Abstract
SLC26A3, or downregulated in adenoma (DRA), plays a major role in mediating Cl− absorption in the mammalian intestine. Disturbances in DRA function and expression have been implicated in intestinal disorders such as congenital Cl− diarrhea and gut inflammation. We previously showed that an increase in DRA function and expression by Lactobacillus acidophilus and its culture supernatant (CS) might underlie antidiarrheal effects of this probiotic strain. However, the effects of Bifidobacterium species, important inhabitants of the human colon, on intestinal Cl−/HCO3− exchange activity are not known. Our current results demonstrate that CS derived from Bifidobacterium breve, Bifidobacterium infantis, and Bifidobacterium bifidum increased anion exchange activity in Caco-2 cells (∼1.8- to 2.4-fold). Consistent with the increase in DRA function, CS also increased the protein, as well as the mRNA, level of DRA (but not putative anion transporter 1). CS of all three Bifidobacterium sp. increased DRA promoter activity (−1,183/+114 bp) in Caco-2 cells (1.5- to 1.8-fold). Furthermore, the increase in DRA mRNA expression by CS of B. breve and B. infantis was blocked in the presence of the transcription inhibitor actinomycin D (5 μM) and the ERK1/2 MAPK pathway inhibitor U0126 (10 μM). Administration of live B. breve, B. infantis, and B. bifidum by oral gavage to mice for 24 h increased DRA mRNA and protein levels in the colon. These data demonstrate an upregulation of DRA via activation of the ERK1/2 pathway that may underlie potential antidiarrheal effects of Bifidobacterium sp.
Keywords: antidiarrheal, Caco-2, chloride absorption, downregulated in adenoma
diarrheal diseases associated with infections by enteric pathogens or with inflammatory bowel disorders cause an enormous health care burden, accounting for ∼4% of overall deaths and ∼1.2 million deaths in children <5 yr of age (20). Diarrhea may result from increased secretion or decreased absorption of solutes and electrolytes. Thus, understanding the regulation of ion transport mechanisms involved in fluid absorption is of critical importance in defining the pathophysiology of diarrheal diseases, as well as developing better therapeutic interventions for treatment of these diseases. Previous studies from our laboratory utilizing purified plasma membranes from organ donor intestines, as well as molecular and cellular approaches, have shown that a major mechanism of electroneutral NaCl absorption in human ileum and colon involves coupling of Na+/H+ (NHE) and Cl−/HCO3− exchangers (7, 12). NHE3 and downregulated in adenoma (DRA) are two major apical ion exchangers implicated in NaCl absorption in the intestine. Putative anion transporter 1 (PAT-1), another apical anion exchanger, is also expressed in the apical membrane of intestinal epithelial cells (IECs); however, its role in diarrheal disorders is not well recognized. For example, PAT-1−/− mice showed a decrease in apical Cl−/HCO3− exchange activity but did not exhibit a diarrheal phenotype (42).
Increasing evidence indicates that disturbances in DRA function or expression play a major role in the pathophysiology of several diarrheal disorders. For example, mutations in DRA lead to a rare genetic disorder, congenital Cl− diarrhea, characterized by metabolic alkalosis, impaired Cl−/HCO3− exchange, and high fecal Cl− concentration (>90 mmol/l) (34). DRA−/− mice exhibit substantial diarrheal phenotype with serum electrolyte imbalances (34). Enteropathogenic Escherichia coli, an important food-borne pathogen causing diarrhea, decreases apical Cl−/HCO3− exchange activity concomitant with a decrease in DRA plasma membrane levels (14). Similarly, diarrhea caused by Citrobacter rodentium infection of mice is associated with a marked reduction in DRA gene expression (4). DRA expression has also been shown to be decreased in intestinal inflammation (2). Therefore, agents that increase DRA offer potential for utilization as antidiarrheal or proabsorptive agents. Probiotics have been shown to have beneficial effects as antidiarrheal agents (11, 17, 37), although the mechanisms underlying their effects are not fully understood.
Probiotics are viable microorganisms with beneficial effects on human health (26). Previous studies have reported that the culture supernatant (CS) of Lactobacillus rhamnosus, Bifidobacterium infantis, and VSL#3 (a mixture of Lactobacillus and Bifidobacterium strains) shows beneficial effects similar to those of live bacteria on intestinal epithelia (27). Multiple mechanisms of action, including suppression of growth or epithelial binding/invasion by pathogenic bacteria, decreased Cl− secretion, improved epithelial barrier function, and immunomodulation, have been suggested to explain the protective effects of probiotics (18, 26). However, studies investigating the mechanism of action of individual probiotic strains on epithelial functions are limited. Our previous studies demonstrated that live bacterium and CS of Lactobacillus acidophilus (LA) increased apical Cl−/HCO3− exchange activity, which may underlie the beneficial effects of LA in several diarrheal disorders (5, 30). The effects of Bifidobacterium species (important inhabitants of human colon) on DRA have not been investigated.
Therefore, in vitro and in vivo mouse models were used to examine the effects of Bifidobacterium sp. on apical Cl−/HCO3− exchangers. Our findings demonstrate that treatment of Caco-2 cells with CS of Bifidobacterium sp. (B. breve, B. infantis, and B. bifidum) increases DRA expression in Caco-2 cells via the ERK1/2 MAPK pathway. DRA promoter activity was also stimulated by CS of Bifidobacterium sp., indicating the involvement of transcriptional mechanisms. Consistent with the in vitro results, in vivo data showed that Bifidobacterium sp. increased DRA mRNA and protein in mouse colon. Our studies suggest that the increase in DRA expression by Bifidobacterium may have therapeutic implications in diarrhea associated with ulcerative colitis or enteric infections.1
MATERIALS AND METHODS
Materials.
