Abstract
Clinical studies indicate that hepatic drug transport may be altered in chronic kidney disease (CKD). Uremic solutes associated with CKD have been found to alter the expression and/or activity of hepatocyte transporters in experimental animals and in cultured cells. However, given the complexity and adaptability of hepatic transport, it is not clear whether these changes translate into significant alterations in hepatic transport in vivo. To directly measure the effect of CKD on hepatocyte transport in vivo, we conducted quantitative intravital microscopy of transport of the fluorescent organic anion fluorescein in the livers of rats following 5/6th nephrectomy, an established model of CKD. Our quantitative analysis of fluorescein transport showed that the rate of hepatocyte uptake was reduced by ∼20% in 5/6th nephrectomized rats, consistent with previous observations of Oatp downregulation. However, the overall rate of transport into bile canaliculi was unaffected, suggesting compensatory changes in Mrp2-mediated secretion. Our study suggests that uremia resulting from 5/6th nephrectomy does not significantly impact the overall hepatic clearance of an Oatp substrate.
Keywords: chronic kidney disease, uremia, cytochrome P-450, hepatic transport, Mrp2, Oatp, sodium fluorescein
patients with end-stage renal disease (ESRD) receiving dialysis take on average 12 medications to manage the complications of their renal failure and their comorbid disease, which places them at a substantial risk for adverse drug events (8, 12, 13). Currently, modifications in drug dosing to improve safety for ESRD patients have largely been confined to those medications excreted renally. This has likely improved the safety of these medications for the ESRD population, yet it remains an incomplete approach, since it does not include the hepatically metabolized medications. This oversight is significant because evidence increasingly indicates that hepatic drug metabolism and transport are reduced in patients with ESRD (3–6, 14–22, 25–28, 30). Consequently, administering the full dose of a hepatically metabolized medication may place an ESRD patient at similar risk for an adverse drug event as administering the full dose of a renally eliminated medication.
Experimental evidence suggests multiple mechanisms by which uremic solutes might influence hepatic drug disposition (20, 25). Studies from the Pichette laboratory demonstrated that the activity and expression of cytochrome P-450 (CYP450) was reduced in the 5/6th nephrectomy (5/6N) rat model of chronic renal failure (6, 10, 11). Their work elucidated a direct role for uremic solutes with the observation that CYP450 activity and expression were reduced in primary rat hepatocytes incubated with human uremic serum (14). More directly relevant to the effects of chronic kidney disease (CKD) on hepatic transport were studies demonstrating that 5/6N induced downregulation of the uptake transporters Oatp1a1, Oatp1a4, and Oatp1b2 in rats (7, 15). This downregulation appeared to reflect a direct effect of uremic solutes on hepatocytes, as serum obtained from 5/6N rats reduced the expression of Oatp1b1 in primary rat hepatocytes (15). In addition, serum obtained from ESRD patients attenuated the expression of OATP1B1 and OATP2B1 in cultured human hepatocytes (27) and diminished the expression of Oatp1a4 in primary rat hepatocytes (18).
Additional studies of cultured cells indicate that uremic solutes not only reduce Oatp expression, but also directly inhibit Oatp activity. Uremic serum and isolated uremic toxins have been found to directly inhibit hepatocyte uptake of the Oatp substrates losartan, pravastatin, and erythromycin in cultured human and rat hepatocytes (22, 26–28). Studies of transfected human embryonic kidney 293 cells have also demonstrated that uremic serum inhibits the transport activity of Oatp1b1, Oatp2b1, and Oatp2b1 (22).
Together, these studies provide a sensible explanation for the observation of reduced hepatic clearance of drugs in individuals with CKD-diminished hepatic drug clearance, which reflects the downregulation and/or inhibition of Oatp resulting from exposure of hepatocytes to uremic solutes. However, the effects of CKD on hepatic transport have, thus far, been evaluated only in studies of cultured cells. While cultured cells provide an experimentally tractable system, their physiological relevance is limited by changes in transporter and enzyme expression that accompany hepatocyte isolation. Isolated hepatocytes lack the dynamic, polarized sinusoid-hepatocyte-canalicular structure that determines in situ hepatocyte transport. Furthermore, isolated hepatocytes do not provide the complex physiological context of the intact animal, which is particularly important in injury models, such as CKD. Moreover, hepatic transport is highly complex, depending upon the activity of both uptake and secretory transporters, whose expression and activity are dynamically regulated, often in compensatory ways. As a result, it is difficult to predict how the effects on Oatp impact overall hepatic transport with existing cell culture methods. In particular, reduced uptake may be compensated by increases in the rates of secretion to the canaliculus or by decreases in the rate of secretion to the sinusoid. Noteworthy in this regard are studies showing upregulation of Mrp2 in rats following 5/6N (7, 9, 15). Thus, testing the effect of 5/6N on hepatic transport can only conclusively be evaluated in vivo.
