Abstract
The cutaneous circulation is used to examine vascular adrenergic function in clinical populations; however, limited studies have examined whether there are regional limb and sex differences in microvascular adrenergic responsiveness. We hypothesized that cutaneous adrenergic responsiveness would be greater in the leg compared with the arm and that these regional limb differences would be blunted in young women (protocol 1). We further hypothesized that cutaneous vasoconstriction to exogenous norepinephrine (NE) during β-adrenergic receptor antagonism would be augmented in young women (protocol 2). In protocol 1, one microdialysis fiber was placed in the skin of the calf and the ventral forearm in 20 healthy young adults (11 men and 9 women). Laser-Doppler flowmetry was used to measure red blood cell flux in response to graded intradermal microdialysis infusions of NE (10−12 to 10−2 M). In protocol 2, three microdialysis fibers were placed in the forearm (6 men and 8 women) for the local perfusion of lactated Ringer (control), 5 mM yohimbine (α-adrenergic receptor antagonist), or 2 mM propranolol (β-adrenergic receptor antagonist) during concurrent infusions of NE (10−12 to 10−2 M). There were no limb or sex differences in cutaneous adrenergic responsiveness (logEC50) to exogenous NE. During α-adrenergic receptor blockade, women had greater exogenous NE-induced cutaneous vasodilation at the lowest doses of NE (10−12 to 10−10 M). Collectively, these data indicate that there are no limb or sex differences in cutaneous adrenergic responsiveness to exogenous NE; however, young women have a greater β-adrenergic receptor-mediated component of the vascular responsiveness to exogenous NE.
Keywords: vasoconstriction, skin blood flow, microdialysis
the human cutaneous circulation is increasingly being used to examine mechanisms underlying vascular dysfunction in clinical populations. The cutaneous circulation is reflexively controlled, in part, by an adrenergic vasoconstrictor branch of the sympathetic nervous system and, at rest, is under tonic adrenergic control (16, 18). Studies investigating cutaneous microvascular dysfunction in pathological conditions have revealed dysregulation in adrenergic signaling pathways similar to that which occurs throughout the systemic vasculature (16, 18). Importantly, the cutaneous circulation is on the efferent arm of both thermoregulatory and nonthermoregulatory reflex control (e.g., baroreflex) (2, 8), and deficits in peripheral vasoconstrictor responsiveness to adrenergic stimuli may contribute to a reduced ability to appropriately respond to challenges to the cardiovascular system, such as orthostasis (2, 5, 25, 35, 36).
In muscle, vascular responsiveness to adrenergic stimuli is greater in the legs (24), likely because greater sympathetic tone may be required in the leg vasculature to maintain proper circulatory function. The majority of studies examining cutaneous adrenergic control mechanisms, in health and in disease, have been performed in the forearm microvasculature (27, 33, 36, 39). The only previous investigation of limb-specific differences in cutaneous adrenergic responsiveness demonstrated greater cutaneous vasoconstriction to exogenous norepinephrine (NE) in the legs than in the arms, likely as a result of increased α-adrenergic receptor reactivity (41); however, this study did not account for the potential impact of sex differences in cutaneous vascular responsiveness to exogenous adrenergic stimuli. Importantly, adrenergic sensitivity in the muscle vasculature is less in women than in men (12, 21). In addition, women exhibit greater β-adrenergic-mediated vasodilation compared with men, demonstrated by blunted forearm vasoconstrictor responses to NE (14, 21). Moreover, differential regulation of cutaneous adrenergic responsiveness has been noted in young women with high and low orthostatic tolerance and appears to be modulated by female sex hormones (36). However, potential sex differences in cutaneous adrenergic control are not currently well understood. Therefore, because deficits in cutaneous adrenergic responsiveness have been documented in several clinical populations, including primary aging (33, 39) and essential hypertension (27), understanding potential limb- and sex-related differences in sympathetic function in the cutaneous microcirculation is clinically relevant.
Given this background, as well as the increasingly widespread use of the cutaneous circulation to examine vascular dysfunction in clinical populations, a more thorough understanding of potential limb and sex differences in cutaneous adrenergic responsiveness is warranted. Therefore, the purpose of the present investigation was to examine limb and sex differences in cutaneous microvascular responsiveness to an adrenergic stimulus (exogenous NE). We tested the hypothesis that NE-mediated cutaneous vasoconstriction would be greater in the leg compared with the arm and that these regional differences would be blunted in young women. We further hypothesized that β-adrenergic receptor antagonism would enhance cutaneous vasoconstriction to exogenous NE and that this vasoconstrictor response would be augmented in young women.
