diarrhea caused by enteric (bacterial/viral/parasitic) infections or diarrhea associated with inflammatory bowel disease represents a major health care burden worldwide (10). In addition, diarrhea associated with antibiotic administration in adults, as well as children, is a significant health care issue (1, 3). Diarrhea is also a common side effect of chemo/radiotherapy during cancer treatment (6). Antidiarrheal drugs generally have been shown to cause nausea, loss of appetite, and constipation, which often hinder treatment protocols (15). Diarrhea is a consequence of the imbalance of water and ion absorption by epithelial cells of the gastrointestinal tract, ultimately leading to enhanced secretion and/or diminished absorption of fluid and electrolytes. Aberrant ion movement across epithelia can occur through a paracellular pathway via tight junctions or a transcellular pathway via membrane transporters such as Na+-dependent glucose transporter 1, Na+/H+ exchanger isoform 3, Cl−/HCO3− exchanger, and downregulated in adenoma (DRA), which can be dysregulated in diarrhea (10). Infectious pathogens can alter ion transporters directly or may induce electrolyte imbalance via increased inflammation or reduction in the absorptive capacity of the epithelial cells (12). Several studies of the functional implication of DRA in diarrheal disorders have been published (4, 7, 13).
A large and growing literature describes studies of the beneficial health effects of live microorganisms in treating gastrointestinal disorders. There are several trials with bacterial formulations of Bifidobacterium in conjunction with other species of probiotic strains to treat diarrheal symptoms, although strong mechanistic studies regarding the usability of individual species of Bifidobacterium by itself are lacking. Bifidobacterium belongs to the class of anaerobic, gram-positive bacteria that are part of the natural microbial flora of the gastrointestinal tract (5). This gap in understanding of the signaling mechanisms associated with the use of a particular probiotic strain poses challenges to the claims made by the food industry.
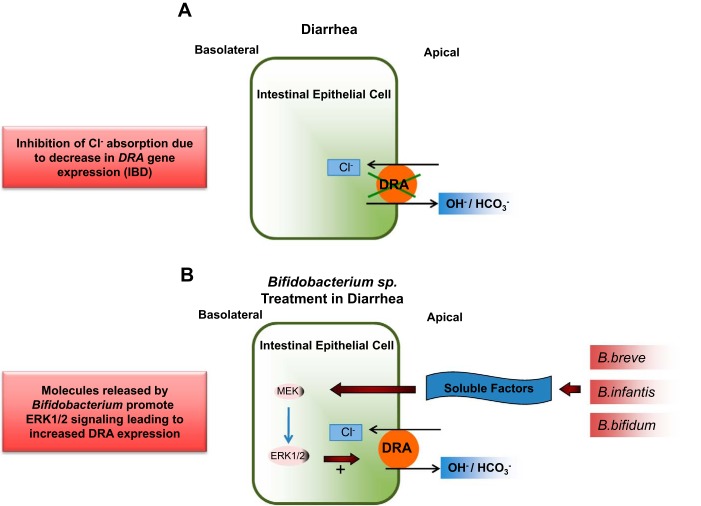
In this issue of American Journal of Physiology-Cell Physiology, Kumar et al. (11a) report that Bifidobacterium species might serve as a potential therapeutic approach for treating diarrhea arising from ulcerative colitis or enteric infections (2, 9). Consistent with previous findings in diarrheal diseases, the in vivo and in vitro studies of Kumar et al. provide strong evidence that enhanced expression of DRA (mediated by treatment with live and culture supernatants of Bifidobacterium, respectively) stimulates Cl−/HCO3− exchanger activity. The authors demonstrate that treatment of Caco-2 cells with supernatants from cultures of various Bifidobacterium species enhances Cl−/HCO3− exchange activity, with a concomitant increase in DRA mRNA and protein expression. Increased DRA mRNA transcription was experimentally blocked by actinomycin D, which resulted in abrogation of the effect of Bifidobacterium breve and Bifidobacterium infantis. Previous studies showed a preferential expression of DRA relative to that of putative anion transporter 1 (PAT-1) in the colonic region (8). Kumar et al. also studied the anion exchanger PAT-1 to determine its role in the stimulation of Cl−/HCO3− exchanger activity. Other studies showed that PAT-1 is refractory to regulation; consistent with such findings, Kumar et al. did not observe an effect of Bifidobacterium treatment on this gene. They also demonstrated the role of the ERK1/2 MAPK signaling pathway in the activation of DRA, thereby advancing the understanding of the potential mechanism of action of probiotic strains on DRA expression. Although treatment of diarrhea with a mixture of probiotic species has been reported (1), the study of Kumar et al. represents a significant advancement toward elucidation of the role of specific species of bacteria as potential probiotic/antidiarrheal agent. The proposed mechanism of action of Bifidobacterium species on the regulation of DRA expression is summarized in Fig. 1.
Fig. 1.
