contained (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose and was aerated with 95% O2-5% CO2 to obtain pH 7.4. Myristoylated PKI 14–22 (Myr-N-Gly-Arg-Thr-Gly-Arg-Arg-Asn-Ala-Ile-NH2), trypsin inhibitor, BSA, and amphotericin B were obtained from Thermo Fisher Scientific (Fair Lawn, NJ). Papain was purchased from Worthington Biochemical (Lakewood, NJ). N-{2-[3-(4-bromophenyl)-2-propenylamino]ethyl}-5-isoquinolinesulfonamide dihydrochloride (H-89), DT-2 trifluoroacetate salt (Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Pro-Pro-Leu-Arg-Lys-Lys-Lys-Lys-Lys-His-NH2), collagenase (type II), and tetrodotoxin (in citrate buffer) were purchased from Sigma-Aldrich (St. Louis, MO). KT-5720 {(9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzo-diazocine-10-carboxylic acid, hexyl ester} was purchased from R & D Systems (Minneapolis, MN). Amphotericin B, H-89, and KT-5720 were dissolved in dimethyl sulfoxide (DMSO) as stock solutions. All other chemicals were dissolved in double-distilled water. The maximum DMSO concentration was <0.1%.
RESULTS
Pharmacological inhibition of constitutive PKA activity reduced the spontaneous TBKCs in UBSM cells.
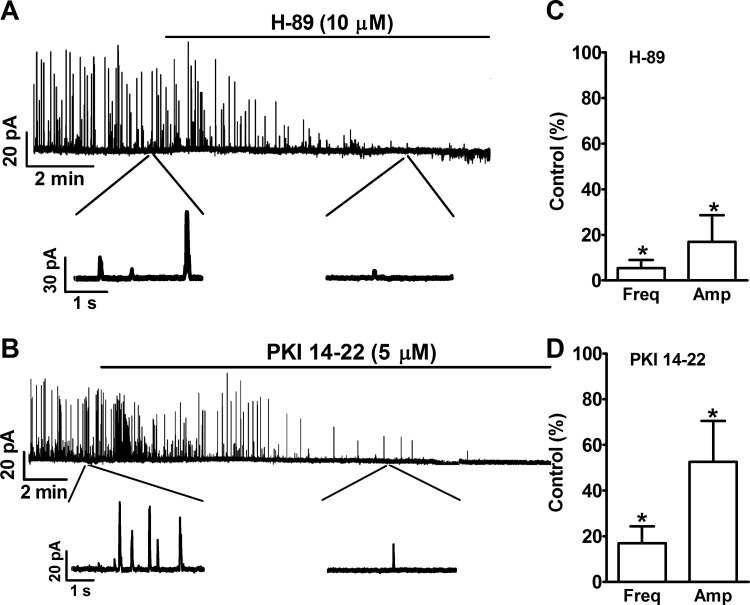
Inhibition of PKA with H-89 (10 μM) led to an inhibition of spontaneous TBKCs in freshly isolated UBSM cells (Fig. 1). In two of the six tested cells, H-89 (10 μM) inhibited TBKCs, and in other four cells, H-89 completely abolished TBKCs. The summary data show that the inhibition of the constitutive PKA activity significantly reduced the frequency and amplitude of spontaneous TBKCs down to 5.4 ± 3.6 and 17.0 ± 11.6% of the control values, respectively (n = 6, N = 6, P < 0.05; Fig. 1).
Fig. 1.
Pharmacological inhibition of PKA with N-{2-[3-(4-bromophenyl)-2-propenylamino]ethyl}-5-isoquinolinesulfonamide dihydrochloride (H-89) or protein kinase A inhibitor PKI 14–22 reduced the spontaneous transient large-conductance voltage- and Ca2+-activated K+ channel (BK) channel currents (TBKCs) in freshly-isolated urinary bladder smooth muscle (UBSM) cells. A and B: original voltage clamp recordings from single UBSM cells illustrating that H-89 (10 μM) or PKI 14–22 (5 μM) inhibited TBKCs. A portion of the recording under each condition is shown on an expanded time scale. C and D: summary data illustrating that H-89 (10 μM; n = 6, N = 6) and PKI 14–22 (5 μM; n = 6, N = 5) significantly decreased the frequency and amplitude of TBKCs (n = no. of cells, and N = no. of guinea pigs). Freq, frequency; Amp, amplitude. *P < 0.05.
To confirm that the effect of H-89 on TBKCs was due to the inhibition of constitutive PKA activity, we applied another highly selective and membrane-permeable peptide inhibitor of PKA, myristoylated PKI 14–22. PKI 14–22 (5 μM) inhibited TBKCs in UBSM cells (Fig. 1). The summary data show that PKI 14–22 decreased the frequency and amplitude of TBKCs down to 17.0 ± 7.4 and 52.6 ± 17.8% of the control, respectively (n = 6, N = 5, P < 0.05; Fig. 1). These data confirm that constitutive PKA activity is essential for the spontaneous TBKCs in UBSM cells.
The inhibitory effect of H-89 on TBKCs was due to the inhibition of PKA.