METHODS
Subjects.
All experimental procedures and protocols were approved by The Pennsylvania State University Institutional Review Board. Verbal and written consent were obtained voluntarily from all subjects before participation. The study conformed to the standards outlined in the Declaration of Helsinki. Twenty young adults (23 ± 1 yr; 11 men and 9 women) participated in the study. Subjects were screened for neurological, cardiovascular, and dermatological diseases and underwent a complete medical screening including a resting 12-lead electrocardiogram, physical examination, and 12-h fasting blood chemistry (Quest Diagnostics, Pittsburgh, PA). All subjects were normotensive, nondiabetic, normally active, and not taking over-the-counter or prescription medications or supplements with primary or secondary cardiovascular effects (e.g., statins, antihypertensives, anticoagulants, antidepressants, etc.). Subjects were nonobese (body mass index < 30 kg/m2) and did not use tobacco products.
Women taking hormonal contraceptives were excluded from the study. All women were normally menstruating and were tested during the early follicular phase (days 1–7) of their menstrual cycle. Before all experimental sessions, subjects abstained from caffeinated and alcoholic beverages for 12 h and strenuous physical activity for 24 h.
Protocol 1: cutaneous adrenergic responsiveness.
All protocols were performed in a thermoneutral laboratory with the subjects in a supine position, with the experimental arm and leg supported at heart level. With the use of sterile technique and after the skin was temporarily anaesthetized with ice (15), one intradermal microdialysis fiber (10 mm, 20 kDa cutoff membrane, MD 2000; Bioanalytical Systems, West Lafayette, IN) was placed in the dermal layer of the ventral forearm and the calf and perfused with lactated Ringer solution (2 μl/min; Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems) for 60–90 min after placement to allow for the resolution of local hyperemia. NE (Sigma, St. Louis, MO) was mixed just before use and dissolved in lactated Ringer solution with 1 mg/ml (5.7 mM) ascorbic acid (Sigma) as a preservative (28, 33). Prolonged infusion of NE at higher concentrations induces uncoupling and desensitization of G protein receptors (1, 4, 26). Ascorbic acid was therefore added to the NE dilutions to act as a preservative, extending the half-life from 8.5 min to over 180 min (20). Although local ascorbate administration has been shown to inhibit the adrenergic vasoconstrictor response to local cooling in human skin (40), in pilot testing for this study, the perfusion of ascorbic acid alone did not induce a cutaneous vascular response. Therefore, it is unlikely that the addition of ascorbic acid to NE contributed to the observed responses in this study. Solutions were filtered using syringe microfilters (Acrodisc; Pall, Ann Arbor, MI) and wrapped in foil to prevent degradation due to light exposure.
Red blood cell flux, an index of skin blood flow, was measured by laser-Doppler flowmeter probes throughout the protocol. The probes were placed in a local heater (MoorLAB, Temperature Monitor SH02; Moor Instruments, Axminster, UK), which was affixed to the skin directly above each microdialysis membrane. Temperature of the local heaters was clamped at 33°C for the duration of the protocol to ensure that changes in skin blood flow were due to the exogenous infusion of vasoactive agents. Brachial artery blood pressure was measured on the contralateral arm every 5 min (Cardiocap 5, GE Healthcare) throughout the protocol.
After the recovery period from probe placement, baseline measurements were made for 20 min while lactated Ringer solution was perfused through each microdialysis fiber. Thereafter, increasing doses of NE were continuously infused for 5 min (10−12, 10−11, 10−10, 10−9, 10−8M, 10−7, 10−6, 10−5, 10−4, 10−3, and 10−2 M), as previously described (39).
Protocol 2: contribution of α- and β-receptors to NE-induced cutaneous vasoconstriction.
Perfusion of the lowest doses of NE (10−12 through 10−8 M) in protocol 1 caused a highly reproducible vasodilation in both the forearm and calf of men and women. Therefore, as an ancillary aim of this study, protocol 2 was developed to begin to probe the relative contribution of α- and β-adrenergic receptors, and potential sex differences, to the cutaneous vascular responses to exogenous NE. For this substudy, we hypothesized that β-adrenergic receptor antagonism would enhance cutaneous vasoconstriction at all concentrations of the NE dose-response protocol and that this vasoconstrictor response would be augmented in young women.
