Abstract
Cigarette smoke is a common environmental insult associated with increased risk of developing airway diseases such as wheezing and asthma in neonates and children. In adults, asthma involves airway remodeling characterized by increased airway smooth muscle (ASM) cell proliferation and increased extracellular matrix (ECM) deposition, as well as airway hyperreactivity. The effects of cigarette smoke on remodeling and contractility in the developing airway are not well-elucidated. In this study, we used canalicular-stage (18–20 wk gestational age) human fetal airway smooth muscle (fASM) cells as an in vitro model of the immature airway. fASM cells were exposed to cigarette smoke extract (CSE; 0.5–1.5% for 24–72 h), and cell proliferation, ECM deposition, and intracellular calcium ([Ca2+]i) responses to agonist (histamine 10 μM) were used to evaluate effects on remodeling and hyperreactivity. CSE significantly increased cell proliferation and deposition of ECM molecules collagen I, collagen III, and fibronectin. In contrast, [Ca2+]i responses were not significantly affected by CSE. Analysis of key signaling pathways demonstrated significant increase in extracellular signal-related kinase (ERK) and p38 activation with CSE. Inhibition of ERK or p38 signaling prevented CSE-mediated changes in proliferation, whereas only ERK inhibition attenuated the CSE-mediated increase in ECM deposition. Overall, these results demonstrate that cigarette smoke may enhance remodeling in developing human ASM through hyperplasia and ECM production, thus contributing to development of neonatal and pediatric airway disease.
Keywords: lung development, prematurity, asthma, collagen
neonatal lung disease, recurrent wheezing, and asthma are significant causes of morbidity and mortality in the pediatric population. Premature infants are at particularly increased risk because of the immaturity of the pulmonary system at birth and interventions in the hospital setting (2, 4, 14, 21, 27). As with adults, diseases such as asthma in the neonatal/pediatric population likely involve inflammation, and alterations in airway structure and function, including that of epithelium and airway smooth muscle (ASM) (7, 25, 43), leading to airway hyperreactivity and airway remodeling. Recently, increased focus has been placed on perinatal factors that may impact pulmonary development and airway remodeling that contribute to both pediatric and (eventually) adult airway disease (2, 4, 12, 21). Our prior work demonstrated the impact of hyperoxia (a common intervention in premature infants) on fetal airway smooth muscle (fASM) proliferation, apoptosis, and mitochondrial function (22). However, numerous other important factors have been implicated in development of airway hyperreactivity (2, 4, 21). One such environmental exposure is cigarette smoke exposure (4, 15, 21), particularly in the setting of premature infants discharged to homes with smoking adults.
Maternal tobacco use during pregnancy has been shown to have negative effects on neonatal and pediatric pulmonary function that may persist into adult years (16, 26, 31). Premature birth is another known complication of maternal tobacco use (30). This risk can be further increased when infants are exposed to numerous factors in the perinatal period, including prematurity itself, hyperoxia in the intensive care unit, and mechanical ventilation (2, 12, 14, 15, 21). Added to these factors is the further secondhand smoke exposure upon discharge from the hospital that may precipitate respiratory problems and repeated hospital visits. These repeated “hits” are certainly detrimental to lung function and development, but the ultimate cellular effects and mechanisms of cigarette smoke exposure have not been well-studied, particularly in the immature airway.
Key mechanisms of airway hyperreactivity in both adult and pediatric airways involve increased intracellular Ca2+ ([Ca2+]i), increased contractility, and ASM hyperplasia (24, 25, 40). In addition, there is evidence that extracellular matrix (ECM) remodeling and increased ECM protein deposition play roles (8, 28, 44). While environmental cigarette smoke exposure is associated with airway hyperreactivity, the mechanisms by which it produces these effects have not been well elucidated, and the potential impact of cigarette smoke on developing ASM cell proliferation or ECM deposition is unknown.
A number of mechanisms control [Ca2+]i and cell proliferation in ASM. Prior data from our lab and others demonstrate that plasma membrane invaginations, termed caveolae, play an important role in regulation of ASM contractility through modulation of [Ca2+]i (38, 42). In adults, the key caveolar protein and signaling molecule caveolin-1 is thought to be a mediator of cell proliferation and ECM deposition, with decreased levels of caveolin-1 resulting in increased cellular proliferation and fibrosis (19, 32, 45, 46). Accordingly, we hypothesized that altered caveolar protein expression and signaling is one mechanism through which cigarette smoke exposure exerts an impact on fASM structure and function in the setting of prematurity. We explored cell proliferation, ECM deposition, [Ca2+]i responses to agonist histamine, and caveolar protein expression.
MATERIALS AND METHODS
Materials.
Tissue culture reagents, including Hanks' Balanced Salt Solution (HBSS), fetal bovine serum (FBS), and DMEM-F-12 were obtained from Invitrogen (Carlsbad, CA). Sigma (St. Louis, MO) was the source for the remaining chemicals and supplies unless otherwise noted.
Summary of methods.
fASM cells were exposed to 0.5, 1, or 1.5% cigarette smoke extract (CSE) for 18–72 h. Western blot analysis was used to determine the level of proliferative, caveolar, and mitogen-activated protein (MAP) kinase pathway protein expression. A nuclear dye-based CyQuant NF proliferation assay was used to assess cellular proliferation. Extracellular matrix deposition was analyzed with a modified in-cell Western technique. fASM cells (control and CSE exposed) were additionally loaded with fura 2-AM, a fluorescent Ca2+ indicator dye, to investigate the effects of CSE on histamine agonist-induced [Ca2+]i responses. Details of these techniques are expanded below.
Isolation of human fASM cells.
Human fASM cells from the canalicular stage (18–20 wk gestation) were provided by Dr. Pandya from the University of Leicester, England, or purchased from Novogenix (Los Angeles, CA) through protocols approved by ethics committees in the United Kingdom (considered exempt by the Mayo Institutional Review Board since the cells were cultured and deidentified when received). Cells were obtained through enzymatic dissociation of fetal tracheobronchial tissue using previously described techniques (33, 34). Standard cell culture techniques were used to grow and maintain cells in a 95% air-5% CO2 humidified incubator. All media were phenol red-free DMEM-F-12 with 10% FBS. Cells were serum starved to arrest growth in 0.5% FBS media for a minimum of 24 h before treatments. Smooth muscle phenotype was frequently verified by confirmation of expression of smooth muscle actin, calponin, and acetylcholine receptor via Western blot. Use of these markers to delineate smooth muscle phenotype has been previously described (5, 22).
Cigarette smoke extract.
The procedure used for CSE preparation is a modification of the Blue and Janoff technique (3, 47). Cigarettes (Kentucky 1RF4 cigarettes) were covered with a sterile tip, and ∼35 cm3 of cigarette smoke were collected from one cigarette using a sterile smoking device (a 50-ml plastic syringe attached to a 3-way stopcock). The cigarette smoke was bubbled in 10 ml sterile PBS over 10 min and filtered through a sterile filter (considered to be 100% CSE stock). The CSE was used either immediately or stored for up to 5 days at 40C. Analysis demonstrated no difference in effects of fresh vs. stored CSE, and data from cells treated with both forms were therefore pooled (data not shown).