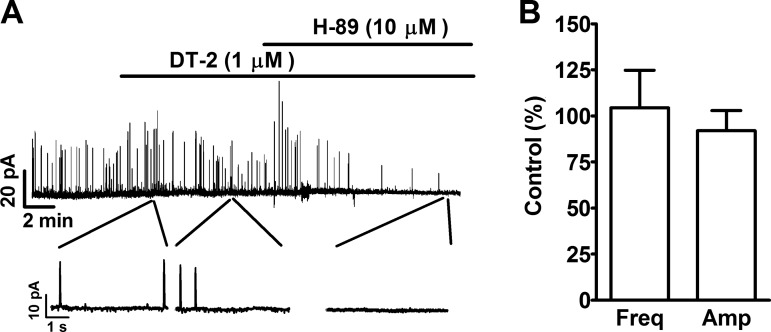
To further clarify that the effect of H-89 on TBKCs was due to the inhibition of PKA and not inhibition of protein kinase G (PKG), we applied a PKG inhibitor prior to the addition of H-89. DT-2 (1 μM), a selective PKG inhibitor, alone did not affect TBKCs in freshly isolated UBSM cells (Fig. 2). The summary data show that, in the presence of DT-2, the frequency and amplitude of TBKCs were 104.4 ± 20.4 and 92.0 ± 10.9% of the control values, respectively (n = 6, N = 5, P > 0.05; Fig. 2). The subsequent addition of H-89 (10 μM) reduced the frequency and amplitude of TBKCs down to 2.7 and 13.8% of the control in one cell, respectively; H-89 completely abolished the TBKCs in the other three cells (n = 4, N = 4). These data support that PKA, but not PKG, played a role in the inhibition of TBKCs caused by H-89 (10 μM).
Fig. 2.
Pharmacological inhibition of PKG with DT-2 did not have any effect on the TBKCs in freshly isolated UBSM cells. A: an original voltage clamp recording illustrating that DT-2 (1 μM) did not affect TBKCs and that the subsequent PKA inhibition with H-89 completely inhibited TBKCs in a UBSM cell. A portion of the recording under each condition is shown on an expanded time scale. B: summary data illustrating that DT-2 (1 μM) did not have a significant effect on the frequency (Freq) and amplitude (Amp) of TBKCs (n = 6, N = 5). P > 0.05.
Pharmacological inhibition of constitutive PKA activity with H-89 decreased Ca2+ spark amplitude and frequency in freshly isolated UBSM cells.
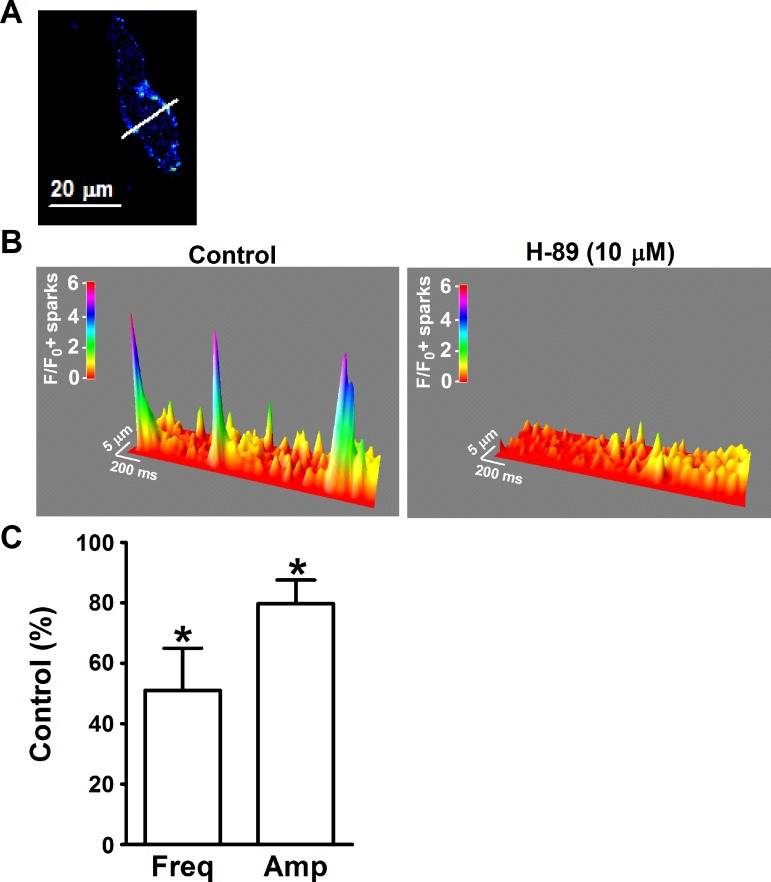
Ca2+ sparks transiently activate BK channels and generate TBKCs in UBSM cells (17, 32, 44). We used high-speed line-scanning confocal microscopy to measure Ca2+ sparks in freshly isolated UBSM cells. H-89 (10 μM) decreased the Ca2+ spark activity (Fig. 3). The summary data demonstrate that H-89 (10 μM) significantly decreased the Ca2+ spark frequency and amplitude down to 58.1 ± 13.9 and 79.7 ± 7.8% of the control values, respectively (n = 8, N = 6, P < 0.05; Fig. 3). These results demonstrate that the constitutively active PKA is critical for the generation of Ca2+ sparks in UBSM cells in the absence of external stimulation. The decrease in the Ca2+ spark activity most likely underlies the inhibition of the spontaneous TBKCs caused by PKA inhibitors.
Fig. 3.