Caco-2 cells, HT-29 cells, and probiotic Bifidobacterium sp. were obtained from American Type Culture Collection (ATCC, Manassas, VA), 125I− from Perkin Elmer, RNeasy kits for RNA extraction from Qiagen (Valencia, CA), and real-time quantitative RT-PCR (qRT-PCR) kits from Stratagene (La Jolla, CA). 4,4′-Diisothiocyanate-stilbene-2,2′-disulfonic acid (DIDS) was purchased from Sigma Aldrich (St. Louis, MO), and ready-made SDS-polyacrylamide gels from Bio-Rad (Hercules, CA). DRA antibody was custom-synthesized against the COOH-terminal amino acid (745–764) sequence INTNGGLRNRVYEPVETKF of SLC26A3 (accession no. BC025671).
Cell lines, cell culture, and treatments.
Caco-2 and HT-29 cells were grown at 37°C in an atmosphere of 5% CO2 in a T-75 flask. Caco-2 cells were maintained in MEM supplemented with 20% fetal bovine serum, and HT-29 cells were maintained in McCoy's medium supplemented with 10% fetal bovine serum. Penicillin (100 IU/ml), streptomycin (100 μg/ml), and gentamicin (2 μg/ml) were added to HT-29 and Caco-2 media. Studies were performed in fully differentiated Caco-2 monolayers grown for 12–14 days postplating on 24-well plastic supports or 12-well Transwell inserts between passages 25 and 45.
Bifidobacterium culture and preparation of conditioned CS.
B. breve (product no. 15700, ATCC), B. infantis (product no. 15697, ATCC), and B. bifidum (product no. 15696, ATCC) were grown in reinforced clostridial medium (Difco, Detroit, MI) for 24 h at 37°C in an anaerobic incubator without shaking to 1.0 optical density at 600 nm (reading taken after dilution in the same medium). The overnight-grown cultures were centrifuged at 3,000 g at 4°C for 10 min. The supernatant was filtered through a 0.22-μm filter (Millex, Millipore, Bedford, MA) to sterilize and remove all bacterial cells and was designated as the CS. For treatment of the cell monolayers, the CS was diluted 1:2–1:10 in DMEM-F12 cell culture medium for 6 or 24 h.
Assessment of Cl−/HCO3− exchange activity.
Cl−/HCO3− exchange activity was measured as described previously by our laboratory (14, 32). Caco-2 cells were incubated with loading buffer, pH 8.5, for 30 min at room temperature. The medium was removed, and the cells were rapidly washed with 1 ml of tracer-free uptake mannitol buffer containing 260 mM mannitol and 20 mM Tris-2-(N-morpholino) ethanesulfonic acid, pH 7.0. The cells were then incubated with the uptake buffer containing 1 μCi of 125I− (3 mM) for 5 min in the absence or presence of 600 μM DIDS. The 5-min time period was chosen, because it falls within the linear range of 125I− uptake in this system. The buffer was removed, and the cells were washed rapidly twice with 1 ml of ice-cold PBS, pH 7.2, to stop the uptake. Finally, the cells were solubilized by incubation with 0.5 N NaOH for 4 h. Protein concentration was measured by the method of Bradford (6), and radioactivity was counted by a liquid scintillation analyzer (TRI-CARB 1600-TR, Packard Instruments, PerkinElmer, Boston, MA). Cl−/HCO3− exchange activity was assessed as DIDS-sensitive 125I− uptake, and values are expressed as nanomoles per milligram protein per 5 min.
RNA extraction and mRNA expression.
RNA was isolated from Caco-2 and HT-29 cells and mouse intestinal tissues using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. An equal amount of RNA for each sample was reverse-transcribed and amplified in a one-step reaction using the Brilliant SYBR Green QRT-PCR Master Mix kit (Stratagene) and Mx 3000 (Stratagene). The gene-specific primers for human or mouse DRA were used for the RT-PCR. ΔCt − DRA and ΔCt − GAPDH represent the difference between the threshold cycle of amplification of DRA and GAPDH, respectively.
Western blotting.
After treatment, Caco-2 cells were washed twice with ice-cold 1× PBS and lysed in 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, and 1× protease cocktail inhibitor mixture. The cells were lysed by sonication, and the lysate was centrifuged at 7,000 rpm for 7 min at 4°C. Protein concentration was determined by the Bradford assay (6). Equal amounts (75 μg) of cell lysates were solubilized in gel loading buffer and boiled for 5 min. Samples were then loaded on ready-made 7.5% SDS-polyacrylamide gels (Bio-Rad) and transblotted to nitrocellulose membranes. The membranes were incubated in blocking buffer containing 1× PBS and 5% nonfat dry milk for 1 h and then with DRA antibody (1:100 dilution) in 1× PBS overnight at 4°C. The membranes were washed four times with the wash buffer containing 1× PBS and 0.1% Tween 20 for 5 min. Finally, the membranes were probed with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2,000 dilution), and the bands were visualized with enhanced chemiluminescence detection reagents.
Assessment of promoter activity.
Caco-2 cells were transfected with DRA promoter (−1183/+114 bp) fragment cloned upstream of the luciferase reporter gene in pGL2-Basic and β-galactosidase expression vector by electroporation using the Amaxa Nucleofactor System, as described previously (30). Activities of firefly luciferase and β-galactosidase were measured according to the manufacturer's instructions (Promega, Madison, WI). DRA promoter activity is expressed in terms of relative luciferase activity normalized to β-galactosidase activity.
In vivo studies.
In vivo studies performed in C57BL/6J mice were approved by the Animal Care Committee of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center. Mice were gavaged with Bifidobacterium sp. (109 colony-forming units) in 200 μl of sterile PBS as vehicle for 24 h. Intestines were resected, and mucosa was scraped for RNA and protein extraction. Sections (∼2 cm) of the different regions of intestine (ileum and colon) were immediately snap-frozen in optimal cutting temperature embedding medium (Tissue-Tek OCT compound, Sakura) for immunofluorescence studies. RNA and protein were extracted, and real-time qRT-PCR and Western blotting were performed as described above.
Statistical analysis.
Values are means ± SE of three to five independent experiments. Differences between controls and various treatments were analyzed using one-way analysis of variance with Tukey's test. Differences were considered significant at P < 0.05.