Western blot analysis.
Standard techniques were used to separate proteins from whole cell lysates on SDS-PAGE (4–15% gradient gels, Criterion Gel System; Bio-Rad, Hercules, CA) at 120 volts for 60 min. A Bio-Rad Trans-Blot Turbo system (Bio-Rad) was used to transfer proteins to nitrocellulose membranes. Membranes were blocked with Odyssey Blocking buffer (Li-Cor Biosciences, Lincoln, NE) and incubated overnight at 4°C in a 1 μg/ml concentration of primary antibody of interest (Santa Cruz Biotechnology, Santa Cruz, CA, unless otherwise noted). Primary antibodies included: cyclin E, proliferating cell nuclear antigen (PCNA), polymerase I and transcript release factor (cavin-1), c-Jun NH2-terminal kinase (JNK, phosphorylated JNK), phosphorylated extracellular signal-related kinase (ERK1/2, phosphorylated ERK1/2), MAP kinase [p-38, phosphorylated p-38 (Cell Signaling Technology, Danvers, MA)], GAPDH (Cell Signaling Technology), caveolin-1, and caveolin-2 (Abcam, Cambridge, MA). Membranes were washed with TBS-Tween 20 (TBST) before a 60-min incubation in secondary antibodies conjugated to 800CW or 680CW IRdye (Li-Cor Biosciences). Imaging was performed via a Li-Cor OdysseyXL system (Li-Cor Biosciences) with quantification performed via densitometry. Protein concentrations were normalized to GAPDH unless noted otherwise. All membranes were stripped and reprobed for loading controls.
Cellular proliferation assay.
In addition to measurement of proliferative protein markers via Western blot, proliferation was assessed using a CyQuant NF fluorescence kit (Invitrogen). Cells were plated in 96-well clear-bottom plates at a density of 5,000 cells/well in serum-free media and incubated overnight. Cells were then treated with vehicle (PBS) or 0.5, 1, or 1.5% CSE in 1% FBS media. Plates were incubated for 48 h after which the media was aspirated and the cells incubated in CyQuant dye in HBSS for 60 min at 37°C. Fluorescence was measured on a Flexstation 3 microplate reader (Molecular Devices, Sunnyvale, CA) at an excitation of 480 nm and emission of 530 nm. Cell proliferation was determined using a standard curve, and proliferation of CSE-treated cells was normalized to vehicle.
Calcium imaging.
[Ca2+]i imaging techniques used by our laboratory have been previously published (38, 42). In brief, cells were incubated in 5 μM fura 2-AM (Invitrogen) in HBSS at room temperature for 60 min. The plates were then washed, and cells were imaged in real time (MetaFluor; Universal Imaging, Downingtown, PA) on a Nikon Diaphot inverted microscope (Nikon Instrument, Melville, NY). Cell responses were based on 20 areas/field and software-defined regions of interest. Agonist stimulation was performed with 10 μM histamine. Amplitudes of responses were calculated as the difference between peak and baseline Ca2+.
Modified In-cell western for ECM proteins.
Cells were grown to 60% confluence in clear-bottom 96-well plates before serum starvation for 24 h. They were then treated with CSE in 0.5% FBS media at final concentrations of 0.5, 1.0, or 1.5% for 72 h. Cells were then lysed and lifted from the plate with 0.016 N NH4OH. Wells were washed with TBS and blocked with Li-Cor Odyssey Blocking Buffer for 60 min before overnight incubation in primary antibody at 4°C. Primary antibodies were used at a concentration of 10 μg/ml and included collagen I and collagen III from Abcam and fibronectin from Santa Cruz. Wells were washed with TBST before 60 min incubation with IR-dye-conjugated secondary antibodies at a concentration of 5 μg/ml. Control wells (without cells) were exposed to media with or without CSE treatments and incubated with primary and secondary antibodies to control for any background noise. Plates were imaged via a Li-Cor OdysseyXL system with quantification performed via densitometry. Protein concentrations were normalized to well cell count determined by performing a CyQuant NF proliferation assay on each plate before cell lysis.
MAP kinase inhibitor studies.
Cells were plated in 96-well plates according to the Cyquant NF proliferation protocol or modified in-cell Western protocol described above. Following serum starvation, cells were then treated for 30 min with either CAS 219138-24-6 (p38 MAP kinase inhibitor; Calbiochem) or ERK activation inhibitor peptide I (Calbiochem). Following inhibitor exposure, cells were treated with 1% CSE for 48–72 h, and proliferation and ECM deposition were assessed as described above.
Statistical analysis.
All experiments included a minimum of four fetal airway samples with a minimum of two replicate experiments per sample. Statistical analysis was performed using a Sigma Plot (SYSTAT, San Jose, CA) software statistical package. Data were analyzed via one-way ANOVA with Student-Newman-Keuls or Dunnets post hoc analysis with statistical significance set at P < 0.05. Values are means ± SE.
RESULTS
CSE increases fASM cell proliferation.
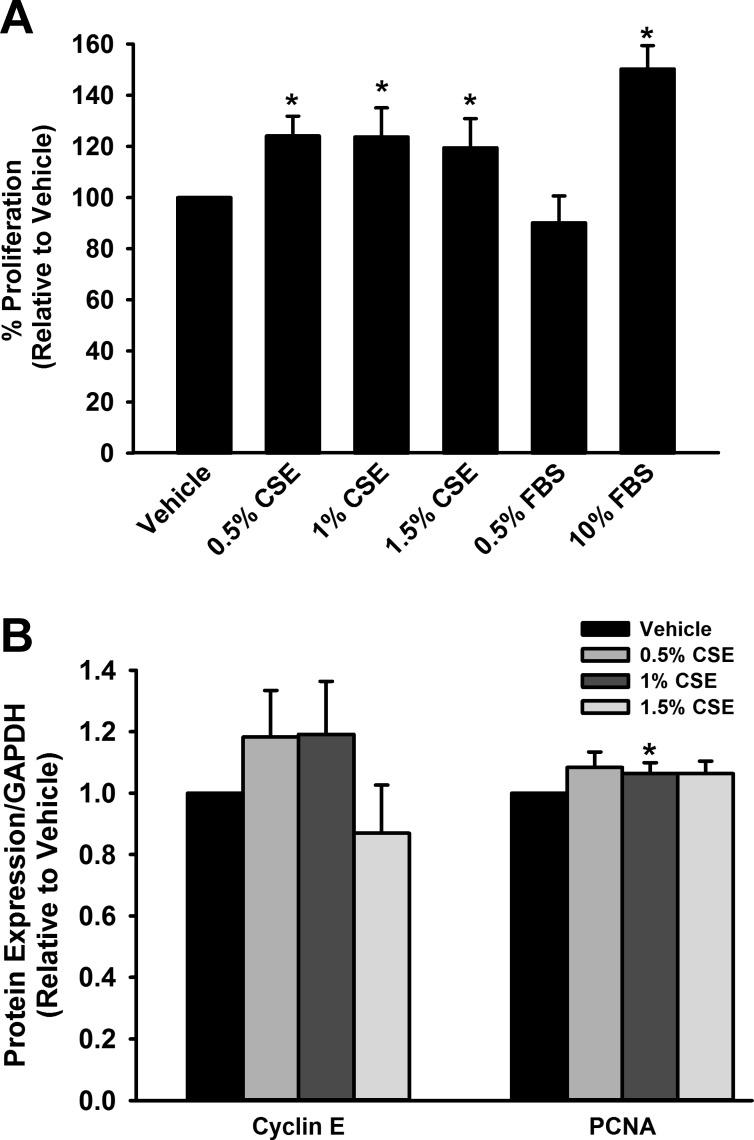
CyQuant analysis demonstrated a significant increase in fASM cell proliferation with CSE exposure (P < 0.05; Fig. 1A). Western blot analysis demonstrated an associated increase in proliferative markers cyclin E and PCNA following 24 h CSE exposure (0.5 and 1%; P < 0.05; Fig. 1B). The proliferative effects of CSE appeared to decrease with higher levels of exposure (1.5% CSE).