Pharmacological inhibition of PKA with H-89 suppressed the basal level of Ca2+ sparks in freshly isolated UBSM cells. A: an image of a freshly isolated UBSM cell loaded with fluo 4-AM. The white line passing through the active site a is the laser beam-scanning pathway (1-pixel width). B: the 3-dimensional view of the recordings illustrate the Ca2+ sparks in the absence and presence of H-89 (10 μM). The color scale indicates the relative fluorescence intensity F/F0. C: summary data illustrating that H-89 (10 μM) decreased the Ca2+ spark frequency (Freq) and amplitude (Amp) in UBSM cells (n = 8, N = 6) *P < 0.05.
Pharmacological inhibition of constitutive PKA activity with H-89 increased the global cytosolic Ca2+ levels in freshly isolated UBSM cells.
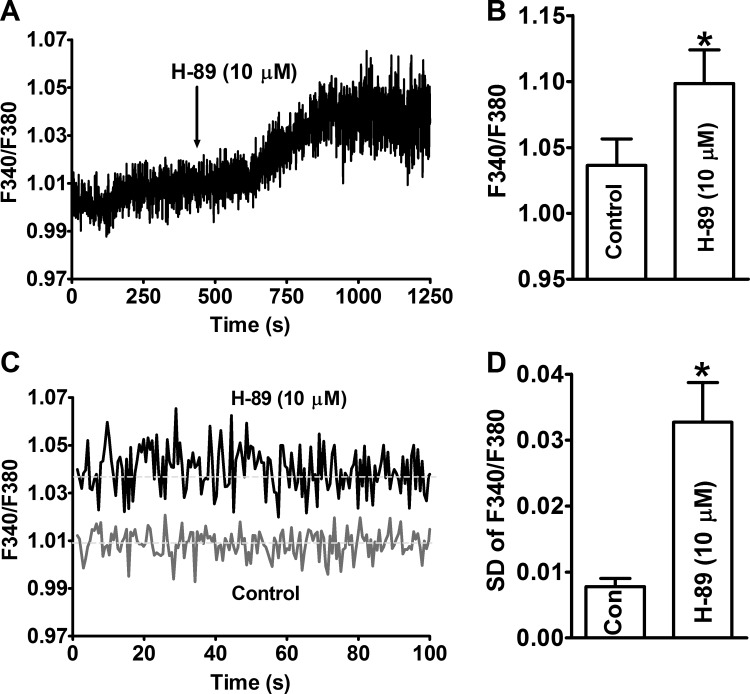
The effect of constitutive PKA activity on global cytosolic Ca2+ levels in UBSM cells was investigated by measuring the fluorescent emission intensity ratio F340/F380 of fura 2. The inhibition of PKA with H-89 (10 μM) increased the levels of cytosolic Ca2+ in UBSM cells (n = 8, N = 4; Fig. 4). The average F340/F380 ratio was 1.036 ± 0.020 under control conditions, and it increased significantly to 1.099 ± 0.025 in the presence of H-89 (10 μM) (n = 8, N = 4, P < 0.05; Fig. 4). H-89 also increased the magnitude of Ca2+ oscillations in UBSM cells (Fig. 4). The magnitude of Ca2+ oscillations was quantified using the standard deviations of F340/F380 ratios during the last 5-min recordings in the absence and presence of H-89 (10 μM). The summary data showed that PKA inhibition increased the Ca2+ oscillations significantly (n = 8, N = 4, P < 0.05; Fig. 4). These results indicate that inhibition of constitutive PKA activity has differential effects on global cytosolic Ca2+ levels and localized Ca2+ signals, i.e., the Ca2+ sparks. These data also indicate that the decrease in Ca2+ spark activity was not due to a reduction of cytosolic Ca2+ levels.
Fig. 4.
Pharmacological inhibition of PKA with H-89 increased the global cellular Ca2+ levels and the magnitude of Ca2+ oscillations in freshly isolated UBSM cells. A: an original recording illustrating that H-89 increased the global Ca2+ levels in a freshly isolated UBSM cell. B: summary data show that the inhibition of PKA significantly increased the global intracellular Ca2+ levels in UBSM cells (n = 8, N = 4). C: the overlay of 100 s of recordings under control conditions and after H-89 treatment shows that H-89 increased the oscillation of the cytosolic Ca2+ in UBSM cells. D: summary data show that the inhibition of PKA significantly increased the magnitude of Ca2+ oscillations, which is measured as the standard deviation (SD) of the F340/F380 ratio during the last 5 min of recording in the absence and presence of H-89 (10 μM; n = 8, N = 4). *P < 0.05. Con, control.
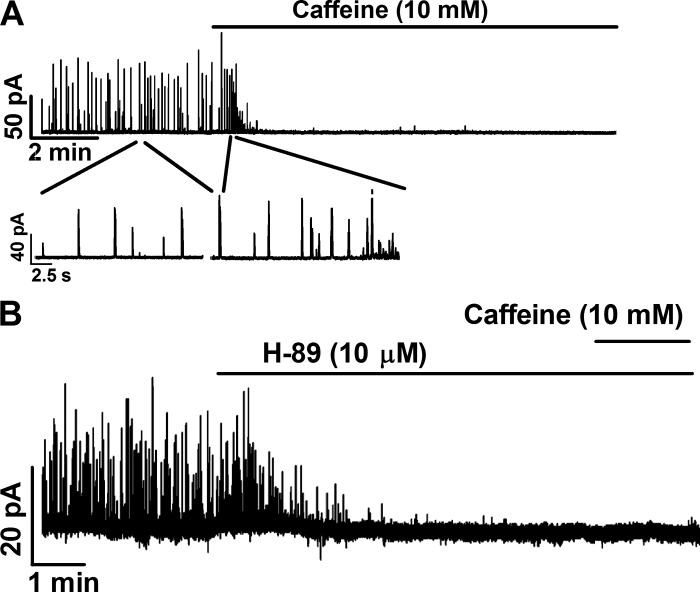
Pharmacological inhibition of PKA with H-89 prevented caffeine-induced increase of TBKCs in freshly isolated UBSM cells.