RESULTS
Bifidobacterium CS increases Cl−/HCO3− activity in Caco-2 cells.
Since Bifidobacterium is an obligate anaerobic bacterium, CS derived from overnight-grown culture medium in an anaerobic chamber, rather than the live bacterium, was used for treatment of cell monolayers. Caco-2 monolayers grown on Transwell inserts or regular plastic supports were treated apically with CS derived from B. breve, B. infantis, or B. bifidum (1:2 dilution) in DMEM-F12 medium for 24 h, and DIDS-sensitive 125I− uptake was measured after base loading the cells. Cl−/HCO3− exchange activity was significantly increased by CS obtained from all species of Bifidobacterium (Fig. 1A). Further dilution of CS to 1:5 or 1:10 diminished the stimulatory effect on DRA. These data indicate that the soluble effector molecules in the CS derived from Bifidobacterium sp. mediate the increase in Cl−/HCO3− exchange activity in Caco-2 cells (Fig. 1B).
Fig. 1.
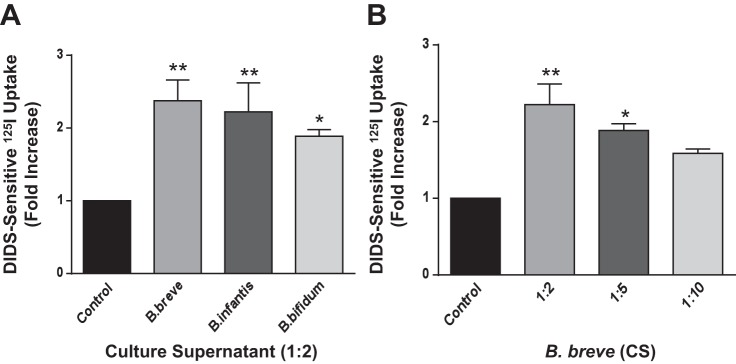
Long-term treatment with Bifidobacterium sp. culture supernatant (CS) stimulates Cl−/HCO3− exchange activity in Caco-2 cells. Fully differentiated Caco-2 cells were treated with a 1:2 dilution of Bifidobacterium breve Bifidobacterium infantis, or Bifidobacterium bifidum CS for 24 h (A) or with different dilutions of B. breve CS (B), and apical Cl−/HCO3− exchange activity (DIDS-sensitive 125I− uptake) was measured. Values are means ± SE of 3–4 separate experiments. *P < 0.05, **P < 0.01 vs. control.
Bifidobacterium sp. CS increases DRA mRNA levels in IECs.
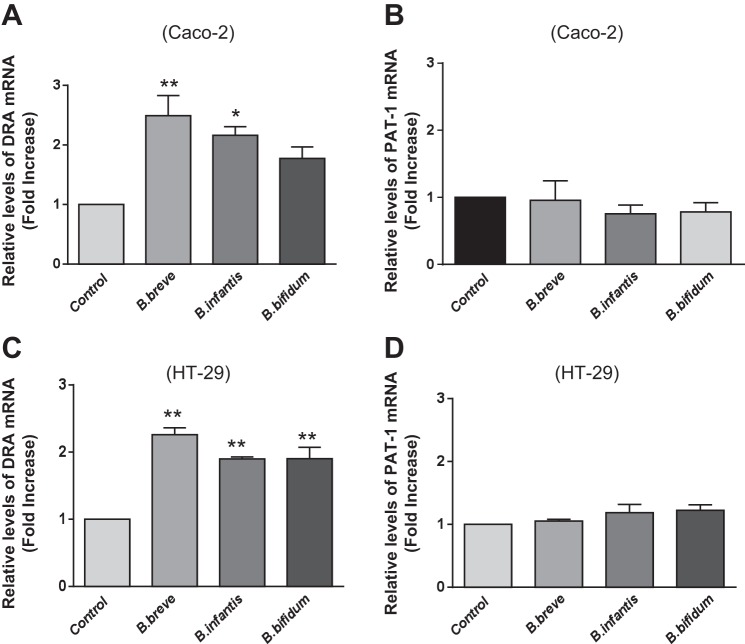
Since long-term treatment with Bifidobacterium CS increased Cl−/HCO3− exchange activity, we next examined whether this stimulation occurs via an increase in expression levels of the apical anion exchangers DRA and/or PAT-1. Caco-2 and HT-29 monolayers were treated with B. breve, B. infantis, or B. bifidum CS for 24 h, and expression of DRA and PAT-1 was determined by real-time qRT-PCR. Consistent with the functional studies, treatment with CS (1:2 dilution) from all Bifidobacterium sp. increased DRA mRNA levels (Fig. 2A). mRNA levels of PAT-1, however, did not change significantly under these conditions (Fig. 2B), indicating that these effects were specific. Furthermore, these effects were not cell line-specific, as treatment of HT-29 cells with Bifidobacterium CS also increased DRA (Fig. 2C), but not PAT-1 (Fig. 2D), mRNA expression, similar to the effects in Caco-2 cells.
Fig. 2.
Bifidobacterium sp. CS increases downregulated in adenoma (DRA), but not putative anion transporter 1 (PAT-1), mRNA levels in Caco-2 and HT-29 cells. Relative mRNA abundance of DRA and PAT-1 in Caco-2 and HT-29 cells was measured using gene-specific primers. GAPDH was used as internal control. Values are means ± SE of 3–4 separate experiments. *P < 0.05, **P < 0.01 vs. control.
Bifidobacterium sp. CS increases DRA protein levels in Caco-2 cells.
To examine whether the increase in DRA mRNA levels by Bifidobacterium CS translates into an increase in protein expression, Western blot studies were performed. Parallel to changes at the mRNA level, CS of Bifidobacterium sp. increased DRA protein levels in Caco-2 cells (Fig. 3A). Densitometric analysis of the protein band shows that CS treatment increases DRA protein levels (∼2-fold) compared with control (Fig. 3B).