Fig. 1.
Cigarette smoke extract (CSE) increases proliferation of human fetal airway smooth muscle (fASM) cells. A: exposure of fASM cells to CSE for 48 h resulted in significantly increased proliferation compared with vehicle control. B: expression of proliferation markers cyclin E and proliferating cell nuclear antigen (PCNA) was increased compared with vehicle following 24 h exposure to low levels (0.5 and 1%) of CSE but not higher levels. Values are means ± SE from n = 4 samples. *Significant difference from vehicle control with P < 0.05.
CSE increases ECM deposition of collagens and fibronectin.
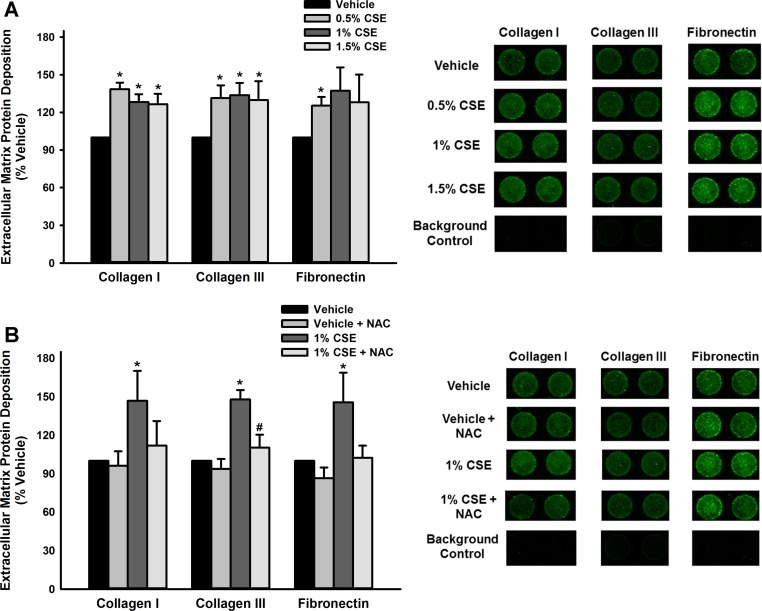
To assess the impact of CSE on ECM deposition, modified In-Cell Westerns were performed to analyze three key molecules: collagen I, collagen III, and fibronectin. CSE exposure (72 h) resulted in increased expression of all three ECM proteins in a non-dose-dependent manner (P < 0.05; Fig. 2A). In contrast, control wells (no cells but media with and without treatment and all primary and secondary antibodies) were blank. To assess the possible contribution of oxidative stress as a mechanism for this increase, cells were pretreated with 10 mM N-acetylcysteine (NAC) for 60 min before CSE exposure. NAC pretreatment resulted in alleviation of the CSE effect on ECM production to near vehicle levels (P < 0.05 for NAC effect; Fig. 2B).
Fig. 2.
CSE increases extracellular matrix (ECM) deposition by human fASM cells. A: a modified LiCor In-Cell Western technique (semiquantitative immunofluorescence) was used to examine the impact of CSE exposure on fASM deposition of ECM molecules collagen I, collagen III, and fibronectin, all of which were significantly increased by 72 h CSE exposure. B: cells were treated with vehicle or N-acetylcysteine (NAC; 10 mM) before CSE exposure. NAC pretreatment prevented CSE-induced increase in ECM deposition. Values are means ± SE from n = 4 samples with proteins normalized to Cyquant NF kit cell count. *Significance from vehicle control with P < 0.05. #Significant difference from 1% CSE group with P < 0.05.
CSE does not increase [Ca2+]i response to agonist.
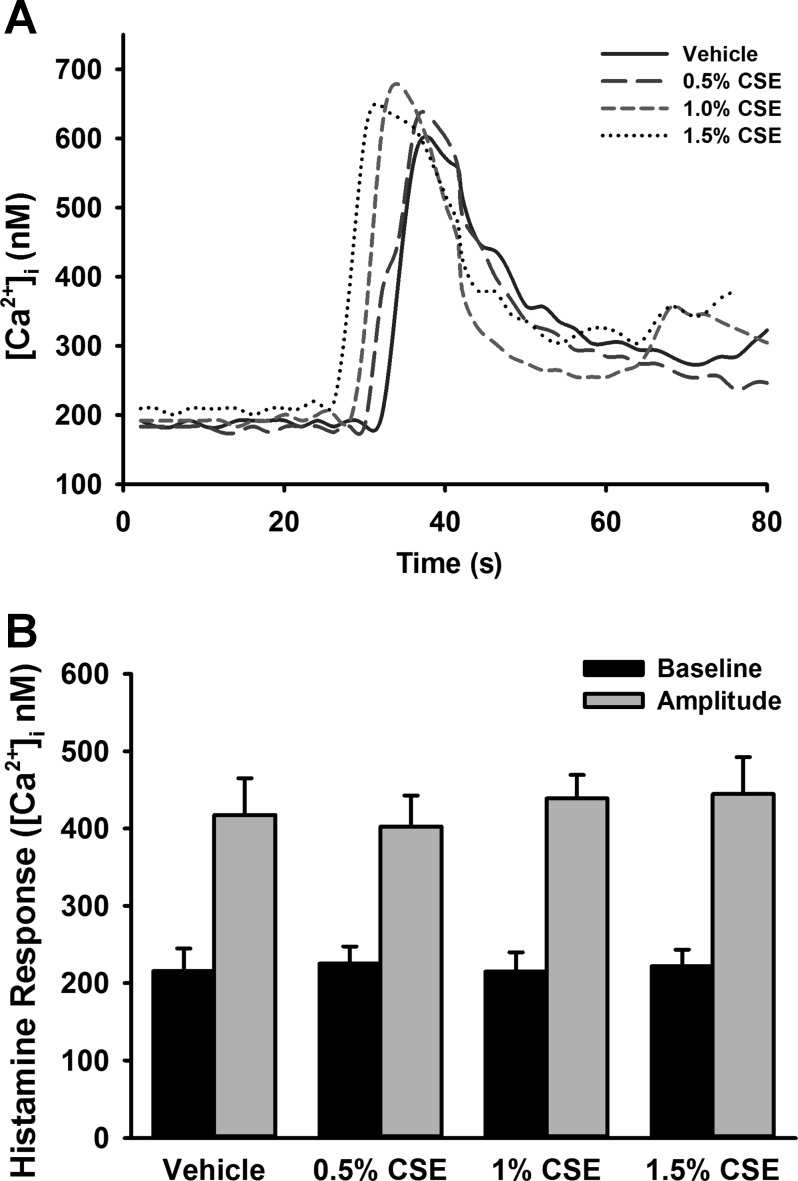
[Ca2+]i responses to histamine in CSE-exposed cells were assessed using fura 2-AM. CSE did not significantly increase [Ca2+]i responses compared with vehicle (no CSE) control (Fig. 3, A and B). Of note, however, increased variability of responses was noted in cells treated with CSE compared with controls.