Caffeine is a potent RyR opener that increases the open probability of RyRs and SR Ca2+ release. Caffeine at a concentration >10 mM can deplete SR Ca2+ by releasing Ca2+ through RyRs (11). In UBSM cells, caffeine (10 mM) transiently increased TBKCs, followed by a complete inhibition (n = 9, N = 6; Fig. 5). However, when the constitutive PKA activity was inhibited with H-89 (10 μM), TBKCs were abolished, and the subsequent addition of caffeine (10 mM) did not induce any TBKCs (n = 5, N = 5; Fig. 5). These data suggest that inhibition of constitutive PKA activity might deplete SR Ca2+, which is necessary for generating Ca2+ sparks and TBKCs.
Fig. 5.
Pharmacological inhibition of PKA with H-89 abolished TBKCs and prevented caffeine-induced outward current in freshly isolated UBSM cells. A: an original voltage clamp recording illustrating that caffeine (10 mM), a ryanodine receptor (RyR) opener, transiently increased the outward currents and then abolished TBKCs in a single UBSM cell (n = 9, N = 6). B: an original recording illustrating that PKA inhibition with H-89 (10 μM) abolished TBKCs and that the subsequent activation of RyR with 10 mM caffeine did not induce any outward currents in single UBSM cells (n = 5, N = 5).
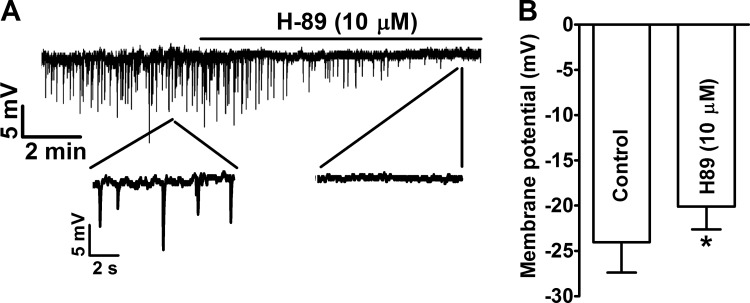
Pharmacological inhibition of constitutive PKA activity with H-89 blocked the spontaneous transient hyperpolarizations and caused membrane depolarization in freshly isolated UBSM cells.
Our previous study demonstrated that spontaneous TBKCs result in spontaneous transient hyperpolarizations in isolated UBSM cells (18, 45). Inhibition of the constitutive PKA activity with H-89 (10 μM) eliminated the spontaneous transient hyperpolarizations of the cell membrane and significantly depolarized the cell membrane potentials from −24.0 ± 3.3 mV under control conditions to −20.1 ± 2.5 mV in the presence of H-89 (n = 11, N = 8, P < 0.05; Fig. 6). These data suggest that constitutive PKA activity is necessary for the spontaneous transient hyperpolarizations in UBSM cells, and therefore, it is important in the regulation of UBSM excitability.
Fig. 6.
Pharmacological inhibition of PKA with H-89 abolished the spontaneous transient hyperpolarizations and depolarized UBSM cell resting membrane potential. A: an original current clamp recording illustrating that H-89 (10 μM) inhibited the spontaneous transient hyperpolarizations in an isolated UBSM cell. A portion of the recording under each condition is shown on an expanded time scale. B: summary data illustrating that H-89 (10 μM) depolarized the membrane potential of UBSM cells (n = 11, N = 8). *P < 0.05.
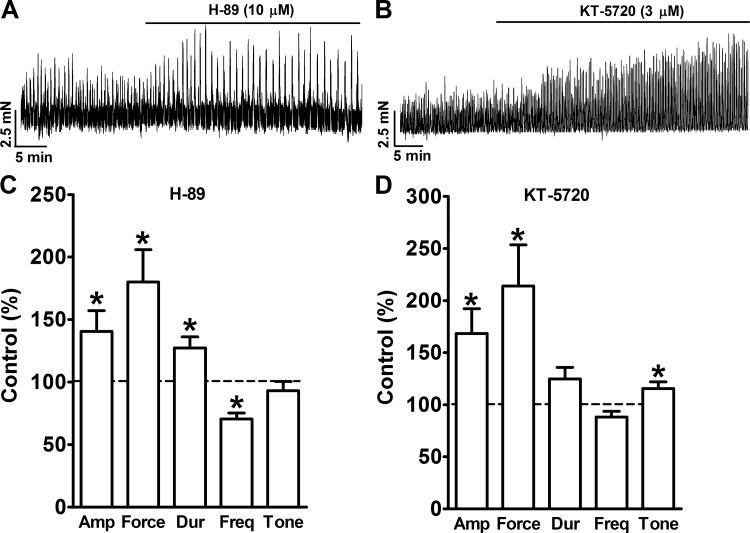
Pharmacological inhibition of constitutive PKA activity leads to an increase in the spontaneous phasic contractions of UBSM.