We have recently developed methods of quantitative intravital microscopy capable of dissecting hepatocyte transport in vivo (1). Time-lapse multiphoton fluorescence microscopy of the liver of living rats following intravenous injection of fluorescent transport substrates is combined with methods of quantitative digital image analysis to quantify the kinetics of transport into the cytosol of individual hepatocytes and into bile canaliculi. The ability to dissect the individual steps of transport makes this method capable of resolving the effects of CKD on hepatocyte uptake, as well as on overall rates of transport to bile canaliculi. Here, we apply this approach to evaluate the effects of CKD on the hepatic transport of fluorescein, a fluorescent substrate specific for Oatp and Mrp2 transporters (2, 29) and to test the hypothesis that Oatp-mediated transport is reduced in the 5/6N rat, resulting in reduced hepatic transport of organic anions.
MATERIALS AND METHODS
5/6th nephrectomy rat model of CKD.
The 5/6N rat model is commonly used as a disease model to study CKD in rats (10). The 5/6N and sham-operated rats (8–10 wk of age, all male) were purchased from Charles River and housed individually in the Indiana University School of Medicine Laboratory Animal Resource Center. The rats were maintained on a diet of Harlan Teklad 4% mouse/rat chow and water ad libitum. An acclimatization interval of at least 4 days was allowed prior to the performance of any animal experiments. All animal experiments were approved and conducted, according to the Institutional Animal Care and Use Committee guidelines. Briefly, 5/6N rats were prepared in a two-part surgery. In the first, surgeries were conducted on a male Sprague-Dawley rat, during which a ventral incision was made and 2/3 of the right kidney was removed. After 5–7 days of recovery, a dorsal incision just below the rib cage is made, and the left kidney was removed. For sham-operated controls, rats were treated to the same anesthesia and surgical processes without the removal of kidneys.
Renal function analyses.
Forty two days following the 5/6N or sham surgery, rats were placed in metabolic cages for 24 h to collect urine samples. Afterward, 0.2-ml blood samples were collected from the tail vein and analyzed for creatinine and blood urea nitrogen (BUN) levels. Prior to imaging, the weights of the rats were recorded.
Multiphoton intravital microscopy.
Our overall approach for analyzing hepatic transport involves quantitative multiphoton microscopy of transport of sodium fluorescein in the intact liver of a living rat. Techniques of quantitative intravital microscopy follow those that we previously described (1). After 24 h in a metabolic cage, the rats were anesthetized with 130 mg/kg of Inactin given intraperitoneally. Subsequently, a venous catheter was placed in the right jugular vein and 2 mg/kg of Hoechst 33342 (Invitrogen) was injected intravenously for the labeling of the nuclei. For access to the liver, a 4-cm ventral incision was placed just 1.5 cm below the rib cage. The liver was carefully placed onto wet gauze and secured to a glass-bottom plate. A preinjection of 0.2 mg/kg of sodium fluorescein (Fluka Analytical) was administered prior to imaging to facilitate positioning of the liver for kinetic studies. The rat was then placed ventral side down on an inverted Olympus FV1000 multiphoton microscope, and an appropriate field of the rat liver was identified. A series of image volumes (6 focal planes, spaced at 1 μm apart) were then collected continuously just before and for 10 min following injection of a 2 mg/kg solution of sodium fluorescein. A high-resolution mosaic, consisting of nine contiguous volumes, was then collected.
Quantification of fluorescein transport.
The image volume from each time point was projected into a maximum-projection image, a procedure that ensures collection of images of the canaliculi, even in the presence of minor vertical motion. The resulting projections were then assembled into a time series of images for analysis. Hepatocyte uptake was measured as the increase in the fluorescence measured over time in 10-pixel regions located over the hepatocyte cytosol. Hepatocyte secretion was measured as the increase in fluorescence in 10-pixel regions located over canaliculi. Measurements were repeated for 20–35 hepatocytes for each animal. Measured values were corrected for background by subtraction of the measurements obtained just prior to addition of fluorescein perfusion. The rate of uptake was calculated from the increase in mean cytosolic fluorescence, measured during the initial interval of linear uptake (in this case, the first 30 s). The rate of secretion was calculated from the increase in canalicular fluorescence, measured during the initial interval of linear secretion (in this case, the subsequent 90 s).