Fig. 3.
CSE does not alter intracellular Ca2+ ([Ca2+]i) responses to histamine in human fASM cells. A: in fura 2-AM-loaded fASM cells, CSE exposure did not significantly affect [Ca2+]i response to 10 μM histamine. Arrow indicates time of histamine administration in representative tracings. B: summary of average baseline and peak amplitude [Ca2+]i responses. Values are means ± SE from n = 4 samples.
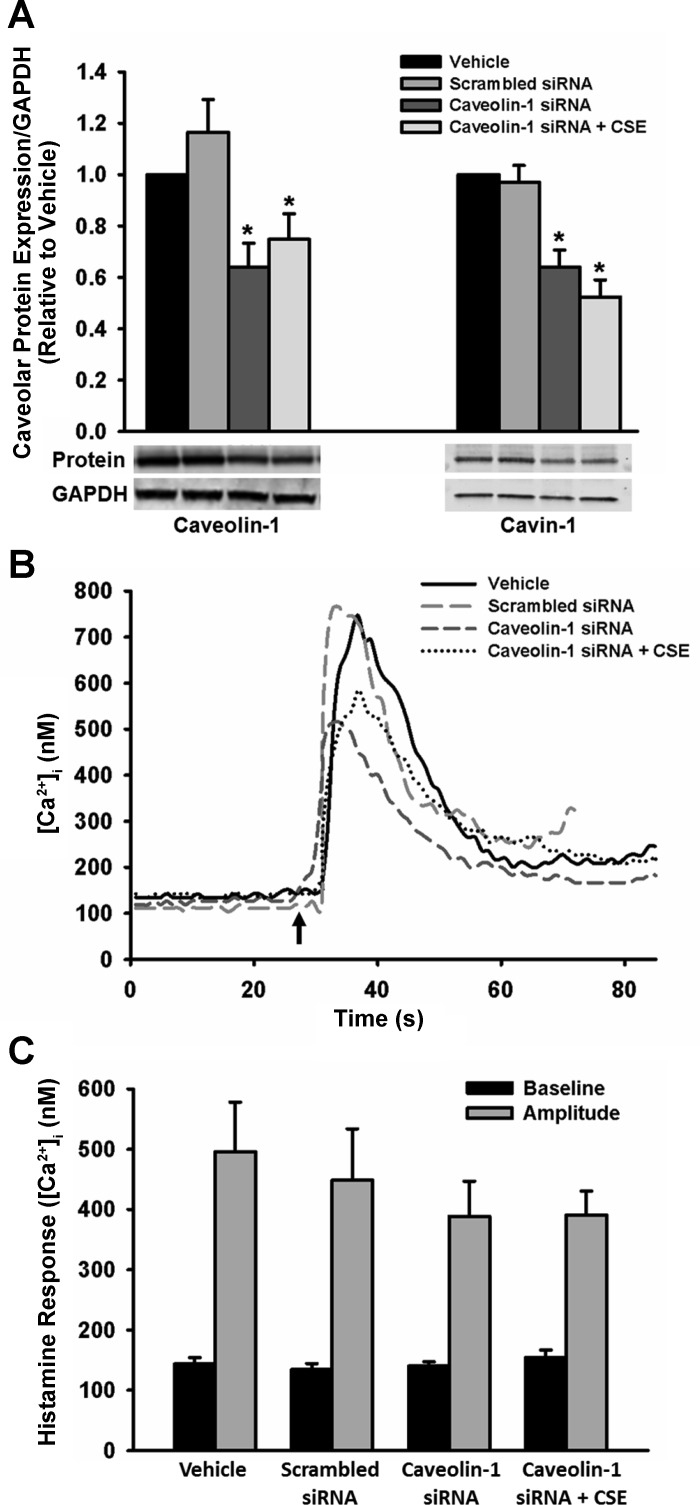
Given our hypothesis that caveolin-1 is involved, we explored the effect of altered caveolin-1 expression in [Ca2+]i responses and CSE exposure. Transfection of fASM cells with caveolin-1 small-interfering RNA (siRNA) resulted in a 40–50% decrease in caveolin-1 and cavin-1 protein levels compared with nontransfected and scrambled siRNA controls. However, CSE treatment of such transfected cells did not significantly impact caveolar protein expression (Fig. 4A). Transfection with caveolin-1 siRNA also did not significantly impact [Ca2+]i responses compared with nontransfected and scrambled siRNA transfected controls (Fig. 4, B and C).
Fig. 4.
Caveolin-1 knockdown does not impact CSE effects on [Ca2+]i responses. A: fASM cells transfected with caveolin-1 small-interfering RNA (siRNA) demonstrated significantly decreased expression of caveolar proteins compared with controls, indicating adequate transfection efficiency. CSE exposure in these transfected cells did not result in significant alteration of caveolar protein expression. B: in fASM cells transfected with caveolin-1 siRNA and loaded with fura 2, [Ca2+]i responses to 10 μM histamine were not influenced by caveolin-1 siRNA compared with nontransfected and scrambled siRNA controls. Arrow indicates time of histamine administration in representative tracings. C: calculated average baseline and peak amplitude [Ca2+]i responses. Values are means ± SE from n = 4 samples. *Significant difference from vehicle control with P < 0.05.
Mechanisms of CSE effects in fASM remodeling.