Functional implications of Bifidobacterium in probiotic therapy for diarrhea. A: downregulated in adenoma (DRA), located on the apical membrane of intestinal epithelial cells. Bifidobacterium promotes reabsorption of Cl−. Diarrhea may occur due to malfunction/underexpression/mutation of DRA, leading to water and electrolyte imbalance. IBD, inflammatory bowel disease. B: treatment in vitro with culture supernatant (CS) of Bifidobacterium species or in vivo with live Bifidobacterium species enhances expression of DRA by an ERK-mediated signaling pathway, which may restore ion-water balance in the intestinal lumen. Molecules in CS causing the effect require further study.
Although several different anion exchangers have been shown to participate in maintaining water and electrolyte balance, increasing evidence for the role of DRA in diarrhea underscores the importance of further studies of this transport protein. For example, DRA expression can be altered in diarrhea associated with infections and antibiotic treatment (3, 4, 7, 10). In addition, the DRA−/− mouse model has been used to characterize the role of DRA in diarrheal disorders (14). Mutations of the transporter slc26a3 (the gene encoding the DRA protein) result in aberrant electrolyte balance in congenital Cl− diarrhea (11). Probiotic treatment of gastrointestinal disorders with diarrheal symptoms is currently under study, including several clinical trials with formulations containing Lactobacillus species and Bifidobacterium species (6). Despite these studies, there are limited reports of the role of Bifidobacterium by itself and also the factors generated by this species that improve diarrheal symptoms. Thus it is important to study the mechanisms of action of possible soluble factors that are secreted from Bifidobacterium species and regulate the transporters involved in anion absorption in the gastrointestinal tract.
The study of Kumar et al. (11a) has a few minor limitations. The mouse models treated with live Bifidobacterium species were not analyzed for side effects on organs other than the gastrointestinal tract. Also Kumar et al. did not identify the bioactive soluble factors secreted by the bacteria. However, their study has the potential to promote research in animal models of diarrhea associated with bacterial/viral pathogens or intestinal inflammation and will reinforce the use of Bifidobacterium formulations as antidiarrheal probiotic agents. Overall, the study strongly suggests that upregulation of DRA expression by bioactive factors secreted by Bifidobacterium may play an important role in treating diarrhea. Also, the use of probiotic bacterial formulations for treatment of infectious diarrhea among younger patients may prevent side effects (e.g., nausea and loss of appetite) of traditional pharmacological drugs, thus making the use of such drugs less challenging. It is quite possible that once the signaling and bioactive factors associated with the action of a probiotic strain are evaluated, the information will give way to development of drugs that would not adversely affect the immune system in patients and would have improved efficacy during the recovery process.
GRANTS
This material is the result of work supported by resources at the Central Texas Veterans Health Care System.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R. and S.G. prepared the figure; D.R. and S.G. drafted the manuscript; D.R., G.A., and S.G. edited and revised the manuscript; G.A. approved the final version of the manuscript.
REFERENCES
- 1.Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W, Dhar A, Brown H, Foden A, Gravenor MB, Mack D. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 382: 1249–1257, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Nomoto K, Shimizu K, Watanuki M, Tanaka R. Increased resistance of mice to Salmonella enterica serovar Typhimurium infection by synbiotic administration of bifidobacteria and transgalactosylated oligosaccharides. J Appl Microbiol 91: 985–996, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG. Antibiotic-associated diarrhea. N Engl J Med 346: 334–339, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase IV as a cause of fatal infectious diarrhea in mice. Infect Immun 77: 3639–3650, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chassard C, de Wouters T, Lacroix C. Probiotics tailored to the infant: a window of opportunity. Curr Opin Biotechnol 26: 141–147, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol 5: 31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas K, Yeruva S, Rakonczay Z, Jr., Ludolph L, Molnar T, Nagy F, Szepes Z, Schnur A, Wittmann T, Hubricht J, Riederer B, Venglovecz V, Lazar G, Kiraly M, Zsembery A, Varga G, Seidler U, Hegyi P. New therapeutic targets in ulcerative colitis: the importance of ion transporters in the human colon. Inflamm Bowel Dis 17: 884–898, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O'Neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54: 242–249, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges K, Gill R. Infectious diarrhea: cellular and molecular mechanisms. Gut Microbes 1: 4–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoglund P. SLC26A3 and congenital chloride diarrhoea. Novartis Found Symp 273: 74–90, 2006. [PubMed] [Google Scholar]
- 11a.Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, Alrefai WA, Gill RK, Dudeja PK. Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol (August 20, 2014). 10.1152/ajpcell.00194.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 34: 346–353, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Musch MW, Arvans DL, Wu GD, Chang EB. Functional coupling of the downregulated in adenoma Cl−/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am J Physiol Gastrointest Liver Physiol 296: G202–G210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Wang HH, Shieh MJ, Liao KF. A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhea in adults. World J Gastroenterol 11: 1540–1543, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]