Inhibition of PKA with H-89 increased the spontaneous phasic contractions of isolated UBSM strips (Fig. 7). The summary data show that the inhibition of PKA with H-89 (10 μM) significantly increased UBSM spontaneous phasic contraction amplitude, muscle force integral, and duration to 140.5 ± 16.8, 180.1 ± 25.9, and 127.2 ± 9.1% of the control, respectively (n = 14, N = 10, P < 0.05; Fig. 7). H-89 (10 μM) also significantly decreased the frequency of spontaneous phasic contractions to 70.6 ± 4.6% of the control (n = 14, N = 10, P < 0.05; Fig. 7). However, H-89 did not have any effect on UBSM tone, which was 93.1 ± 7.4% of the control (n = 14, N = 10, P > 0.05; Fig. 7).
Fig. 7.
Pharmacological inhibition of PKA with H-89 or KT-5720 significantly increased spontaneous phasic contractions of isolated UBSM strips. A and B: representative recordings of UBSM strips illustrating that pharmacological PKA inhibition increases UBSM spontaneous phasic contractions. C and D: summary data illustrating the effects of PKA inhibition with H-89 (10 μM; n = 14, N = 10) or KT-5720 (3 μM; n = 8, N = 5) on the amplitude (Amp), muscle force integral (Force), duration (Dur), frequency (Freq), and tone of UBSM spontaneous phasic contractions. *P < 0.05.
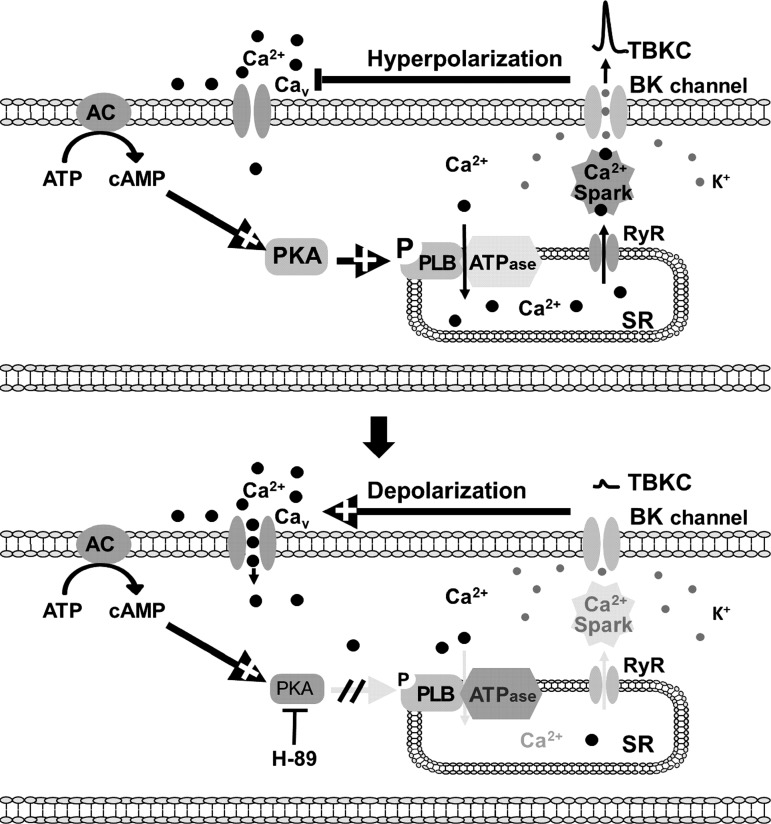
To confirm that the effect of H-89 on the spontaneous phasic contractions of UBSM was indeed caused by the inhibition of PKA, we used another selective PKA inhibitor, KT-5720. Figure 7 illustrates that KT-5720 (3 μM) increased the amplitude, muscle force integral, duration, and tone of the spontaneous phasic contractions to 168.4 ± 23.8, 214.0 ± 39.6, 124.8 ± 11.0, and 115.4 ± 6.6% of the control values, respectively (n = 8, N = 5, P < 0.05; Fig. 7). These data suggest that the constitutive PKA activity is important for regulating the spontaneous phasic contractions of the UBSM, as illustrated in Fig. 8.
Fig. 8.
A diagram depicting the signaling pathway by which the constitutive PKA activity regulates the spontaneous TBKCs and thus UBSM excitability and contractility. AC, adenylyl cyclase; CaV, L-type voltage-gated Ca2+ channel; pPLB, phosphorylated phospholamban; SR, sarcoplasmic reticulum.
DISCUSSION
We tested the hypothesis that constitutively active PKA is essential for the spontaneous TBKCs in UBSM cells and spontaneous phasic contractions of UBSM tissue. We provided direct evidence at the cellular and tissue levels that constitutive PKA activity controls spontaneous TBKCs by regulating their functionally coupled Ca2+ sparks in guinea pig UBSM cells. Therefore, we conclude that constitutive PKA activity is fundamentally important for the regulation of the excitability and spontaneous phasic contractions of UBSM.