Mosaic analysis.
As described above, a mosaic of nine contiguous volumes (each 224 by 224 by 15 μm) was collected for each rat. Each volume was projected into a single image (using Metamorph, Sunnyvale, CA), and the resulting images were assembled into a single square image (using Photoshop, Adobe, San Jose, CA), representing a hyper-field ∼650 μm on a side. Using Metamorph, images were scaled to 8 bits and smoothed (2 × 2, low-pass filter). A median image (using a 24 by 24 region) was subtracted from this image, and the quotient was binarized (using a threshold of 50). The binarized image was skeletonized, and single pixels were removed, resulting in a binary image that reproduced the distribution of canaliculi in the original image. The volume density of canaliculi was quantified as the fraction of image pixels occupied by the binarized canaliculi.
Statistical analyses.
Graphical and statistical analyses were conducted using Kaleidagraph 4.03 (Synergy). Data are presented as means ± SE. Significance testing was conducted using one-tailed Student's t-test, and random probability values (P values) are provided as exact values.
RESULTS
CKD in a 5/6N rat.
Studies were conducted using the 5/6N rat model of CKD (10), using male Sprague-Dawley rats. Consistent with previous studies, the characteristics of CKD were apparent 42 days after 5/6N, as evidenced by reduced weight, increased BUN, and increased creatinine (Table 1).
Table 1.
Serum creatinine, BUN, weight, and urine volume of 5/6th nephrectomy and control rats
| 5/6N Rats | Control Rats | |
|---|---|---|
| Creatinine, mg/dl | 0.65 ± 0.278 | 0.20 ± 0.107 |
| BUN, mg/dl | 42.03 ± 25.76 | 18.63 ± 2.35 |
| Weight, g | 355.63 ± 17.12 | 472.38 ± 13.63 |
| Urine, ml | 48.13 ± 3.80 | 23.25 ± 4.83 |
Renal function in 5/6th nephrectomy (5/6N) and control rats. At day 42 after 5/6N surgery (CKD) or sham surgery (control), blood creatinine and blood urea nitrogen (BUN) levels were measured (mg/dl). The creatinine (P = 0.00102, one-tailed Student's t-test) and BUN (P = 0.0384, one-tailed Student's t-test) levels were significantly higher in CKD rats when compared to control rats.
Fluorescein transport in the livers of 5/6N rats.
The organic anion fluorescein has been widely used as a fluorescent probe in studies of hepatic transport (2, 24) and has recently been identified as a specific substrate of human and rat organic anion (OATP/Oatp) transporters (2). In previous studies (1), we demonstrated that quantitative intravital microscopy of the livers of rats injected with fluorescein can be used to sensitively detect alterations in Oatp function in vivo, demonstrating a 70% inhibition in the rate of hepatocyte uptake in rats treated with the Oatp inhibitor, rifampin. To evaluate the functional consequences of the 5/6N on hepatic transport, we conducted similar quantitative intravital microscopy studies of the liver of 5/6N and sham-operated rats following intravenous injection of sodium fluorescein.
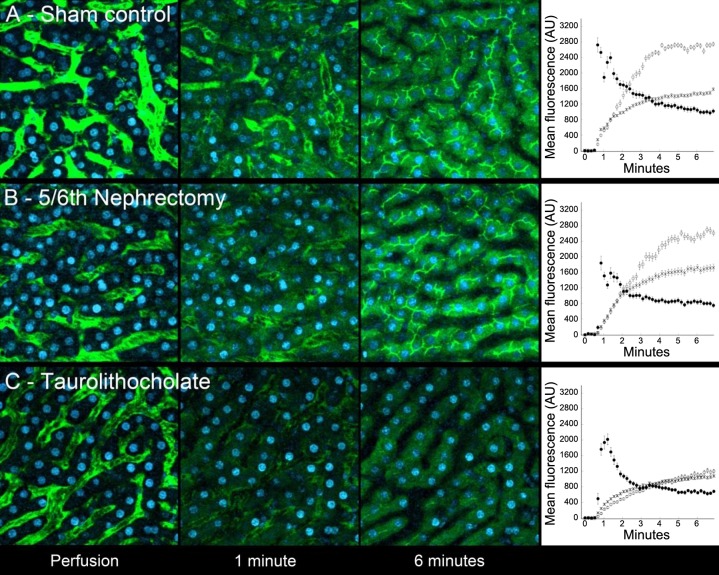
Figure 1A shows example images from a series of images collected from the liver of a sham-operated rat during and following intravenous injection of sodium fluorescein. Initially restricted to the sinusoids, fluorescein starts to appear in the hepatocytes and even in the canaliculi within 1 min of injection, reflecting rapid transport by Oatp and Mrp2, respectively. The speed of transport is apparent in the graph shown in the far-right panel of Fig. 1A, which shows the mean fluorescence levels in the cytosol, canaliculi, and adjacent sinusoids measured for 22 hepatocytes.