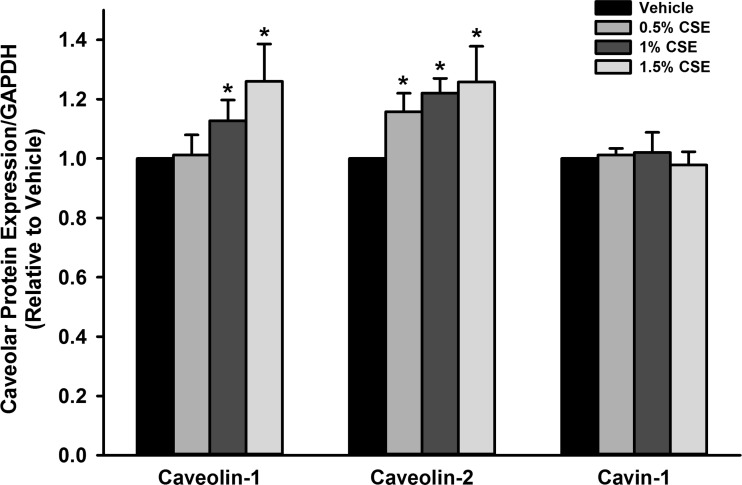
To examine whether CSE exposure resulted in decreased caveolar protein expression that could contribute to the observed changes in proliferation and ECM deposition, caveolar protein expression was examined through Western blot. Exposure of fASM cells to CSE (0.5–1.5%) for 24 h in fact did result in significantly increased expression of caveolar proteins caveolin-1 and caveolin-2 compared with vehicle (P < 0.05; Fig. 5). Cavin-1 did not demonstrate significant change in expression (Fig. 5).
Fig. 5.
CSE exposure increases caveolar protein expression. Exposure of fASM cells (24 h) to 0.5, 1, and 1.5% CSE increased caveolar proteins caveolin-1 and caveolin-2 but had no significant effect on cavin-1. Values are means ± SE from n = 4 samples. *Significant difference from vehicle control with P < 0.05.
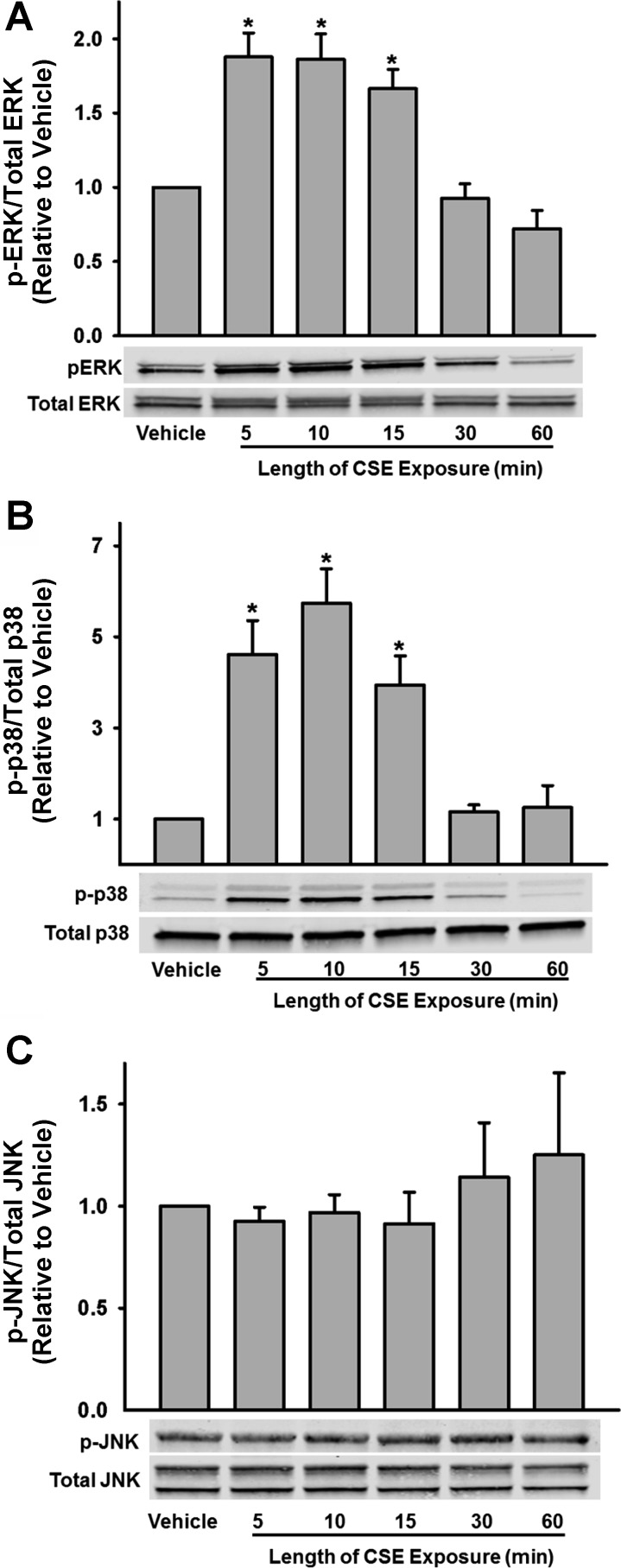
In light of the fact that CSE resulted in increased caveolar protein expression rather than an expected decreased expression (and thus, consistent with the data of Fig. 4, was unlikely to be the contributory mechanism for CSE effect on proliferation), we explored additional mechanisms important to cellular proliferation. We specifically examined MAP kinase pathway induction with focus on JNK, ERK1/2, and MAP kinase (p-38). Protein phosphorylation was examined 5, 10, 15, 30, and 60 min after cell treatment with CSE. fASM exposure to CSE resulted in a rapid induction of ERK and p-38 phosphorylation with significant increase in p-ERK and phospho-p-38 by 5 min, with peak phosphorylation at 10 min (P < 0.05; Fig. 6, A and B). There was no significant phosphorylation of JNK noted (Fig. 6C).
Fig. 6.
Mechanisms of CSE action in fASM. Exposure of fASM cells to CSE resulted in a rapid phosphorylation of extracellular signal-related kinase (ERK, A) and p38 (B) within 5 min, with peak phosphorylation at 10 min, but did not affect c-Jun NH2-terminal kinase (JNK) phosphorylation (C). Values are means ± SE from n = 4 samples. *Significant difference from vehicle control with P < 0.05.