Previous studies suggest that β-adrenergic receptor activation or PDE inhibition stimulates BK channel activity through a Ca2+-dependent mechanism rather than a direct regulation of the channels (19, 43). In particular, when RyRs are inhibited, β-adrenergic receptor activation or PDE1 inhibition does not have any effect on UBSM excitability (17, 19, 45). These findings indicate that the cAMP/PKA signaling pathway regulates BK channel activity by controlling intracellular Ca2+ dynamics instead of directly phosphorylating the channels. These previous studies further demonstrated that elevated PKA activity increases Ca2+ sparks in guinea pig UBSM cells when β-adrenergic receptors are activated or PDE4 is inhibited (32, 44). Ca2+ sparks transiently activate BK channels, generating TBKCs, and thus regulate UBSM excitability (8, 17, 27, 30–32, 44). Increased Ca2+ spark activity is likely due to elevated SR Ca2+ content and an increase in RyR activity (5, 12). The reduction of the SR Ca2+ load inhibits RyR and Ca2+ spark activity (21). The present study demonstrates the novel finding that constitutive PKA activity is essential for spontaneous TBKCs in UBSM cells (Figs. 1 and 2). Pharmacological inhibition of constitutive PKA activity depleted the SR Ca2+ content, leading to a reduction of Ca2+ sparks and their functionally coupled spontaneous TBKCs (Fig. 3). The dependency of basal Ca2+ spark frequency on constitutive PKA activity is also seen in mouse ventricular myocytes (29). Specifically, inhibition of constitutive PKA activity attenuates Ca2+ spark activity and decreases the excitation-contraction coupling in ventricular myocytes (29).
The continuous refilling of the SR is required to maintain its Ca2+ content and basal Ca2+ spark activity (7). Pharmacological inhibition of PKA reduced Ca2+ spark activity, which might be caused by inhibiting SR Ca2+-ATPase and depleting SR Ca2+. In fact, pharmacological inhibition of PKA induces dephosphorylation of serine 16 of PLB, leading to an increased inhibitory effect of PLB on Ca2+-ATPase and a reduction in the ability of Ca2+-ATPase to refill the SR with Ca2+ (29). Without sufficient SR Ca2+ refilling, the Ca2+ release will eventually deplete SR Ca2+ and diminish Ca2+ sparks, as illustrated in Fig. 8 (7, 34). It is unlikely that the lack of SR Ca2+ results from a decrease in cytosolic Ca2+ levels. It is more likely that the decreased Ca2+ spark activity leads to an increase in global Ca2+ levels. As our data show, the inhibition of PKA causes an increase in the global intracellular Ca2+ levels and the magnitude of Ca2+ oscillation (Fig. 4). Similarly, decreased Ca2+ spark activity is functionally associated with increased global Ca2+ as a result of reduced BK channel activity in arterial and airway smooth muscle cells (20, 35, 42, 46). Taken together, the present study demonstrates that the localized intracellular Ca2+ signals, Ca2+ sparks, are critically important in the regulation of global intracellular Ca2+ levels. More importantly, this study is in line with our recent report, which showed that increased Ca2+ spark activity can occur simultaneously with a reduction of global Ca2+ levels (44).
Furthermore, when constitutive PKA activity was inhibited with H-89, caffeine (10 mM), a RyR opener, did not induce any TBKCs, supporting the hypothesis that the inhibition of constitutive PKA activity depletes SR Ca2+, resulting in attenuated Ca2+ spark activity (Fig. 5). Caffeine (10 mM) transiently increases TBKCs and is followed by a complete inhibition of TBKCs due to the increased release of Ca2+ and subsequent depletion of SR Ca2+ content (5, 47). The caffeine-induced inhibition of TBKCs has been used as an indication of the depletion of SR Ca2+ in smooth muscle cells (3, 41). Likewise, in UBSM cells, caffeine (10 mM) initially transiently increased but then completely abolished TBKCs (Fig. 5). When the constitutive PKA activity was inhibited and TBKCs were abolished, caffeine (10 mM) was no longer able to induce any TBKCs in UBSM cells (Fig. 5), suggesting that the inhibition of constitutive PKA activity can deplete SR Ca2+ prior to the application of caffeine. These results emphasize the essential role of constitutive PKA activity in maintaining SR Ca2+ content and the spontaneous TBKC activity.
Spontaneous TBKCs caused by basal Ca2+ spark activity regulate the excitability and spontaneous phasic contractions of UBSM (17, 23, 30, 32, 44). The spontaneous TBKCs and transient membrane hyperpolarizations are highly correlated when measured by voltage clamp and current clamp techniques in the same UBSM cells (18, 45). Inhibition of the constitutive PKA activity abolished the spontaneous transient hyperpolarizations and significantly depolarized the membrane potential of isolated UBSM cells (Fig. 6). The essential role of constitutive PKA activity in controlling UBSM excitability is supported by the observation that an increase in the expression of PKA compensates for the permanent loss of BK channels and the gain of excitability in UBSM upon genetic deletion of the BK channels (4, 39). These findings demonstrate that constitutive PKA activity is fundamentally important for the physiological functions of UBSM.
The major finding of the current study is that constitutive PKA activity is essential for the basal TBKCs in UBSM cells (Fig. 1), and this was confirmed by using the highly selective peptide PKA inhibitor PKI 14–22 in addition to H-89 (Fig. 1). It has been reported that H-89 has in vivo IC50 values ranging from 10 to 30 μM, depending on the intracellular ATP levels, and 10 μM H-89 has been used to inhibit PKA activity in most cell-based studies (6, 16). H-89 at low micromolar range may not be sufficient to inhibit endogenous PKA activity (10). In the present study, we used 10 μM H-89 to investigate the effects of constitutive PKA activity on TBKCs, which was further examined using a highly selective peptide inhibitor, PKI 14–22.