Because there were no limb or sex differences in cutaneous adrenergic responsesiveness (see results), this protocol was performed in the forearm of a subset of men (n = 6; 24 ± 1 yr) and women (n = 8; 23 ± 1 yr) who previously participated in protocol 1. After the skin was temporarily anaesthetized with ice (15), three intradermal microdialysis fibers were placed in the ventral forearm skin for the local delivery of 1) lactated Ringer (control), 2) 5 mM yohimbine (Sigma) for local blockade of α-adrenergic receptors (34), and 3) 2 mM propranolol (Sigma) for local blockade of β-adrenergic receptors (7, 34). Although yohimbine is traditionally employed as a selective α2-adrenergic receptor antagonist, it antagonizes both α1- and α2-adrenergic receptors at the concentration used in this study (13, 30–32). All pharmacological inhibitors were mixed just before use, dissolved in lactated Ringer solution, filtered using syringe microfilters, and wrapped in foil to prevent degradation due to light exposure. After insertion of the fibers, inhibitors were perfused through the fibers (2 μl/min; Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems) during the resolution of the fiber insertion trauma (∼60–90 min).
Similar to protocol 1, temperature of the local heaters was clamped at 33°C throughout the protocol. After the recovery period from probe placement, baseline measurements were made for 20 min while inhibitors or lactated Ringer solution were perfused through each microdialysis fiber. Following baseline, increasing doses of NE were continuously infused for 5 min (10−12, 10−11, 10−10, 10−9, 10−8, 10−7, 10−6, 10−5, 10−4, 10−3, 10−2 M), as described above. A 5-min infusion of the inhibitors or lactated Ringer was used between each dose of NE.
Data acquisition and statistical analysis.
Data were collected at 40 Hz (Powerlab and LabChart; ADInstruments, Bella Vista, NSW, Australia). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by mean arterial pressure. CVC data were normalized and expressed as a percentage of baseline (%CVCbase; protocol 1) or absolute change from baseline (ΔCVCbase; protocol 2). CVC was averaged over the last minute of each NE dose.
Subject characteristics were compared using unpaired t-tests (SPSS 19.0). For protocol 1, NE doses were transformed to logarithmic concentrations, and CVC was normalized such that baseline CVC = 100% (i.e., pre-NE). Sigmoidal dose-response curves with variable slope were generated using four-parameter nonlinear regression modeling (36–38), with constraints set for the top (100) to best fit parameters of the model (Prism v. 5.0, GraphPad, San Diego, CA). Responsiveness to NE was determined by the effective concentration causing 50% of the maximal response (logEC50) and the extent of maximal vasoconstrictor capacity. The differences within anatomical region between sexes were analyzed using an F-test for repeated-measures comparisons (Prism v. 5.0) (36, 37), which takes into account all points over the entire curve as opposed to each specific dose (6). For protocol 2, the primary interest was the vasoconstrictor response at a given concentration of NE, in addition to the maximal NE-induced vasoconstricting capability. Therefore, these data were analyzed using a two-way repeated-measures ANOVA, followed by post hoc testing with Bonferroni corrections when warranted (SPSS 19.0). Results are reported as means ± SE, and the α-level was set at P < 0.05.
RESULTS
NE-induced cutaneous vasoconstriction.
Subject characteristics are presented in Table 1. There were no sex differences in body mass index or blood biochemistry. Resting blood pressure was lower in women (P < 0.05). Baseline CVC was not different between sexes or between limbs in protocol 1 (P > 0.05).
Table 1.
Subject characteristics
| Baseline Characteristic | Men | Women |
|---|---|---|
| n | 11 | 9 |
| Age, yr | 23 ± 1 | 23 ± 1 |
| Height, cm | 180 ± 3 | 165 ± 2* |
| Mass, kg | 79 ± 4 | 63 ± 3* |
| Body mass index, kg/m2 | 24.4 ± 1.3 | 23.4 ± 0.7 |
| Systolic BP, mmHg | 121 ± 3 | 108 ± 4* |
| Diastolic BP, mmHg | 74 ± 2 | 68 ± 2 |
| Heart rate, beats/min | 63 ± 3 | 59 ± 2 |
| HbA1c, % | 5.3 ± 0.1 | 5.3 ± 0.1 |
| Fasting total cholesterol, mg/dl | 161.1 ± 11.4 | 187.1 ± 9.6 |
| Fasting HDL, mg/dl | 55.5 ± 4.6 | 69.3 ± 5.1 |
| Fasting LDL, mg/dl | 88.9 ± 10.1 | 97.8 ± 6.4 |
| Fasting triglycerides, mg/dl | 83.5 ± 10.5 | 73.0 ± 7.0 |
| Basal CVC | ||
| Forearm | 0.21 ± 0.02 | 0.27 ± 0.09 |
| Calf | 0.25 ± 0.04 | 0.30 ± 0.07 |
Values are means ± SE.
BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CVC, cutaneous vascular conductance.
P < 0.05 vs. men.
NE dose-response curves for each limb in men and women are presented in Fig. 1. There were no differences in cutaneous adrenergic responsiveness to exogenous NE between limbs in either men or women (Table 2 and Fig. 1). There was also no regional limb difference in the maximal vasoconstrictor response to exogenous NE in either men or women (Table 2). Furthermore, there were no sex differences in the parameters of the NE dose-response curves (Table 2 and Fig. 1).
Fig. 1.
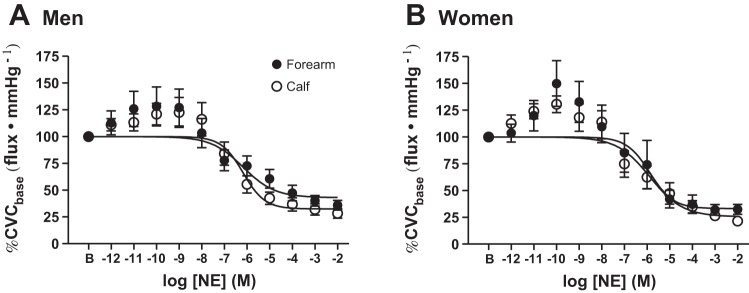
Group summary data for exogenous norepinephrine (NE)-induced cutaneous vasoconstriction in the forearm (●) and calf (○) of men (A) and women (B). There were no regional limb or sex differences in cutaneous adrenergic responsiveness. CVCbase, baseline cutaneous vascular conductance.
Table 2.
Modeling parameters for norepinephrine-mediated cutaneous vasoconstriction in the forearm and calf of men and women
| Men |
Women |
|||
|---|---|---|---|---|
| Forearm | Calf | Forearm | Calf | |
| Minimum | 38.6 ± 9.9 | 33.8 ± 5.1 | 32.8 ± 9.4 | 26.3 ± 7.2 |
| LogEC50 | −5.73 ± 0.57 | −6.31 ± 0.25 | −5.84 ± 0.44 | −5.94 ± 0.35 |
Values are means ± SE.
Contribution of α- and β-receptors to NE-induced cutaneous vasoconstriction.
As depicted in Fig. 1, perfusion of the lowest doses of NE (10−12 through 10−8 M) caused an unexpected, but highly reproducible, vasodilation in both limbs in men and women. Baseline CVC values were different between drug treatments in protocol 2 (Ringer, 0.24 ± 0.04; yohimbine, 0.26 ± 0.13; propranolol, 0.63 ± 0.17; ANOVA main effect, P < 0.01); therefore, the data for this protocol are presented as an absolute change from baseline. For this analysis, because we were interested in potential treatment differences at subsequent doses of NE, CVC was plotted across NE concentrations without modeling a dose-response curve (Fig. 2). When men and women were pooled, infusion of propranolol enhanced cutaneous vasoconstriction and effectively eliminated any NE-mediated vasodilation, as evidenced by the progressive decrease in CVC throughout infusion of increasing concentrations of NE (Fig. 2A). Infusion of yohimbine blunted vasoconstriction in both men and women at the highest doses of NE but had no effect at doses lower than 10−7 M (Fig. 2B).
Fig. 2.
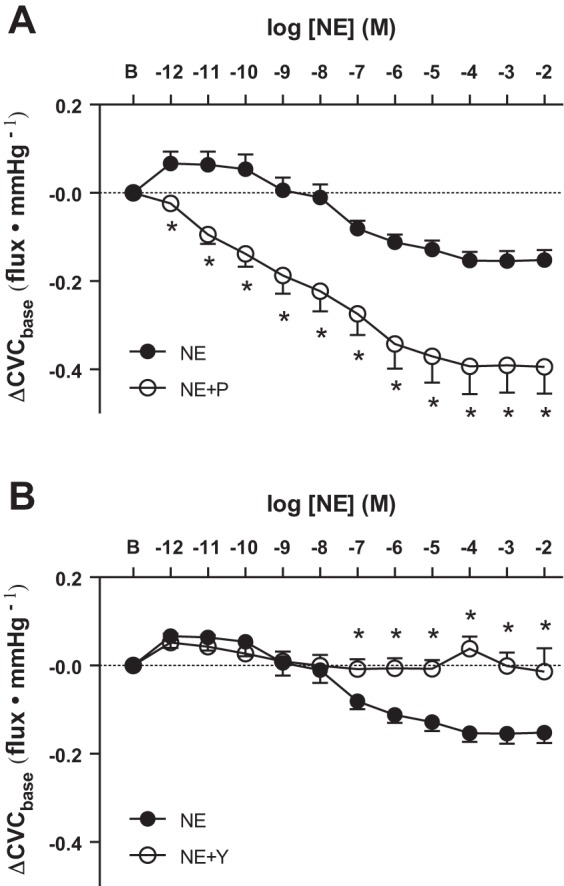
Group summary data for exogenous NE-induced cutaneous vasoconstriction during β-adrenergic receptor antagonism [NE + propranolol (P); A] and during α-adrenergic receptor antagonism [NE + yohimbine (Y); B]. Antagonism of β-receptors enhanced vasoconstriction, whereas antagonism of α-adrenergic receptors blunted NE-induced cutaneous vasoconstriction. *P < 0.05 vs. NE.