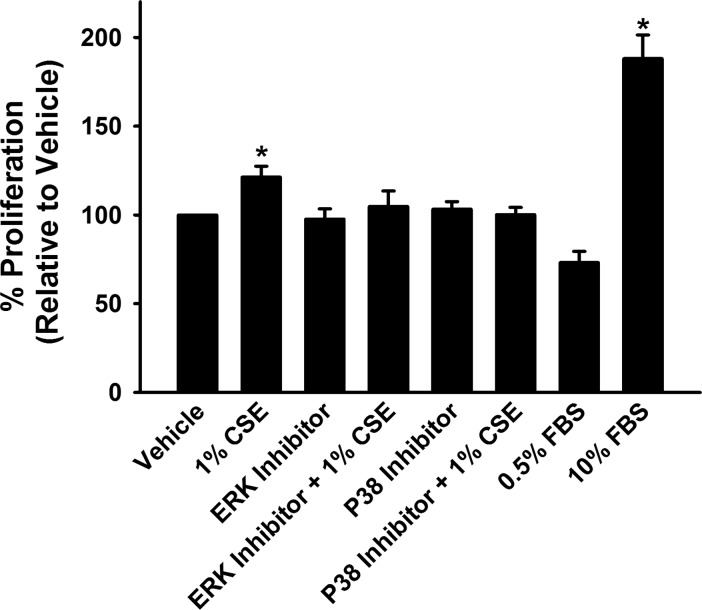
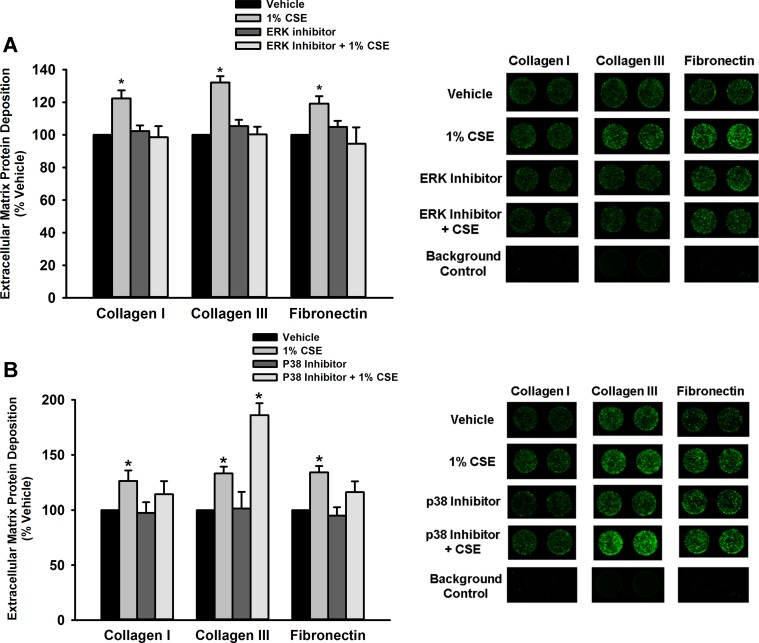
MAP kinase pathway inhibition prevents CSE-mediated remodeling.
To further assess the potential role of MAP kinase pathway signaling in fASM proliferation and ECM deposition, cells were treated with either p38 or ERK inhibitors before CSE exposure. ERK inhibition prevented CSE-mediated proliferation and increased deposition of collagen I, collagen III, and fibronectin (P < 0.05; Figs. 7 and 8A). p38 inhibition also resulted in prevention of CSE-mediated proliferation but did not have a significant effect on ECM deposition (P < 0.05; Figs. 7 and 8B).
Fig. 7.
ERK and p38 inhibition prevents CSE-mediated fASM proliferation. Pretreatment of fASM cells with 10 μM ERK activation inhibitor peptide 1 or 1 μM CAS 219138-24-6 (p38 inhibitor) abrogated CSE-mediated proliferation. Values are means + SE from n = 4 samples. *Significant difference from vehicle control with P < 0.05.
Fig. 8.
ERK inhibition prevents CSE-mediated increase in extracellular matrix deposition. A: a modified LiCor In-Cell Western technique (semiquantitative immunofluorescence) was used to examine fASM deposition of ECM molecules collagen I, collagen III, and fibronectin, all of which were significantly increased by 72 h CSE exposure. Pretreatment for 30 min with 10 μM ERK activation inhibitor peptide 1 prevented these CSE-mediated increases in ECM deposition. B: cells were treated with 1 μM CAS 219138-24-6 (p38 inhibitor) for 30 min before 72 h of CSE exposure. In contrast to the ERK inhibitor effects, p38 inhibition did not have a significant effect on reducing CSE-mediated ECM deposition. Values are means ± SE from n = 4 samples with proteins normalized to Cyquant NF kit cell count. *Statistical significance from vehicle control with P < 0.05.
DISCUSSION
Wheezing, asthma, and airway disease remain significant health burdens in the neonatal and pediatric population. Environmental exposures such as allergens, pollution, and cigarette smoke are important risk factors in development and exacerbation of these diseases (4, 6, 16, 21). In this study, we investigated the impact of cigarette smoke exposure on key properties of fASM cells that may contribute to development of reactive airway disease in the neonatal population. CSE exposure resulted in increased fASM proliferation and increased deposition of ECM molecules collagen I, collagen III, and fibronectin. Surprisingly, there was no significant impact of CSE exposure on [Ca2+]i responses to agonist (histamine). Overall, these results imply that cigarette smoke exposure may lead to airway remodeling, which may have long-term consequences on airway structure and function relevant to diseases such as asthma.
Airway wall thickening is a key feature of reactive airway diseases such as asthma and neonatal lung disease (13, 43). Postmortem examinations of infants with chronic lung disease of prematurity and bronchopulmonary dysplasia have demonstrated significant airway wall thickening (29). These wall changes can result from proliferation of ASM cells as well as increased deposition and alteration in composition of the ECM, resulting in thickening and stiffening of the airways (13, 35, 43). Based on our findings, it appears that cigarette smoke exposure may result in altered proliferation of fASM cells, with low levels of exposure leading to increased proliferation. This proliferative trend begins to decrease with higher levels (1.5%) of CSE exposure and may indicate a toxic effect with increased exposure. These changes in fASM proliferation may result in alteration of airway development and decreased airway caliber, potentially contributing to airway thickening and stiffness, particularly in the setting of an otherwise compliant chest wall and lung during postnatal development. Interestingly, our prior investigations demonstrated higher fASM proliferation in cells exposed to hyperoxia (22). Thus, in preterm infants exposed to hyperoxia in the hospital followed by secondhand environmental smoke exposure at home, the issue of airway thickening due to ASM proliferation may be further compounded and the risk of developing airway disease increased.
Extracellular matrix composition has been shown to significantly impact cellular morphology, properties, and cell responses to stress largely through connection of the internal cellular cytoskeleton to the ECM via focal adhesion complexes (13, 35, 37). Matrix metalloproteinases (MMPs) are endopeptidases responsible for breaking down and regulating the components of the ECM, particularly collagens and fibronectin (10, 11). Numerous studies have demonstrated alterations in MMP expression in bronchoalveolar lavage specimens from neonates with bronchopulmonary dysplasia, implying that dysregulation of ECM remodeling contributes to neonatal lung disease (8, 44). Expression of ECM by human fASM cells has not been previously explored. Our findings demonstrate that fASM cells are capable of building and remodeling the extracellular matrix, with collagen I, collagen III, and fibronectin significantly expressed. Furthermore, exposure to cigarette smoke results in increased expression of these molecules, whereas pretreatment with the antioxidant NAC prevents these changes. This implies that oxidative stress plays a role in the CSE-mediated increase of fASM production and remodeling of the ECM. In terms of further potential mechanisms, TGF-β and Smad pathways have been previously demonstrated to be important in ECM remodeling, and it is possible that CSE exposure could impact these signaling pathways in fASM (1, 49).