As for many ATP-competitive kinase inhibitors, the specificity and the nonkinase-dependent effects of H-89 have raised concerns in studies investigating the function of PKA (24, 26, 36). In the present study, we measured TBKCs, which are activated solely by the localized Ca2+ sparks in freshly isolated UBSM cells (8, 17). The Ca2+ spark activity decreased in the presence of H-89, leading to the reduction of TBKCs' frequency and amplitude (Figs. 1 and 2). These results suggest that the inhibitory effect of H-89 on TBKCs was mediated by PKA, resulting in a decrease in Ca2+ spark activity. Thus, the inhibitory effect of H-89 on TBKCs was unlikely to be caused by a direct action on BK channels in UBSM cells (28). PKA-independent inhibitory effect of H-89 on voltage-gated Na+ channels has been reported in rat alveolar type II epithelial cells; however, this should not be a concern for the present study since there are no voltage-gated Na+ channels expressed in guinea pig UBSM cells (25).
PKI 14–22 is a heat-stable peptide inhibitor of PKA, an endogenous polypeptide that interacts specifically with the free catalytic subunit of PKA after dissociation of the holoenzyme (24, 37, 38, 40). PKI 14–22 is a nine-amino acid segment of the polypeptide that resembles the sequence in the “hinge regions” of the PKA regulatory subunits I and II, which bind to the PKA catalytic subunits in vivo and inhibit their enzyme activity (38). Therefore, PKI 14–22 is a highly potent and specific PKA inhibitor (24, 38). The attenuation of TBKCs by PKI 14–22 confirmed that the effect of H-89 was indeed due to the inhibition of the constitutive PKA activity (Fig. 1).
UBSM spontaneous phasic contractions are triggered by spontaneous action potentials (13, 14). Inhibition of BK channels increases both the action potential amplitude and duration and decreases action potential frequency; consequently, this increases the UBSM spontaneous phasic contractions (13, 15, 31). The effects of the constitutive PKA activity on UBSM phasic contractions are consistent with data showing that inhibition of the constitutive PKA activity inhibited the spontaneous TBKCs in UBSM cells (Figs. 1, 2, and 6). The physiological importance of the constitutive activity of PKA in the regulation of UBSM spontaneous contractility was confirmed by the two PKA inhibitors H-89 and KT-5720. We used KT-5720 instead of PKI 14–22 because of its higher membrane permeability. Both H-89 and KT-5720 are ATP-competitive PKA inhibitors; however, they have substantial differences in their chemical structures. The structure of H-89 is much simpler and has more similarity with ATP than KT-5720. KT-5720 increased the spontaneous phasic contractions of freshly isolated UBSM strips, as H-89 did. These data indicate that constitutive PKA activity is functionally important to control the spontaneous contractile activity of UBSM (Fig. 7). The critical role of constitutive PKA activity in maintaining UBSM excitability and contractility is also supported by the observation that a gain of PKA expression counterbalances the increased contractility of mouse UBSM caused by genetic deletion of BK channel pore-forming α-subunit (4, 39).
In conclusion, constitutive PKA activity is fundamentally important for maintaining spontaneous TBKCs and cellular Ca2+ homeostasis in UBSM cells as well as the spontaneous contractility of UBSM, as depicted in Fig. 8.
GRANTS
This study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-084284) to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.X. and G.V.P. conception and design of research; W.X., N.L., Q.C., V.S.F., and G.V.P. performed experiments; W.X., N.L., Q.C., V.S.F., and G.V.P. analyzed data; W.X., N.L., Q.C., and G.V.P. interpreted results of experiments; W.X., N.L., Q.C., V.S.F., and G.V.P. prepared figures; W.X. and G.V.P. drafted manuscript; W.X., N.L., Q.C., V.S.F., and G.V.P. edited and revised manuscript; W.X., N.L., Q.C., V.S.F., and G.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. John Malysz, Shankar Parajuli, and Kiril L. Hristov and Aaron Provence for critical evaluation of the manuscript.