Baseline CVC during propranolol (0.52 ± 0.14 men vs. 0.43 ± 0.07 women; P = 0.50) and yohimbine infusion (0.23 ± 0.09 men vs. 0.21 ± 0.03 women; P = 0.82) were not different between men and women. There were no sex differences in the cutaneous vasoconstrictor response during infusion of propranolol (Fig. 3A). However, during α-adrenergic receptor antagonism with yohimbine, women had greater NE-mediated cutaneous vasodilation at the lowest doses of NE (10−12 through 10−10 M; Fig. 3B), suggesting a greater β-adrenergic component of the cutaneous vascular responsiveness to exogenous NE in the forearm.
Fig. 3.

Group summary data for exogenous NE-induced cutaneous vasoconstriction during β-adrenergic (A) and α-adrenergic (B) receptor antagonism in a subset of men (n = 6; ●) and women (n = 8; ○) at the doses of NE during which cutaneous vasodilation was observed (10−12 through 10−7 M). There were no sex differences in the vasoconstrictor response during β-adrenergic receptor blockade; however, during α-adrenergic receptor blockade, women had greater NE-mediated cutaneous vasodilation at the lowest doses of NE (10−12 through 10−10 M) compared with men. *P < 0.05 vs. men; †P = 0.059 vs. men.
DISCUSSION
The primary finding of the current study was that there is no difference in the cutaneous vasoconstrictor response to exogenous NE between the arm and leg. In addition, there are no sex differences in exogenous NE-mediated cutaneous vasoconstriction in either limb. It appears both α- and β-adrenergic receptors have an important functional role in the human cutaneous vasculature and differentially mediate the microcirculatory response to exogenous NE. Furthermore, young women have a greater β-adrenergic receptor-mediated component of the vascular responsiveness to low concentrations of exogenous NE. Collectively, these data indicate that there are no limb or sex differences in cutaneous adrenergic responsiveness and further suggest an important role of sex differences in adrenergic receptor subtypes mediating exogenous NE-induced cutaneous vascular responses.
Regional limb differences in the vascular responses to adrenergic stimuli have been documented in the human skeletal muscle arterial vasculature (24). Specifically, phenylephrine-induced decreases in vascular conductance were greater in the calf compared with the forearm, and the authors speculate that greater adrenergic sensitivity in the legs is a consequence of repeated exposure to both hemodynamic and hydrostatic pressure gradients when upright (24). Because orthostatic stress elicits differential regional vascular responses (10, 11) and given the important role of the cutaneous vasculature in the peripheral vasoconstrictor response to orthostatic challenge (2, 25, 35), it is therefore plausible that cutaneous adrenergic responsiveness may also be greater in the lower leg. However, perhaps surprisingly, we found no evidence for limb differences in the cutaneous vasoconstrictor responses to exogenous NE. Indeed, the sensitivity to adrenergic stimuli, quantified as the logEC50 of the NE dose-response curves, was remarkably similar between the forearm and calf in both men and women. Furthermore, there were no differences in the exogenous NE-induced maximal vasoconstrictor responses between limbs. Taken together, the results of the present study suggest that cutaneous adrenergic responsiveness is similar in the forearm and calf of healthy young adults. This lack of a regional difference in cutaneous adrenergic sensitivity may be due to the low pressure and compliant nature that are characteristic of the human cutaneous circulation. In the only previous study to examine limb-specific differences in cutaneous adrenergic responsiveness, the authors report an augmented cutaneous vasoconstrictor response to exogenous NE in the calf and, through the use of selective adrenergic receptor agonists, suggest that this is due to greater α1- and α2-adrenergic receptor sensitivity in the lower leg (41). Although the reason(s) for the contrasting conclusions between the aforementioned study (41) and the present investigation is not clear, methodological differences such as differing NE mixing and dilution techniques, study design (the use of agonists compared with the use of antagonists in the current study), and mathematical modeling and statistical analyses may have contributed.