Fig. 3.
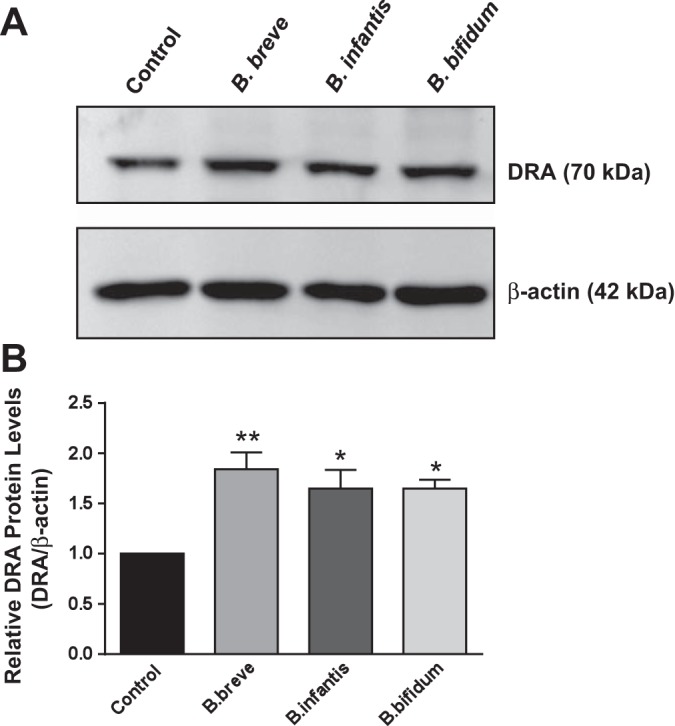
DRA protein expression in response to long-term treatment with Bifidobacterium sp. CS. A: Western blot analysis of postconfluent Caco-2 cells treated with B. breve, B. infantis, or B. bifidum CS (diluted 1:2 in DMEM-F12 medium) for 24 h. B: densitometry analysis of intensity of DRA protein normalized to β-actin. Values are means ± SE of 4 independent experiments. *P < 0.05, **P < 0.01 vs. control.
Bifidobacterium sp. CS activates DRA promoter activity in Caco-2 cells.
To elucidate the mechanisms underlying the increase in DRA function and expression, we further investigated the effects of Bifidobacterium sp. CS on DRA promoter activity in Caco-2 cells. Caco-2 cells were transiently cotransfected with DRA promoter construct along with pCMV-β-galactosidase vector as a control for transfection efficiency. At 24 h posttransfection, cells were treated with CS (1:2 dilution) derived from B. breve, B. infantis, or B. bifidum for 6 and 24 h, and DRA promoter activity was assessed. The results demonstrate an increase in DRA promoter activity in response to CS of all Bifidobacterium sp. at 6 and 24 h (Fig. 4). These data indicate that the Bifidobacterium sp. CS-mediated increase in DRA gene expression was via an increase in DRA promoter activity.
Fig. 4.
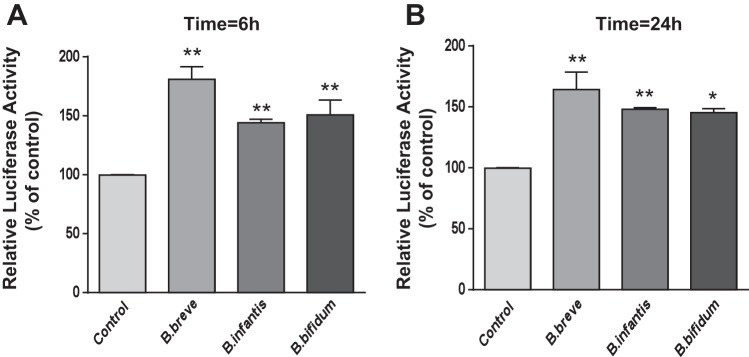
Bifidobacterium sp. CS stimulates DRA promoter activity in Caco-2 cells. Caco-2 cells were transiently transfected with a 1.3-kb fragment of the promoter region of the DRA gene cloned into pGL2-Basic vector. At 24 h posttransfection, cells were treated with Bifidobacterium sp. CS (1:2 dilution) from the apical surface for 6 h (A) and 24 h (B). Promoter activity was measured by luciferase assay and normalized to β-galactosidase activity. Values are means ± SE of 3–4 independent experiments. *P < 0.05, **P < 0.01 vs. control.
Actinomycin D blocks effects of B. breve and B. infantis CS on DRA mRNA expression.
To further confirm whether the Bifidobacterium sp.-mediated increase in DRA mRNA expression occurs at a transcriptional level, we used actinomycin D (5 μm), which blocks the newly synthesized mRNA. Caco-2 cells were pretreated with actinomycin D for 1 h and then coincubated in the presence or absence of CS of B. breve or B. infantis (1:2 dilution) for 24 h. The increase in DRA mRNA levels in response to B. breve or B. infantis CS was abrogated in the presence of actinomycin D (Fig. 5). These data suggest that de novo synthesis of RNA is essential to elicit the effects of B. breve and B. infantis on DRA expression.
Fig. 5.
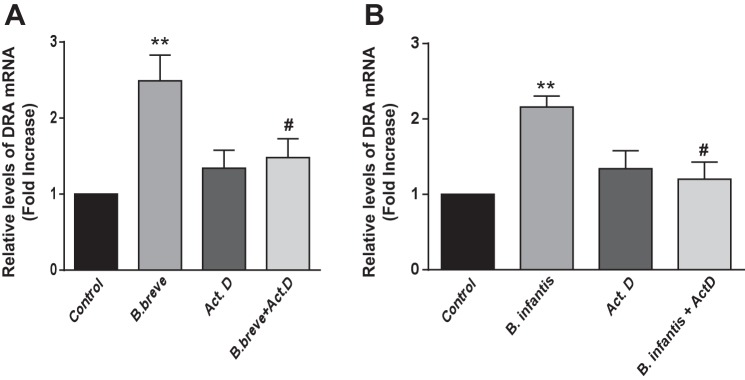
Actinomycin D blocks B. breve- and B. infantis-induced increases in DRA mRNA expression. Caco-2 cells were pretreated with the transcription inhibitor actinomycin D (ActD, 5 μM) for 1 h and then cotreated with B. breve (A) or B. infantis (B) CS (1:2 dilution) for 24 h. DRA mRNA levels were measured using gene-specific primers and normalized to GAPDH mRNA as the internal control. Values are means ± SE of 4 experiments performed in triplicate. **P < 0.01 vs. control. #P < 0.05 vs. B. breve (A) or B. infantis (B).