REFERENCES
- 1.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Beca S, Ahmad F, Shen W, Liu J, Makary S, Polidovitch N, Sun J, Hockman S, Chung YW, Movsesian M, Murphy E, Manganiello V, Backx PH. Phosphodiesterase type 3A regulates basal myocardial contractility through interacting with sarcoplasmic reticulum calcium ATPase type 2a signaling complexes in mouse heart. Circ Res 112: 289–297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borisova L, Shmygol A, Wray S, Burdyga T. Evidence that a Ca2+ sparks/STOCs coupling mechanism is responsible for the inhibitory effect of caffeine on electro-mechanical coupling in guinea pig ureteric smooth muscle. Cell Calcium 42: 303–311, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient K(Ca) currents. J Physiol 544: 71–84, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265: 5267–5272, 1990. [PubMed] [Google Scholar]
- 7.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol 587: 5197–5209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, Indolfi C, Cable NT, Goldspink DF, Nadal-Ginard B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem 282: 11397–11409, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev 91: 651–690, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Fredriksson JM, Thonberg H, Ohlson KB, Ohba K, Cannon B, Nedergaard J. Analysis of inhibition by H89 of UCP1 gene expression and thermogenesis indicates protein kinase A mediation of β3-adrenergic signalling rather than β3-adrenoceptor antagonism by H89. Biochim Biophys Acta 1538: 206–217, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Viquez NL, Guerrero-Serna G, Arvizu F, García U, Guerrero-Hernández A. Inhibition of SERCA pumps induces desynchronized RyR activation in overloaded internal Ca2+ stores in smooth muscle cells Am J Physiol Cell Physiol 298: C1038–C 1046, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Guo T, Gillespie D, Fill M. Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ Res 111: 28–36, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Herbst KJ, Allen MD, Zhang J. The cAMP-dependent protein kinase inhibitor H-89 attenuates the bioluminescence signal produced by Renilla Luciferase. PLoS One 4: e5642, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koide M, Nystoriak MA, Brayden JE, Wellman GC. Impact of subarachnoid hemorrhage on local and global calcium signaling in cerebral artery myocytes. Acta Neurochir Suppl 110: 145–150, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kur J, Bankhead P, Scholfield CN, Curtis TM, McGeown JG. Ca2+ sparks promote myogenic tone in retinal arterioles. Br J Pharmacol 168: 1675–1686, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masterson LR, Yu T, Shi L, Wang Y, Gustavsson M, Mueller MM, Veglia G. cAMP-dependent protein kinase A selects the excited state of the membrane substrate phospholamban. J Mol Biol 412: 155–164, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Murray AJ. Pharmacological PKA Inhibition: All May Not Be What It Seems. Sci Signal. 1: re4, 2008. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- 25.Niisato N, Ito Y, Marunaka Y. Effects of PKA inhibitors, H-compounds, on epithelial Na+ channels via PKA-independent mechanisms. Life Sci 65: PL109–PL114, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Norman RA, Toader D, Ferguson AD. Structural approaches to obtain kinase selectivity. Trends Pharmacol Sci 33: 273–278, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol 534: 313–326, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park WS, Son YK, Kim N, Youm JB, Warda M, Ko JH, Ko EA, Kang SH, Kim E, Earm YE, Han J. Direct modulation of Ca2+-activated K+ current by H-89 in rabbit coronary arterial smooth muscle cells. Vascul Pharmacol 46: 105–113, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Parks RJ, Howlett SE. H-89 decreases the gain of excitation-contraction coupling and attenuates calcium sparks in the absence of beta-adrenergic stimulation. Eur J Pharmacol 691: 163–172, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Petkov GV. Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am J Physiol Regul Integr Comp Physiol 307: R571–R584, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 293: C1073–C1081, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Rainbow RD, Macmillan D, McCarron JG. The sarcoplasmic reticulum Ca2+ store arrangement in vascular smooth muscle. Cell Calcium 46: 313–322, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Roberts OL, Kamishima T, Barrett-Jolley R, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) induces vascular relaxation by activating Ca2+-sensitive K+ channels in rat mesenteric artery. J Physiol 591: 5107–5123, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scapin G. Protein kinase inhibition: different approaches to selective inhibitor design. Curr Drug Targets 7: 1443–1454, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Scott JD, Fischer EH, Demaille JG, Krebs EG. Identification of an inhibitory region of the heat-stable protein inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci USA 82: 4379–4383, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott JD, Fischer EH, Takio K, Demaille JG, Krebs EG. Amino acid sequence of the heat-stable inhibitor of the cAMP-dependent protein kinase from rabbit skeletal muscle. Proc Natl Acad Sci USA 82: 5732–5736, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprossmann F, Pankert P, Sausbier U, Wirth A, Zhou XB, Madlung J, Zhao H, Bucurenciu I, Jakob A, Lamkemeyer T, Neuhuber W, Offermanns S, Shipston MJ, Korth M, Nordheim A, Ruth P, Sausbier M. Inducible knockout mutagenesis reveals compensatory mechanisms elicited by constitutive BK channel deficiency in overactive murine bladder. FEBS J 276: 1680–1697, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh DA, Ashby CD, Gonzalez C, Calkins D, Fischer EH. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3′,5′-monophosphate-dependent protein kinases. J Biol Chem 246: 1977–1985, 1971. [PubMed] [Google Scholar]
- 41.Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am J Physiol Cell Physiol 281: C1029–C1037, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol 590: 1849–1869, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin W, Cheng Q, Soder RP, Petkov GV. Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage- and Ca2+-activated K+ channel. Am J Physiol Cell Physiol 302: C1361–C1370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xin W, Li N, Cheng Q, Petkov GV. BK channel-mediated relaxation of urinary bladder smooth muscle: a novel paradigm for phosphodiesterase type 4 regulation of bladder function. J Pharmacol Exp Ther 349: 56–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin W, Soder RP, Cheng Q, Rovner ES, Petkov GV. Selective inhibition of phosphodiesterase 1 relaxes urinary bladder smooth muscle: role for ryanodine receptor-mediated BK channel activation. Am J Physiol Cell Physiol 303: C1079–C1089, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhuge R, Bao R, Fogarty KE, Lifshitz LM. Ca2+ sparks act as potent regulators of excitation-contraction coupling in airway smooth muscle. J Biol Chem 285: 2203–2210, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol 113: 215–228, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]