Fig. 1.
Results of studies of fluorescein transport in the livers of rats 42 days following sham surgery (A) or 5/6th nephrectomy (5/6N; B). Micrographs show projections of image volumes collected from the livers of living rats at the indicated times after perfusion. Imaged areas are 224 μm across. Graphs show quantifications of mean fluorescence (± SE) measured in the cytosol (○), canaliculi (X), and adjacent sinusoid (●) of 22 hepatocytes (A, sham-operated) and 31 hepatocytes (B, 5/6th nephrectomy). C: assay sensitivity is demonstrated, which shows results obtained 15 min after intravenous injection of taurolithocholate, an established inhibitor of hepatocyte transport. As shown in both the micrographs and the associated graph, uptake is profoundly slowed, and secretion is essentially blocked with canalicular fluorescence exceeding cytosolic levels only slightly and slowly.
A comparable study conducted in a 5/6N rat yielded very similar results (Fig. 1B), suggesting that 5/6N modestly reduces organic anion transport in rats. The sensitivity of the technique is demonstrated in studies of a rat treated with the cholestatic agent taurolithocholate (23), which profoundly reduces the rate of fluorescein uptake and essentially blocks secretion into canaliculi (Fig. 1C).
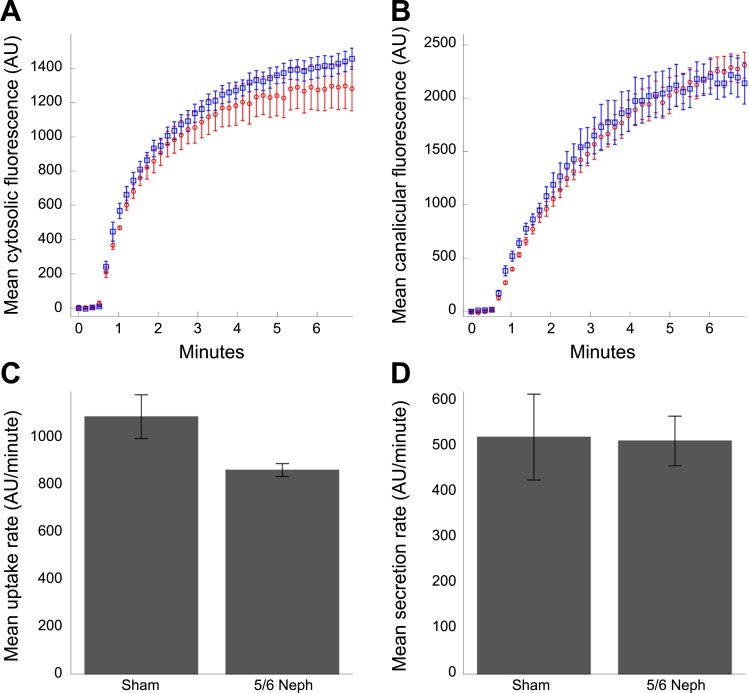
These studies were repeated for five sham and five 5/6N rats, and measurements were obtained for 22–36 hepatocytes for each rat. These studies demonstrate that 5/6N had a modest effect on the rate at which fluorescein accumulated in the hepatocyte cytosol (Fig. 2A). Quantification of the rate of uptake during the initial linear period of accumulation demonstrated that the 5/6N decreased the rate of hepatocyte uptake of fluorescein (20.7%), an effect that was nonetheless statistically significant (P = 0.024, one-tailed t-test) (Fig. 2C). In contrast, 5/6N had no detectible effect on the rate of fluorescence accumulation in canaliculi (Fig. 2B) and had no significant effect on the rate of canalicular secretion (P = 0.47, one-tailed t-test) (Fig. 2D).
Fig. 2.