Although the effects of CSE on developing ASM proliferation and ECM deposition have not been previously studied, similar studies have been performed in mature ASM with comparable results. A number of prior papers have demonstrated increased mature ASM proliferation in the setting of CSE exposure (23, 36, 39, 51). The etiology of CSE-mediated ASM proliferation is complex, with multiple pathways implicated in previous investigations. A number of studies have demonstrated an Akt pathway-mediated increase in cyclin D1 expression, leading to ASM proliferation in mature ASM cell lines (23, 51). Other studies in adult human tracheal smooth muscle and mature bovine tracheal smooth muscle have focused on reactive oxygen species-mediated effects of CSE and induction of the ERK1/2 pathway as a cause of ASM proliferation (36, 39). These studies demonstrating ERK-mediated proliferation in mature ASM support our data demonstrating ERK-mediated proliferation in developing ASM. The effects of CSE on ECM deposition are less well elucidated, even in mature ASM. One study in guinea pigs exposed to cigarette smoke demonstrated increased airway wall thickening with increased collagen deposition, consistent with the increased collagen deposition we observed with CSE exposure in developing ASM (50).
In light of the remodeling changes with CSE exposure characterized by increased proliferation and ECM deposition, we evaluated caveolar protein expression as a possible mechanism for these changes. Caveolin-1 has been shown to normally have a suppressive effect on ASM proliferation, at least in adults (17, 18, 20). Additionally, caveolin-1 is an important mediator of fibrosis, with decreased levels of caveolin-1 associated with increased fibrosis and collagen deposition in the mature lung (17, 32, 45, 46, 48). Intriguingly, in this study, both caveolin-1 and caveolin-2 protein levels were increased in fASM cells exposed to CSE, and thus the opposite to the expected trend. Whereas we did not specifically explore any potential effect of this increased caveolin-1 on proliferation or remodeling, our data suggest that caveolar proteins may play a less important role at this stage of development or may represent dysfunctional or decreased insertion of caveolins in the cell membrane. Regardless, caveolin-1 may not play a role in ECM modulation per se, at least in the context of remodeling.
To further assess possible mechanisms contributing to the observed fASM remodeling changes, we assessed induction of key MAP kinase pathways, specifically ERK, JNK, and p38. Both ERK and p38 phosphorylation were rapidly and significantly increased with CSE exposure, whereas JNK induction was not affected. A key role for ERK and p38 activation is further supported by the inhibitor data demonstrating decreased fASM proliferation with both p38 and ERK inhibition. ECM changes appear to be more strongly influenced by ERK inhibition, since collagen I, collagen III, and fibronectin deposition were decreased with ERK inhibition but no significant changes were noted with inhibition of p38. These data are consistent with a prior paper in mature bovine ASM demonstrating ERK and p38 induction in the setting of CSE and LPS exposure, although at a higher concentration of CSE (36). It is likely that free radical and oxidant stress induced by CSE exposure contribute to the observed ERK and p38 activation, as has been noted in mature human ASM cells exposed to CSE (9, 36, 39). The import of oxidant stress as a major mechanism in causing the effects of CSE is further supported by the fact that pretreatment with the antioxidant NAC prevented increased ECM deposition in fASM, as previously noted.
fASM cells have been previously shown to respond to bronchoconstriction agonists such as acetylcholine and histamine, although the responses are smaller in amplitude and delayed when compared with adult ASM cells (22). The mechanisms of calcium regulation in these cells have not been previously closely examined. We have previously demonstrated the importance of caveolar protein caveolin-1 in regulation of [Ca2+]i in mature ASM (38, 41, 42). In adult ASM, knockdown of caveolin-1 abrogates cytokine-mediated increases in [Ca2+]i (40, 41). In this study, we hypothesized that similar effects would occur in developing ASM. However, although exposure to CSE increased caveolin-1 protein expression and resulted in increased variability of fASM histamine response, we did not find significant changes in [Ca2+]i with CSE exposure. Similarly, knockdown of fASM caveolin-1 with siRNA did not result in a statistically significant decrease in [Ca2+]i response to histamine. These data imply that fASM [Ca2+]i regulation is not as dependent on caveolar mechanisms and may rely on other mechanisms of calcium regulation at this stage of development. In terms of additional control points, the balance between sarcoplasmic calcium release vs. extracellular calcium influx has not been closely examined in these cells. Whereas fASM cells do respond to histamine, the decreased amplitude of response compared with adult ASM cells noted in prior studies may indicate that the fASM cells are more dependent on other sources for calcium influx.
In summary, our findings demonstrate that cigarette smoke exposure modulates key components of airway remodeling in developing human ASM. Specifically, CSE exposure results in increased proliferation and increased deposition of key ECM molecules, including collagen I, collagen III, and fibronectin. These remodeling changes may lead to development of a thicker more fibrotic airway that can contribute to airway diseases such as wheezing and asthma. These remodeling changes may also result in a particular burden of increased work of breathing in an already vulnerable neonatal and pediatric population. MAP kinase pathway induction, specifically ERK and p38 induction, likely plays a significant role in the etiology of these observed changes, with ERK signaling playing a particularly key role in mediating both proliferation and ECM effects. Antioxidants may represent a potential avenue for amelioration of these effects. Overall, these results indicate that environmental tobacco smoke may significantly contribute to development of airway disease in the neonatal and pediatric population.
GRANTS
This study was funded by Clinical Innovator Grants through the Flight Attendant Medical Research Institute (C. M. Pabelick, R. Vassallo). C. M. Pabelick's Flight Attendant Medical Research Institute support is through a grant to the American Academy of Pediatrics Julius B. Richmond Center. Further support was provided by the National Heart Lung and Blood Institute of the National Institutes of Health (T3-HL-105355, E. R. Vogel; HL-056470, Y. S. Prakash; HL-090595, C. M. Pabelick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.R.V., R.V., H.C.P., Y.S.P., and C.M.P. conception and design of research; E.R.V., S.K.V., M.A.H., and D.D.H. performed experiments; E.R.V., S.K.V., M.A.H., and D.D.H. analyzed data; E.R.V., M.A.T., Y.S.P., and C.M.P. interpreted results of experiments; E.R.V., S.K.V., Y.S.P., and C.M.P. prepared figures; E.R.V., Y.S.P., and C.M.P. drafted manuscript; E.R.V., M.A.T., Y.S.P., and C.M.P. edited and revised manuscript; E.R.V., S.K.V., M.A.H., D.D.H., M.A.T., R.V., H.C.P., Y.S.P., and C.M.P. approved final version of manuscript.