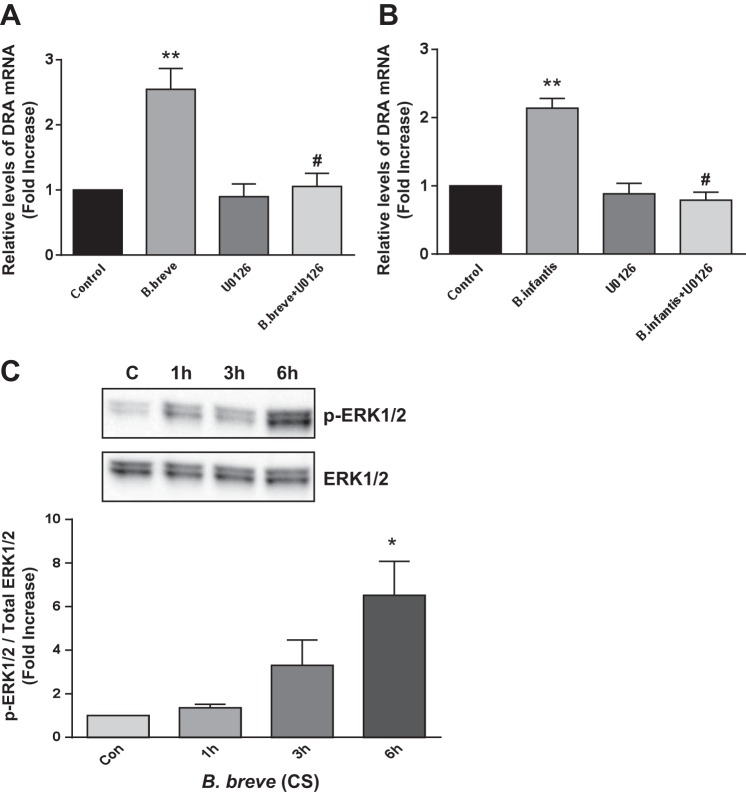
Bifidobacterium CS effects on DRA expression are ERK1/2 MAPK-dependent.
Previous studies showed that soluble factors of Bifidobacterium sp. activate ERK1/2 MAPK-dependent signaling pathways (18, 31). We thus examined the role of the ERK1/2 MAPK pathway in upregulation of DRA by Bifidobacterium sp. The specific ERK1/2 MAPK inhibitor U0126 (10 μM) blocked the stimulatory effects of B. breve and B. infantis CS on DRA mRNA level in Caco-2 cells (Fig. 6, A and B), suggesting involvement of ERK1/2 MAPK in Bifidobacterium sp. effects on DRA expression. This was further confirmed by examining the phosphorylation levels of ERK1/2 in response to B. breve treatment of Caco-2 cells. Interestingly, activation of ERK1/2 occurred as early as 3 h and persisted for 6 h posttreatment (Fig. 6C).
Fig. 6.
Bifidobacterium CS effects on DRA expression are ERK1/2 MAPK-dependent. A and B: Caco-2 cells were pretreated with the specific ERK1/2 MAPK inhibitor U0126 (10 μM) for 1 h and then cotreated with B. breve (A) or B. infantis (B) CS (1:2 dilution) for 24 h. DRA mRNA levels were measured using gene-specific primers and normalized to GAPDH mRNA as the internal control. Values are means ± SE of 4 experiments. **P < 0.01 vs. control. #P < 0.05 vs. B. breve (A) or B. infantis (B). C: phosphorylation levels of ERK1/2 in response to B. breve CS for 1–6 h in Caco-2 cells. Levels of phosphorylated ERK1/2 (p-ERK1/2) protein were measured using specific antibodies and normalized to total ERK1/2 levels. Values are means ± SE of 4 separate experiments. *P < 0.05 vs. control (Con).
Oral administration of live Bifidobacterium sp. enhances DRA expression.