Kinetics of the increase in fluorescence measured in the cytosol (A) and canaliculi (B), measured in images collected from the livers of living rats 42 days following either 5/6N (red symbols) or sham surgery (blue symbols). Each point represents the mean ± SE of the measurements collected from five rats, each representing the mean of measurements of 22–32 hepatocytes. Overall transport kinetics are very similar for the two conditions. C: mean initial rates of uptake (measured during the first 30 s following perfusion). D: mean rates of secretion (measured during the subsequent 90-s interval). The mean rate of uptake was reduced by 20.7% in the 5/6th nephrectomy group (P = 0.024, one tailed Student's t-test). The mean rate of secretion did not differ between the two groups (P = 0.470, one-tailed Student's t-test).
DISCUSSION
Emerging evidence indicates that chronic kidney disease attenuates the nonrenal clearance of medications. Experimental animal models have demonstrated that CKD is associated with reduced hepatic expression of CYP450 (6, 10, 11) and Oatp transporters (7, 15). Similar results have been obtained in studies of cultured cells, which have also demonstrated that uremic solutes directly inhibit Oatp-mediated transport (18, 22, 26–28). Taken together, these studies suggest that the reduced nonrenal drug clearance associated with CKD may reflect reduced Oatp transport function. The studies described here represent the first direct, in vivo measurement of the impact of uremia on the hepatic transport of fluorescein, an Oatp substrate.
Our study identified a 20.7% decrease in the rate of fluorescein uptake in hepatocytes of 5/6N rats, a modest decrease that did not translate into a detectable decrease in the overall rate of fluorescein secretion into canaliculi. Although speculative, the lack of an effect on overall transport may reflect upregulation of Mrp2, consistent with previous studies of 5/6N rats (7, 9), supporting the idea that increased Mrp2 function may compensate for reduced Oatp activity in CKD (7, 25). Regardless of the mechanism, our study indicates that uremia resulting from a 5/6th nephrectomy does not significantly impact the overall hepatic clearance of fluorescein, an Oatp substrate.
Perspectives and Significance
This research provides further evidence of the complex, integrated relationship between the kidney and liver in the clinically important area of CKD and drug disposition. The significance of our findings transcends earlier research, which merely demonstrated a decrement in hepatic transport of substrates in CKD. Our research corroborated this reduction in hepatic transport, but more significantly, did not find a significant difference in the overall hepatic clearance of an Oatp substrate in CKD. This finding suggests that the reduced Oatp function in CKD may not be a clinically important factor in drug disposition. This is a compelling finding since it may indicate an adaptive response on the part of the liver to ameliorate the deleterious effect of the uremic milieu. Future research in our laboratory will focus on further elucidating hepatic transport in CKD with a specific focus on the efflux of substrates and this potential adaptive response.
GRANTS
This work was supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Disease Grant 1K23KD084260-01A1, the Clinical and Translational Sciences Institute, NIH Grant TR000006, and the Dialysis Clinic (C-3681).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C.R. performed experiments; J.C.R. prepared figures; J.C.R. drafted manuscript; J.C.R., K.W.D., and B.S.D. approved final version of manuscript; K.W.D. and B.S.D. analyzed data; K.W.D. and B.S.D. interpreted results of experiments; K.W.D. and B.S.D. edited and revised manuscript; B.S.D. conception and design of research.
ACKNOWLEDGMENTS
Studies were conducted at the Indiana Center for Biological Microscopy.