Interestingly, there appear to be regional differences in the local vasodilatory responses of the cutaneous vasculature during local skin warming (9, 29). Specifically, the axon reflex, which is dependent on both sensory and sympathetic nerves (22), appears to be lower in the leg compared with the arm (9). NE contributes to this initial vasodilatory response to local heating (17, 19), and the authors of these studies speculate that a blunted initial peak response (i.e., axon reflex) may be suggestive of increased tonic sympathetic vasoconstriction in the lower legs (9). However, the results of the present study suggest that limb differences in cutaneous vasoconstrictor responses to exogenous NE may not fully account for these previously reported regional differences in microvascular function. As such, further investigation regarding potential limb differences in the contribution of sympathetic neurotransmitters to cutaneous vasodilatory function during thermal hyperemia—specifically a role in the axon reflex—is therefore warranted.
This study was also designed to examine potential sex differences in cutaneous adrenergic responsiveness. Importantly, adrenergic sensitivity of the resistance vasculature is less in women compared with men (12, 21). For instance, women have a blunted vasoconstrictor response to brachial artery infusion of phenylephrine compared with men, whereas there were no sex differences in the response to reflex vasoconstriction or to intra-arterial tyramine (12), suggesting sex differences in adrenergic receptor sensitivity/density and not in the responsiveness to neurally mediated stimuli. Sex differences in adrenergic responsiveness are further substantiated by reduced NE-mediated vasoconstriction in the forearm of women compared with men (14, 21). However, to date, limited studies have examined sex differences in the responsiveness of the cutaneous microvasculature to exogenous NE. Our results in protocol 1 demonstrate that exogenous NE-induced cutaneous vasoconstriction was not different between men and women, in either the forearm or the calf. While these findings were perhaps surprising in light of the evidence demonstrating clear sex differences in adrenergic sensitivity in the resistance vasculature (12, 14, 21), the present results instead indicate similar cutaneous vasoconstrictor responsiveness to exogenous NE between sexes. It is important to note that all of the women who participated in the current study were normally menstruating and not using hormonal contraceptives and were tested during the early follicular phase of the menstrual cycle, when female reproductive hormones are at their lowest circulating concentrations. Interestingly, there is evidence that women in the high-hormone phase of oral contraceptives have the same ratio of NE-to-cotransmitter-mediated reflex cutaneous vasoconstriction as men but that women in the low-hormone placebo phase tend to rely almost entirely on NE to effect the same magnitude of vasoconstriction (31). However, when normally menstruating women were tested during the early follicular phase of their menstrual cycle, there was no difference in the magnitude of reflex cutaneous vasoconstriction or the individual contributions of sympathetic cotransmitters compared with men (34), and although methodological differences (i.e., physiological vs. pharmacologically-induced cutaneous vasoconstriction) must be considered, the results of the present investigation extend these previous findings and likewise demonstrate no sex differences in exogenous NE-mediated cutaneous vasoconstriction.
Importantly, the present investigation used a purposefully wide-dosage range of NE in an effort to more precisely detect any limb or sex differences in cutaneous vasoconstriction. The lowest concentrations of NE caused an unexpected, but highly reproducible, dilator response. This consistent early dilator portion of the response, which occurred at NE concentrations that are known to elicit reflex vasoconstriction, is essentially absent from our previously published work using a similar protocol (33). Nevertheless, its consistency and magnitude suggests that a genuine physiological event underlies this response. In addition, because blunted adrenergic sensitivity in women may by explained, in part, by increased sensitivity of β-adrenergic receptors balancing the α-adrenergic vasoconstrictor effect of NE (14, 21), an ancillary protocol was developed to begin to probe the relative contribution of adrenergic receptor subtypes, along with potential sex differences, in this response.