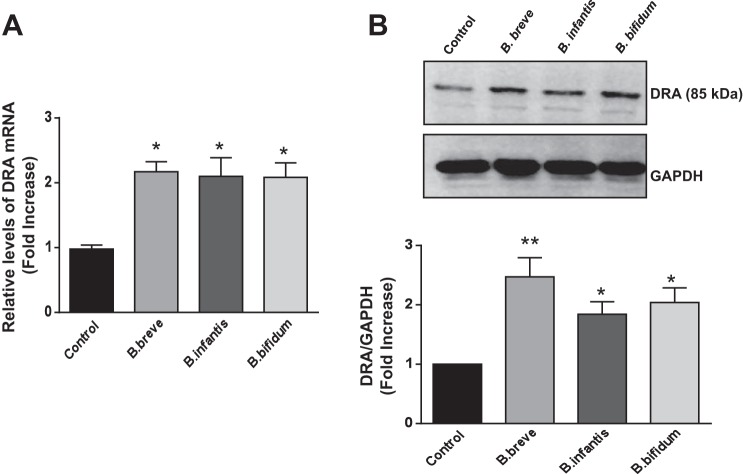
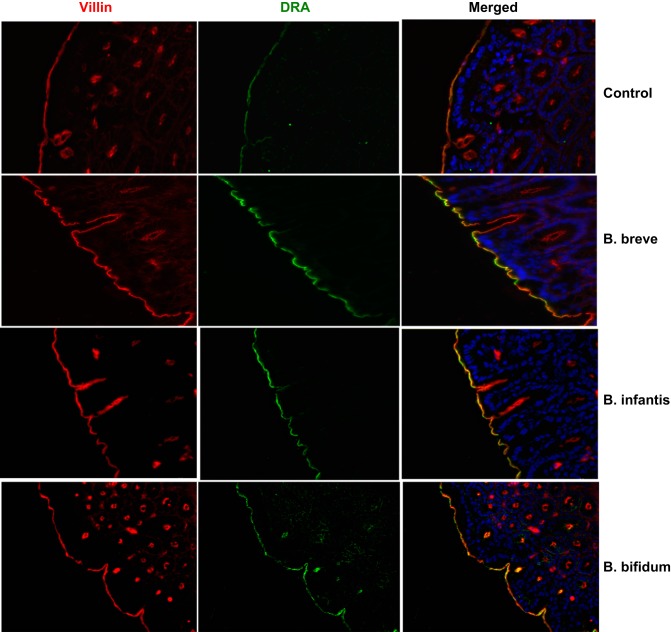
Because study of live Bifidobacterium is limited to in vitro studies due to oxygen sensitivity, we next examined the effects of Bifidobacterium sp. on DRA expression in an in vivo mouse model. Live B. breve, B. infantis, and B. bifidum (109 colony-forming units in 200 μl of PBS) or vehicle alone was administered by oral gavage to C57BL/6 mice. After 24 h, the mice were euthanized, the intestine was removed, and mucosa was scraped from ileal and colonic regions for RNA isolation and preparation of protein lysates. As shown in Fig. 7, colonic DRA mRNA and protein levels were significantly increased in response to Bifidobacterium sp. However, Bifidobacterium sp. did not change mRNA levels of DRA in the ileal region. Bifidobacterium sp. effects on DRA protein expression were further examined by immunofluorescence staining of colonic sections. As shown in Fig. 8, all three Bifidobacterium sp. increased DRA levels on the apical plasma membrane, as evidenced by increased colocalization (yellow) with villin compared with control.
Fig. 7.
Oral administration of Bifidobacterium sp. increases DRA expression in mouse colon. A: relative abundance of DRA mRNA was measured using gene-specific primers in ileal and colonic mucosa of mice treated with vehicle and live Bifidobacterium sp. Values are means ± SE of 5 mice. *P < 0.01 vs. control. B: representative Western blot for DRA protein levels in mucosal tissue lysates (top) and densitometric analysis of relative band intensities (bottom). Values are means ± SE of 4 mice. *P < 0.05, **P < 0.01 vs. control.
Fig. 8.
Oral administration of Bifidobacterium sp. increases DRA immunostaining. Green, DRA; red, villin; blue, nuclei.
DISCUSSION
Diarrhea generally occurs via dysregulation of normal intestinal electrolyte transport processes (19, 33, 36). The predominant route of Na+ and Cl− absorption in mammalian ileum and colon involves coupled operation of Na+/H+ and Cl−/HCO3− exchangers (12, 13). The gene slc26a3, the mutations of which are linked to congenital Cl− diarrhea, encodes a protein product, DRA, that mediates Cl−/HCO3− exchange and, thus, plays a critical role in intestinal Cl− absorption (22, 25). DRA−/− mice exhibit substantial diarrheal phenotype with serum electrolyte imbalances (34). Recent genome-wide association studies and animal models of inflammation and infection linked the dysregulated DRA function or expression to diarrhea associated with infection and inflammatory bowel diseases (4, 8, 43, 44). Thus, DRA is emerging as an important target for intervention of diarrheal disorders and, therefore, could be a novel target for antidiarrheal therapy or interventions.
Accumulating evidence suggests that probiotics may provide an effective adjunct to the management of infectious and inflammatory diarrhea (16, 29). Several clinical trials attest to the efficacy of probiotics as a promising complementary approach for treatment of various forms of diarrheal disorders (11, 16, 17, 28, 29, 40). However, many studies showing the beneficial effects of probiotics have utilized a mixture of strains of probiotics, making interpretation of the data difficult, especially with regard to the mechanisms underlying their antidiarrheal effects. Thus, to fully exploit the potential of probiotics as antidiarrheals, a complete understanding of the cellular and molecular mechanisms of a given probiotic species is critical.
In the current report we show, for the first time, that the bacteria-free CS of the probiotic Bifidobacterium sp. stimulates DIDS-sensitive 125I− uptake via upregulation of DRA expression in Caco-2 cells. Interestingly, all three species of Bifidobacterium (B. breve, B. infantis, and B. bifidum) had a similar effect on apical Cl−/HCO3− exchange activity and DRA expression. In addition to Caco-2 monolayers, Bifidobacterium sp. exhibited stimulatory effects in another colonic cell line, HT-29, further ruling out the notion that these effects are not cell line-specific. It is important to note that the apical Cl−/HCO3− exchange activity in IECs is contributed by two apical anion exchangers: DRA and PAT-1. The effects of Bifidobacterium sp. were specific to DRA, as the expression of SLC26A6 (PAT-1) remained unaltered in response to Bifidobacterium CS treatment. It is worth mentioning here that DRA−/− (but not PAT-1−/−) mice exhibit diarrheal phenotype, further emphasizing the important role of DRA in NaCl absorption and implying a role for DRA in diarrheal disorders. Also, several lines of evidence have shown that DRA is subject to extensive regulation, whereas PAT-1 remains unaltered in response to many physiological or pathophysiological stimuli, including lysophosphatidic acid, LA, or enteropathogenic E. coli infection (14, 30, 38, 39).