REFERENCES
- 1.Alejandre-Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 11: e1001596, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blue ML, Janoff A. Possible mechanisms of emphysema in cigarette smokers. Release of elastase from human polymorphonuclear leukocytes by cigarette smoke condensate in vitro. Am Rev Respir Dis 117: 317–325, 1978. [DOI] [PubMed] [Google Scholar]
- 4.Britt RD, Jr, Faksh A, Vogel E, Martin RJ, Pabelick CM, Prakash YS. Perinatal factors in neonatal and pediatric lung diseases. Exp Rev Respir Med 7: 515–531, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt RD, Jr, Faksh A, Vogel ER, Thompson MA, Chu V, Pandya HC, Amrani Y, Martin RJ, Pabelick CM, Prakash YS. Vitamin D attenuates cytokine-induced remodeling in human fetal airway smooth muscle cells. J Cell Physiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 129: 735–744, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Bush A, Menzies-Gow A. Phenotypic differences between pediatric and adult asthma. Proc Am Thoracic Soc 6: 712–719, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, Andersson S. Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics 108: 686–692, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cheng SE, Luo SF, Jou MJ, Lin CC, Kou YR, Lee IT, Hsieh HL, Yang CM. Cigarette smoke extract induces cytosolic phospholipase A2 expression via NADPH oxidase, MAPKs, AP-1, and NF-kappaB in human tracheal smooth muscle cells. Free Radic Biol Med 46: 948–960, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Dancer RC, Wood AM, Thickett DR. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J 38: 1461–1467, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Davey A, McAuley DF, O'Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J 38: 959–970, 2011. [DOI] [PubMed] [Google Scholar]
- 12.De Luca G, Olivieri F, Melotti G, Aiello G, Lubrano L, Boner AL. Fetal and early postnatal life roots of asthma. J Maternal-Fetal Neonatal Med 23, Suppl 3: 80–83, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thoracic Soc 6: 683–692, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Deuber C, Terhaar M. Hyperoxia in very preterm infants: a systematic review of the literature. J Perinatal Neonatal Nursing 25: 268–274, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol 27: 5–14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duijts L, Jaddoe VW, van der Valk RJ, Henderson JA, Hofman A, Raat H, Steegers EA, Moll HA, de Jongste JC. Fetal exposure to maternal and paternal smoking and the risks of wheezing in preschool children: the Generation R Study. Chest 141: 876–885, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med 8: 741–753, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Gvaramia D, Blaauboer ME, Hanemaaijer R, Everts V. Role of caveolin-1 in fibrotic diseases. Matrix Biol 32: 307–315, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc Am Thoracic Soc 5: 80–88, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Harding R, Maritz G. Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med 17: 67–72, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L711–L719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He F, Li B, Zhao Z, Zhou Y, Hu G, Zou W, Hong W, Zou Y, Jiang C, Zhao D, Ran P. The pro-proliferative effects of nicotine and its underlying mechanism on rat airway smooth muscle cells. PLos One 9: e93508, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 30: 114–133, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology 20: 28–35, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Hylkema MN, Blacquiere MJ. Intrauterine effects of maternal smoking on sensitization, asthma, and chronic obstructive pulmonary disease. Proc Am Thoracic Soc 6: 660–662, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, Jaakkola MS. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 118: 823–830, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kaarteenaho-Wiik R, Paakko P, Herva R, Risteli J, Soini Y. Type I and III collagen protein precursors and mRNA in the developing human lung. J Pathol 203: 567–574, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kwinta P, Pietrzyk JJ. Preterm birth and respiratory disease in later life. Exp Rev Respir Med 4: 593–604, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol 20: 115–126, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Landau LI. Tobacco smoke exposure and tracking of lung function into adult life. Paediatr Respir Rev 9: 39–43, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Le Saux O, Teeters K, Miyasato S, Choi J, Nakamatsu G, Richardson JA, Starcher B, Davis EC, Tam EK, Jourdan-Le Saux C. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am J Physiol Lung Cell Mol Physiol 295: L1007–L1017, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandya HC, Innes J, Hodge R, Bustani P, Silverman M, Kotecha S. Spontaneous contraction of pseudoglandular-stage human airspaces is associated with the presence of smooth muscle-alpha-actin and smooth muscle-specific myosin heavy chain in recently differentiated fetal human airway smooth muscle. Biol Neonate 89: 211–219, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Pandya HC, Snetkov VA, Twort CH, Ward JP, Hirst SJ. Oxygen regulates mitogen-stimulated proliferation of fetal human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L1220–L1230, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Parameswaran K, Willems-Widyastuti A, Alagappan VK, Radford K, Kranenburg AR, Sharma HS. Role of extracellular matrix and its regulators in human airway smooth muscle biology. Cell Biochem Biophys 44: 139–146, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Pera T, Gosens R, Lesterhuis AH, Sami R, van der Toorn M, Zaagsma J, Meurs H. Cigarette smoke and lipopolysaccharide induce a proliferative airway smooth muscle phenotype (Abstract). Respir Res 11: 48, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, Geiger B, Bershadsky AD. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat Cell Biol 13: 1457–1465, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L1126, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ravasi S, Citro S, Viviani B, Capra V, Rovati GE. CysLT1 receptor-induced human airway smooth muscle cells proliferation requires ROS generation, EGF receptor transactivation and ERK1/2 phosphorylation (Abstract). Respir Res 7: 42, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi J.F. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thoracic Soc 5: 23–31, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Sathish V, Thompson MA, Sinha S, Sieck GC, Prakash YS, Pabelick CM. Inflammation, caveolae and CD38-mediated calcium regulation in human airway smooth muscle. Biochim Biophys Acta 1843: 346–351, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sathish V, Yang B, Meuchel LW, VanOosten SK, Ryu AJ, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 and force regulation in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 300: L920–L929, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddiqui S, Martin JG. Structural aspects of airway remodeling in asthma. Curr Allergy Asthma Rep 8: 540–547, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Sweet DG, McMahon KJ, Curley AE, O'Connor CM, Halliday HL. Type I collagenases in bronchoalveolar lavage fluid from preterm babies at risk of developing chronic lung disease. Arch Dis Child Fetal Neonatal Ed 84: F168–F171, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson MA, Prakash YS, Pabelick CM. The role of caveolae in the pathophysiology of lung diseases. Exp Rev Respir Med 8: 111–122, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Tourkina E, Richard M, Gooz P, Bonner M, Pannu J, Harley R, Bernatchez PN, Sessa WC, Silver RM, Hoffman S. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 294: L843–L861, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 175: 2684–2691, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, Choi AM. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 203: 2895–2906, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warburton D, Shi W, Xu B. TGF-beta-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol 304: L83–L85, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright JL, Postma DS, Kerstjens HA, Timens W, Whittaker P, Churg A. Airway remodeling in the smoke exposed guinea pig model. Inhal Toxicol 19: 915–923, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Xu GN, Yang K, Xu ZP, Zhu L, Hou LN, Qi H, Chen HZ, Cui YY. Protective effects of anisodamine on cigarette smoke extract-induced airway smooth muscle cell proliferation and tracheal contractility. Toxicol Appl Pharmacol 262: 70–79, 2012. [DOI] [PubMed] [Google Scholar]