Functional β-adrenergic receptors are present in human skin (7), and when stimulated by NE, cutaneous vasodilation occurs. Therefore, to test the contribution of β-adrenergic receptors to the initial vasodilatory response to NE, the dose-response protocol was repeated during concurrent β-adrenergic receptor blockade in a subset of subjects. Nonspecific β-adrenergic receptor blockade completely abolished the early vasodilation in response to exogenous NE and significantly augmented the vasoconstrictor response, confirming an important role for β-adrenergic receptors in mediating cutaneous vascular responsiveness to adrenergic stimuli. Collectively, these findings suggest that β-adrenergic-mediated vasodilation is present in the human cutaneous vasculature and can be stimulated by administration of physiologically relevant concentrations of exogenous NE (10−12 to 10−8M). In light of the aforementioned findings (14, 21), it is perhaps suprising that in the present study we did not observe any sex differences in the forearm cutaneous vasoconstrictor response to exogenous NE during β-adrenergic receptor blockade, which we interpret as suggesting that there are no sex differences in the functional role of cutaneous α-adrenergic receptors to this response. However, the significant augmentation in cutaneous vasoconstriction to exogenous NE during propranolol infusion in both men and women suggests that β-adrenergic receptor-mediated buffering of exogenous NE-induced vasoconstriction occurs in the skin microcirculation of both sexes.
Interestingly, despite the fact that α-adrenergic receptor blockade during exogenous NE administration did not appear to entirely recapitulate the early vasodilatory response, there were no statistical differences between NE alone and with concurrent α-adrenergic receptor blockade. Furthermore, α-adrenergic receptor blockade completely prevented vasoconstriction at the much higher pharmacological concentrations of NE, likewise substantiating a crucial role for α-adrenergic receptors in mediating the cutaneous vascular responses to exogenous NE in both men and women. However, during nonselective α-adrenergic receptor antagonism with yohimbine, women had greater exogenous NE-induced cutaneous vasodilation at the lowest physiological doses of NE (10−12 through 10−10 M), suggesting that young women have a greater β-adrenergic component of cutaneous vascular responsiveness to exogenous NE in the forearm compared with young men. These novel findings, which are consistent with those reported using forearm venous occlusion plethysmography (14, 21), further validate the use of the cutaneous circulation as a model with which to study alterations in vascular function in both health and disease.
Limitations.
It is important to note that nonselective receptor antagonists were used in protocol 2. However, because this protocol was designed only to initially probe the relative contribution of α- and β-adrenergic receptors to the cutaneous vascular responses to exogenous NE, nonspecific antagonists were used. Given the relatively more important role for α2-adrenergic receptors in the human cutaneous vasculature compared with α1-adrenergic receptors (3, 23), further studies are necessary to more specifically delineate the contribution of each receptor subtype to NE-induced microvascular responses in both men and women, as well to determine any potential sex differences in the sensitivity of these receptors in the cutaneous vasculature.
Perspectives and significance.
In conclusion, the primary novel finding of this study was that there are no limb or sex differences in the cutaneous vascular responses to exogenous NE. These findings were contrary to our initial hypotheses and instead suggest cutaneous adrenergic responsiveness is similar in the forearm and calf of both men and women. Additionally, it appears that both α- and β-adrenergic receptors have an important functional role in mediating the responses of the human cutaneous microvasculature to exogenous NE; however, young women have a greater β-adrenergic component of the cutaneous vascular responsiveness to exogenous NE in the forearm compared with young men. Given the growing use of the cutaneous circulation as a means to probe mechanisms of vascular function in health and in disease, it is important to characterize regional and sex-specific differences in sympathetic control of the cutaneous microvasculature. Collectively, our findings contribute to the growing body of literature characterizing adrenergic function in the human cutaneous vasculature and provide new insights regarding NE-mediated vasoconstriction in the forearm and calf of healthy young men and women.
GRANTS
This research was supported by National Institutes of Health Grants HL120471-01 (to J. L. Greaney), AG007004-23 (to W. L. Kenney), and HL093-238-04 (to L. M. Alexander).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.L.G., A.E.S., and L.M.A. conception and design of research; J.L.G. and A.E.S. performed experiments; J.L.G. analyzed data; J.L.G., A.E.S., W.L.K., and L.M.A. interpreted results of experiments; J.L.G. prepared figures; J.L.G. drafted manuscript; J.L.G., A.E.S., W.L.K., and L.M.A. edited and revised manuscript; J.L.G., A.E.S., W.L.K., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The time and effort expended by all the volunteer subjects are greatly appreciated. We are grateful for the assistance of Susan Slimak and Jane Pierzga.