Since a decrease in DRA function is implicated in infectious diarrhea, as well as in diarrhea associated with inflammatory bowel diseases, our studies demonstrating upregulation of DRA may underlie the potential beneficial effects of Bifidobacterium. The efficacy of Bifidobacterium in the prevention of antibiotic-associated diarrhea and Clostridium difficile diarrhea in older patients has recently been shown (1). Similarly, Bifidobacterium strains prevented the effects associated with C. difficile in a hamster model of enterocolitis (41). A very recent study showed that B. infantis modulates host inflammatory processes by reducing the TNF and IL-6 levels in ulcerative colitis (15). In addition, B. infantis was shown to suppress Peyer's patch macrophage inflammatory protein-1α and -1β secretion during Salmonella infection (35). Bifidobacterium has also been shown to be protective against enteropathogenic infection through production of acetate and also via upregulation of the Toll-like receptor-negative regulators (10, 21). The soluble factor(s) in Bifidobacterium that upregulate DRA expression is not known and will be subject of future investigations.
Previous studies from our laboratory have demonstrated that short-term treatment of IECs with the probiotic LA strain 4357 (ATCC) substantially increased Cl−/HCO3− exchange activity by increasing apical membrane DRA levels via a phosphatidylinositol 3-kinase-dependent pathway (5). Importantly, the bacteria-free CS of LA was equally effective in stimulating DRA function and apical membrane localization, indicating that LA-secreted soluble factor(s) mediates the effects on DRA trafficking and function. In addition to short-term modulation of DRA trafficking, long-term treatment of IECs with LA CS increased DRA expression and function via modulation of DRA gene transcription (30). Our studies show that long-term treatment of IECs with Bifidobacterium sp. CS increases DRA expression and function via modulation of DRA gene transcription (30). This was evident, as the transcriptional inhibitor actinomycin D was able to block the stimulatory effect of B. breve and B. infantis on DRA mRNA expression. These studies indicate that de novo RNA synthesis is involved in the effects of Bifidobacterium sp. CS on DRA expression. Whether the effects of Bifidobacterium sp. on DRA mRNA occur solely via transcriptional mechanisms is still unclear. DRA promoter activity was significantly increased as early as 6 h after treatment with Bifidobacterium sp. However, the effect on DRA promoter activity was less than the increase in DRA mRNA or function in response to Bifidobacterium. Other mechanisms, such as an increase in mRNA stability, may also be involved.
Bifidobacterium sp. have been shown to activate various signaling pathways or transcription factors in the host cytosol. For instance, administration of B. breve to preterm infants resulted in an upregulation of transforming growth factor-β1 and Smad3 expression (9). B. breve has been shown to decrease proinflammatory cytokines in human dendritic cells challenged with Salmonella typhi through Toll-like receptor activation (3). B. infantis CS prevented cytokine-induced decrease in transepithelial resistance and rearrangement of tight junction proteins via the ERK1/2 MAPK pathway in colonic T84 cells (27). In the current study we used the specific ERK1/2 MAPK pathway inhibitor U0126 (10 μM), which blocked the stimulatory effect of B. breve and B. infantis CS on DRA mRNA expression in Caco-2 cells. Our data suggest that the effects of B. breve and B. infantis CS on DRA expression are ERK1/2 MAPK-dependent. This idea was further confirmed by examining phosphorylation levels of ERK1/2 in response to B. breve treatment of Caco-2 cells. Interestingly, activation of ERK1/2 occurred as early as 3 h and persisted to 6 h.
B. infantis and VSL#3 were shown to attenuate the degree of colitis in IL-10−/− mice (27). To validate the effects of Bifidobacterium sp. in an in vivo model, we further examined DRA expression in C57BL/6J mice gavaged with B. breve, B. infantis, or B. bifidum. DRA mRNA and protein expression was significantly enhanced in the colon of C57BL/6 mice gavaged with B. breve, B. infantis, or B. bifidum, whereas mRNA levels of PAT-1 were unchanged in the colon. No effect of Bifidobacterium sp. was observed in the ileum. This regional difference could be due to the higher relative expression of DRA and its functional role mainly in the colon compared with the small intestine (2, 23, 24). Another reason for the effects in the colon could be the rapid transit of probiotics from the small intestine compared with the colon and that Bifidobacterium sp. more predominantly colonize the colon than the ileum.
In summary, our findings, for the first time, demonstrate an upregulation of DRA function and expression by Bifidobacterium sp. via mechanisms involving ERK1/2 activation and increase in DRA promoter activity. Since DRA is one of the key transporters involved in coupled electroneutral NaCl absorption in the intestine, our findings represent a significant contribution to the study of molecular mechanisms underlying the beneficial effects of probiotics in treatment of diarrheal disorders. Further studies are needed to identify the bioactive soluble factors and study their effects on NaCl absorption, as well as to delineate mechanisms used by the bioactive factors of probiotics that produce these beneficial effects.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54016, DK-81858, and DK-92441 (to P. K. Dudeja), DK-71596 (to W. A. Alrefai), and DK-096258 (to R. K. Gill), Bill and Melinda Gates Foundation Grant OPP1058288 (to A. Borthakur), and Department of Veteran Affairs Grants BX 002011 (to P. K. Dudeja) and BX 000152 (to W. A. Alrefai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K., C.H., S.P., A.N.A., and A.A. performed the experiments; A.K., A.B., W.A.A., and P.K.D. analyzed the data; A.K., W.A.A., R.K.G., and P.K.D. interpreted the results of the experiments; A.K. prepared the figures; A.K. drafted the manuscript; A.K., W.A.A., R.K.G., and P.K.D. edited and revised the manuscript; A.K., C.H., S.P., A.N.A., A.A., A.B., W.A.A., R.K.G., and P.K.D. approved the final version of the manuscript; R.K.G. and P.K.D. are responsible for conception and design of the research.
Footnotes
This article is the topic of an Editorial Focus by Debolina Ray, Gianfranco Alpini, and Shannon Glaser (30a).