REFERENCES
- 1.Babbey C, Ryan JC, Gill EM, Gharbril MS, Burch CR, Paulman A, Dunn KW. Quantitative intravital microscopy of hepatic transport. Intravital 1: 44–53, 2012. [Google Scholar]
- 2.De Bruyn T, Fattah S, Stieger B, Augustijns P, Annaert P. Sodium fluorescein is a probe substrate for hepatic drug transport mediated by OAP1B1 and OATP1B3. J Pharm Sci 100: 5018–5030, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Dowling TC, Briglia AE, Fink JC, Hanes DS, Light PD, Stackiewicz L, Karyekar CS, Eddington ND, Weir MR, Henrich WL. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther 73: 427–434, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Dreisbach AW, Japa S, Gebrekal AB, Mowry SE, Lertora JJ, Kamath BL, Rettie AE. Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther 73: 475–477, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Dreisbach AW, Lertora JJ. The effect of chronic renal failure on hepatic drug metabolism and drug disposition. Semin Dial 16: 45–50, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Guevin C, Michaud J, Naud J, Leblond FA, Pichette V. Down-regulation of hepatic cytochrome p450 in chronic renal failure: role of uremic mediators. Br J Pharmacol 137: 1039–1046, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzer B. Expression and Function of Hepatocellular Organic Anion Transport Proteins in a Rat Model of Chronic Renal Failure. Zurich, Switzerland: ETH Zürich, 2006. [Google Scholar]
- 8.Kaplan B, Shimp LA, Mason NA, Ascione FJ. Chronic hemodialysis patients. Part II: Reducing drug-related problems through application of the focused drug therapy review program. Ann Pharmacother 28: 320–324, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Laouari Yang R D, Veau C, Blanke I, Friedlander G. Two apical multidrug transporters, P-gp and MRP2, are differently altered in chronic renal failure. Am J Physiol Renal Physiol 280: F636–F645, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Leblond F, Guevin C, Demers C, Pellerin I, Gascon-Barre M, Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol 12: 326–332, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Leblond FA, Giroux L, Villeneuve JP, Pichette V. Decreased in vivo metabolism of drugs in chronic renal failure. Drug Metab Dispos 28: 1317–1320, 2000. [PubMed] [Google Scholar]
- 12.Manley HJ, Drayer DK, Muther RS. Medication-related problem type and appearance rate in ambulatory hemodialysis patients. BMC Nephrol 4: 10, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manley HJ, McClaran ML, Overbay DK, Wright MA, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS. Factors associated with medication-related problems in ambulatory hemodialysis patients. Am J Kidney Dis 41: 386–393, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Michaud J, Dube P, Naud J, Leblond FA, Desbiens K, Bonnardeaux A, Pichette V. Effects of serum from patients with chronic renal failure on rat hepatic cytochrome P450. Br J Pharmacol 144: 1067–1077, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naud J, Michaud J, Leblond FA, Lefrancois S, Bonnardeaux A, Pichette V. Effects of chronic renal failure on liver drug transporters. Drug Metab Dispos 36: 124–128, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Naud J, Nolin TD, Leblond FA, Pichette V. Current understanding of drug disposition in kidney disease. J Clin Pharmacol 52: 10S–22S, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Nolin TD, Appiah K, Kendrick SA, Le P, McMonagle E, Himmelfarb J. Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J Am Soc Nephrol 17: 2363–2367, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, Pichette V, Himmelfarb J. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol 20: 2269–2276, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolin TD, Frye RF, Matzke GR. Hepatic drug metabolism and transport in patients with kidney disease. Am J Kidney Dis 42: 906–925, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 83: 898–903, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Pichette V, Leblond FA. Drug metabolism in chronic renal failure. Curr Drug Metab 4: 91–103, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Reyes M, Benet L. Effects of uremic toxins on transport and metabolism of different biopharmaceutics drug dispositio classification system xenobiotics. J Pharm Sci 100: 3831–3842, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roma MG, Penalva GL, Aguero RM, Rodriguez Garay EA. Hepatic transport of organic anions in taurolithocholate-induced cholestasis in rats. J Hepatol 20: 603–610, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Sherman IA, Fisher MM. Hepatic transport of fluorescent molecules: in vivo studies using intravital TV microscopy. Hepatology 6: 444–449, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther 109: 1–11, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Sun H, Huang Y, Frassetto L, Benet LZ. Effects of uremic toxins on hepatic uptake and metabolism of erythromycin. Drug Metab Dispos 32: 1239–1246, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimoto M, Hatozaki D, Shima D, Yokota H, Furukubo T, Izumi S, Yamakawa T, Minegaki T, Nishiguchi K. Influence of serum in hemodialysis patients on the expression of intestinal and hepatic transporters for the excretion of pravastatin. Ther Apher Dial 16: 580–587, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Tsujimoto M, Kinoshita Y, Hirata S, Otagiri M, Ohtani H, Sawada Y. Effects of uremic serum and uremic toxins on hepatic uptake of digoxin. Ther Drug Monit 30: 576–582, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Van der Kolk DM, de Vries EG, Koning JA, Van den Berg E, Muller M, Vellenga E. Activity and expression of the multidrug resistance proteins MRP1 and MRP2 in acute myeloid leukemia cells, tumor cell lines and normal hematopoietic CD35+ peripheral blood cells. Clin Cancer Res 4: 1727–1736, 1998. [PubMed] [Google Scholar]
- 30.Yeung CK, Shen DD, Thummel KE, Himmelfarb J. Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int 85: 522–528, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]