REFERENCES
- 1.Akinaga J, Lima V, Kiguti LR, Hebeler-Barbosa F, Alcantara-Hernandez R, Garcia-Sainz JA, Pupo AS. Differential phosphorylation, desensitization, and internalization of alpha1A-adrenoceptors activated by norepinephrine and oxymetazoline. Mol Pharmacol 83: 870–881, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Beiser GD, Zelis R, Epstein SE, Mason DT, Braunwald E. The role of skin and muscle resistance vessels in reflexes mediated by the baroreceptor system. J Clin Invest 49: 225–231, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borbujo J, Garcia-Villalon AL, Valle J, Gomez B, Diequez G. Postjunctional alpha-1 and alpha-2 adrenoceptors in human skin arteries. An in vitro study. J Pharmacol Exp Ther 249: 284–287, 1989. [PubMed] [Google Scholar]
- 4.Cabrera-Wrooman A, Romero-Avila MT, Garcia-Sainz JA. Roles of the α1A-adrenergic receptor carboxyl tail in protein kinase C-induced phosphorylation and desensitization. Naunyn Schmiedebergs Arch Pharmacol 382: 499–510, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Cook DA, Bielkiewicz B. A computer-assisted technique for analysis and comparison of dose-response curves. J Pharmacol Methods 11: 77–89, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Crandall CG, Etzel RA, Johnson JM. Evidence of functional beta-adrenoceptors in the cutaneous vasculature. Am J Physiol Heart Circ Physiol 273: H1038–H1043, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Del Pozzi AT, Carter SJ, Collins AB, Hodges GJ. The regional differences in the contribution of nitric oxide synthase to skin blood flow at forearm and lower leg sites in response to local skin warming. Microvasc Res 90: 106–111, 2013. [PubMed] [Google Scholar]
- 10.Essandoh LK, Duprez DA, Shepherd JT. Postural cardiovascular reflexes: comparison of responses of forearm and calf resistance vessels. J Appl Physiol 63: 1801–1805, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Essandoh LK, Houston DS, Vanhoutte PM, Shepherd JT. Differential effects of lower body negative pressure on forearm and calf blood flow. J Appl Physiol 61: 994–998, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res 61: 581–585, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg MR, Robertson D. Yohimbine: a pharmacological probe for study of the alpha 2-adrenoreceptor. Pharmacol Rev 35: 143–180, 1983. [PubMed] [Google Scholar]
- 14.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol 589: 5285–5297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodges GJ, Chiu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol 106: 1112–1118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges GJ, Johnson JM. Adrenergic control of the human cutaneous circulation. Appl Physiol Nutr Metab 34: 829–839, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol 109: 1538–1544, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes IE, Smith JA. The stability of noradrenaline in physiological saline solutions. J Pharm Pharmacol 30: 124–126, 1978. [DOI] [PubMed] [Google Scholar]
- 21.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen H, Mortensen FV, Mulvany MJ. Responses to noradrenaline in human subcutaneous resistance arteries are mediated by both alpha 1- and alpha 2-adrenoceptors. Br J Pharmacol 99: 31–34, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol 92: 2105–2113, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Rowell LB, Wyss CR, Brengelmann GL. Sustained human skin and muscle vasoconstriction with reduced baroreceptor activity. J Appl Physiol 34: 639–643, 1973. [DOI] [PubMed] [Google Scholar]
- 26.Seasholtz TM, Gurdal H, Wang HY, Johnson MD, Friedman E. Desensitization of norepinephrine receptor function is associated with G protein uncoupling in the rat aorta. Am J Physiol Heart Circ Physiol 273: H279–H285, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Smith CJ, Santhanam L, Alexander LM. Rho-kinase activity and cutaneous vasoconstriction is upregulated in essential hypertensive humans. Microvasc Res 87: 58–64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol 115: 1025–1031, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanhewicz AE, Greaney JL, Kenney WL, Alexander LM. Sex- and limb-specific differences in the nitric oxide-dependent cutaneous vasodilation in response to local heating. Am J Physiol Regul Integr Comp Physiol 307: R914–R919, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol 280: H1496–H1504, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Stephens DP, Bennett LA, Aoki K, Kosiba WA, Charkoudian N, Johnson JM. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol 282: H264–H272, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287: H1404–H1409, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol 288: R1108–R1113, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558: 697–704, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathi A, Nadel ER. Forearm skin and muscle vasoconstriction during lower body negative pressure. J Appl Physiol 60: 1535–1541, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol 589: 975–986, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenner MM, Wilson TE, Davis SL, Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol 111: 1703–1709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci 97: 122–128, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Wilson TE, Monahan KD, Short DS, Ray CA. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol 287: R1230–R1234, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki F. Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J Appl Physiol 108: 328–333, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki F, Yuge N. Limb-specific differences in the skin vascular responsiveness to adrenergic agonists. J Appl Physiol 111: 170–176, 2011. [DOI] [PubMed] [Google Scholar]