REFERENCES
- 1.Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W, Dhar A, Brown H, Foden A, Gravenor MB, Mack D. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 382: 1249–1257, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am J Physiol Gastrointest Liver Physiol 293: G923–G934, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez-Brito M, Munoz-Quezada S, Gomez-Llorente C, Matencio E, Bernal MJ, Romero F, Gil A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLos One 8: e59370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase IV as a cause of fatal infectious diarrhea in mice. Infect Immun 77: 3639–3650, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 7.Dudeja PK, Gill RK, Ramaswamy K. Absorption-Secretion and Epithelial Cell Function. Clifton, NJ: Humana, 2003. [Google Scholar]
- 8.Farkas K, Yeruva S, Rakonczay Z, Jr, Ludolph L, Molnar T, Nagy F, Szepes Z, Schnur A, Wittmann T, Hubricht J, Riederer B, Venglovecz V, Lazar G, Kiraly M, Zsembery A, Varga G, Seidler U, Hegyi P. New therapeutic targets in ulcerative colitis: the importance of ion transporters in the human colon. Inflamm Bowel Dis 17: 884–898, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Fuji T, Ohtsuka Y, Lee T, Kudo T, Shoji H, Sato H, Nagata S, Shimizu T, Yamashiro Y. Bifidobacterium breve enhances transforming growth factor beta 1 signaling by regulating Smad7 expression in preterm infants. J Pediatr Gastroenterol Nutr 43: 83–88, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 7: 503–514, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill R, Alrefai W, Ramaswamy K, Dudeja P. Mechanisms and regulation of NaCl absorption in the human intestine. Recent Res Dev Physiol 1: 643–677, 2003. [Google Scholar]
- 13.Gill RK, Alrefai WA, Dudeja PK. Intestinal anion absorption. In: Physiology of the Gastrointestinal Tract. II. Neurogastroenterology. New York: Elsevier, 2012, p. 1819–1847. [Google Scholar]
- 14.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groeger D, O'Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, Shanahan F, Quigley EM. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 4: 325–339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol 45 Suppl: S149–S153, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol 25: 18–23, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Heuvelin E, Lebreton C, Bichara M, Cerf-Bensussan N, Heyman M. A Bifidobacterium probiotic strain and its soluble factors alleviate chloride secretion by human intestinal epithelial cells. J Nutr 140: 7–11, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Hoque KM, Chakraborty S, Sheikh IA, Woodward OM. New advances in the pathophysiology of intestinal ion transport and barrier function in diarrhea and the impact on therapy. Expert Rev Anti Infect Ther 10: 687–699, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Kent AJ, Banks MR. Pharmacological management of diarrhea. Gastroenterol Clin North Am 39: 495–507, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Murata K, Villena J, Tomosada Y, Hara R, Chiba E, Shimazu T, Aso H, Suda Y, Iwabuchi N, Xiao J-Z, Saito T, Kitazawa H. Bifidobacteria upregulate expression of Toll-like receptor negative regulator counteracting entrotoxigenic Escherichia coli mediated inflammation in bovine intestinal epitheliocytes. Open J Vet Med 3: 143–155, 2013. [Google Scholar]
- 22.Makela S, Kere J, Holmberg C, Hoglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat 20: 425–438, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem 274: 22855–22861, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 276: G185–G192, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Musch MW, Arvans DL, Wu GD, Chang EB. Functional coupling of the downregulated in adenoma Cl−/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am J Physiol Gastrointest Liver Physiol 296: G202–G210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15: 300–310, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298: G807–G819, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Preidis GA, Hill C, Guerrant RL, Ramakrishna BS, Tannock GW, Versalovic J. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology 140: 8–14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quigley EM. Prebiotics and probiotics: their role in the management of gastrointestinal disorders in adults. Nutr Clin Pract 27: 195–200, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Ray D, Alpini G, Glaser S. Probiotic Bifidobacterium species: potential beneficial effects in diarrheal disorders. Focus on “Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells.” Am J Physiol Cell Physiol (September 10, 2014). 10.1152/ajpcell.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-α- and IFN-γ-induced dysfunction in human intestinal epithelial cells. Gastroenterology 130: 731–746, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Saksena S, Tyagi S, Goyal S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Stimulation of apical Cl−/HCO3− OH− exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent. Am J Physiol Gastrointest Liver Physiol 299: G1334–G1343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandle GI. Infective and inflammatory diarrhoea: mechanisms and opportunities for novel therapies. Curr Opin Pharmacol 11: 634–639, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Scully P, Macsharry J, O'Mahony D, Lyons A, O'Brien F, Murphy S, Shanahan F, O'Mahony L. Bifidobacterium infantis suppression of Peyer's patch MIP-1α and MIP-1β secretion during Salmonella infection correlates with increased local CD4+CD25+ T cell numbers. Cell Immunol 281: 134–140, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Seidler U, Lenzen H, Cinar A, Tessema T, Bleich A, Riederer B. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann NY Acad Sci 1072: 262–275, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Shanahan F. Probiotics in inflammatory bowel disease—therapeutic rationale and role. Adv Drug Deliv Rev 56: 809–818, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Singla A, Dwivedi A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl−/OH− exchange. Am J Physiol Gastrointest Liver Physiol 298: G182–G189, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singla A, Kumar A, Priyamvada S, Tahniyath M, Saksena S, Gill RK, Alrefai WA, Dudeja PK. LPA stimulates intestinal DRA gene transcription via LPA2 receptor, PI3K/AKT, and c-Fos-dependent pathway. Am J Physiol Gastrointest Liver Physiol 302: G618–G627, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sleator RD. Probiotics—a viable therapeutic alternative for enteric infections especially in the developing world. Discov Med 10: 119–124, 2010. [PubMed] [Google Scholar]
- 41.Trejo FM, De Antoni GL, Pérez PF. Protective effect of Bifidobacteria in an experimental model of Clostridium difficile associated colitis. J Dairy Res 80: 263–269, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, Silberg DG, Antalis TM, Schweinfest CW, Wu GD. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 275: G1445–G1453, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Yang SK, Jung Y, Kim H, Hong M, Ye BD, Song K. Association of FCGR2A, JAK2 or HNF4A variants with ulcerative colitis in Koreans. Dig Liver Dis 43: 856–861, 2011. [DOI] [PubMed] [Google Scholar]