Abstract
Endothelial barrier function is an essential and tightly regulated process that ensures proper compartmentalization of the vascular and interstitial space, while allowing for the diffusive exchange of small molecules and the controlled trafficking of macromolecules and immune cells. Failure to control endothelial barrier integrity results in excessive leakage of fluid and proteins from the vasculature that can rapidly become fatal in scenarios such as sepsis or the acute respiratory distress syndrome. Here, we highlight recent advances in our understanding on the regulation of endothelial permeability, with a specific focus on the endothelial glycocalyx and endothelial scaffolds, regulatory intracellular signaling cascades, as well as triggers and mediators that either disrupt or enhance endothelial barrier integrity, and provide our perspective as to areas of seeming controversy and knowledge gaps, respectively.
Keywords: endothelial cell, permeability, edema, acute lung injury/acute respiratory distress syndrome
the primordial function of the vascular endothelium is the maintenance of an effective barrier for fluids, proteins, and cells while concomitantly allowing for efficient gas transfer and the regulated transport of solutes as well as trafficking of inflammatory cells (112). The endothelium allows for unrestricted transfer of low-molecular-weight substances (<3 nm in radii) such as dissolved gases or ions through passive diffusion. In contrast, transendothelial trafficking of plasma proteins such as albumin or immunoglobulins (ranging from 7 to 11.5 nm radii) is dynamically controlled. Failure to maintain an intact barrier results in leakage of solutes, proteins, and fluid into the interstitial space and, in the case of the lung, alveolar space with detrimental and frequently fatal sequelae for lung mechanics and alveolo-capillary gas exchange (100). Mechanisms regulating barrier integrity, failure, or reconstitution and therapeutic strategies aimed at the preservation or recovery of an intact endothelial barrier function have therefore been a topic of intense research over past years (64).
Barrier function of the endothelial monolayer is regulated by cell-cell and cell-extracellular matrix adhesion as well as a wide variety of biological, chemical, or physical stimuli, and endogenous mediators, respectively. The glycocalyx, an extracellular covering on the apical side of endothelium, along with the monolayer adhesive property provided by the intercellular endothelial junctions, integrin receptors, and their protein partners, maintains the albumin-impermeable nature of the vessel wall under basal conditions. Barrier disruption often stems from compromised interendothelial junctions, resulting in the formation of gaps between normally contiguous cells, while barrier reinforcement arises from stabilization of junctional complexes (86). Canonically, barrier disruptive substances have included thrombin, platelet activating factor, tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), histamine and bradykinin, while sphingosine-1-phosphate (S1P) and angiopoietin-1 are regarded as barrier stabilizing (87). Endothelial barrier function is critically regulated by small GTPases such as RhoA, Rac1, and Cdc42, that serve as key integrators of signaling events between the plasma membrane and the endothelial cytoskeleton. In broadest terms, signaling via Rac1 and Cdc42 enhances cortical actin formation leading to increased barrier stability, while RhoA activation reorganizes actin filaments into stress fibers, destabilizing VE-cadherin and increasing barrier permeability (51). In parallel, changes in the intracellular Ca2+ concentration ([Ca2+]i) regulate endothelial permeability via activation of myosin light chain kinase and subsequent actin-myosin-induced endothelial cell contraction (152). The relative contribution of both GTPase and [Ca2+]i signaling varies considerably under different experimental conditions as well as in response to different stimuli, and this variability likely accounts for some of the existing heterogeneity in regulation of endothelial barrier function by these mechanisms (141).
Over the past years, a series of seminal studies focusing on the regulation of lung endothelial barrier function and edema formation have been published in the American Journal of Physiology - Lung Cellular and Molecular Physiology. Herein, we discuss these results and provide our perspective, which may shed new light into the basic mechanisms regulating barrier function and/or may have important therapeutic implications for the prevention or treatment of diseases associated with lung endothelial leak and edema formation.
ENDOTHELIAL CELL SURFACE AND SCAFFOLDS REGULATING PULMONARY BARRIER FUNCTION
Glycocalyx
The negatively charged glycocalyx is a surface layer of proteoglycans, glycosaminoglycans, and adsorbed plasma proteins on the apical side of the endothelial monolayer (84, 113). The thickness of the glycocalyx varies considerably depending on vascular beds as well as tissue types e.g., lungs, skeletal muscle, or heart (59, 67, 146). Additionally, studies indicate that negative charge distribution on the glycocalyx may be uneven, forming heterogeneous microdomains on the luminal cell surface (147, 148). Based on studies from vascular beds of systemic vessels, the glycocalyx has been shown to act as a sieve allowing transendothelial transport of low-molecular-weight solutes, inhibiting red blood cell and neutrophil adhesion and extravasation, and as a mechanotransducer (67, 113). However, characterization of the glycocalyx in the pulmonary microcirculation remained controversial as most of the studies relied on using enzymatic degradation of glycocalyx constituents (24, 31, 35, 37, 45, 71, 102, 130). Recently, Schmidt and coworkers (125) used a model of intravital microscopy in the intact, ventilated mouse (136) to demonstrate that the pulmonary endothelial glycocalyx is ∼1.7 μm thick, i.e., around two to three times the value of the glycocalyx in the systemic circulation. Schmidt and colleagues showed that the glycocalyx is rapidly lost following endotoxemia due to posttranslational activation of heparanase, resulting in exposure of adhesive molecules such as intercellular adhesion molecule-1 (ICAM-1) to circulating neutrophils and their extravasation into the interstitium, leading thereby to inflammatory injury. However, previous studies showed that formyl-methionyl-leucyl-phenylalanine-activated neutrophils trigger shedding of the glycocalyx around the postcapillary venules (97), raising the possibility that shedding of the glycocalyx may be a general phenomenon for regulating transendothelial migration of neutrophils following sepsis.
Neutrophil transmigration across the endothelium and the associated release of cytokines and chemokines are known to disrupt endothelial barrier function (138). Counterintuitively, Schmidt and colleagues (125) showed that loss of glycocalyx following endotoxemia had no effect on protein permeability of the pulmonary vessel wall. In contrast, in 2012 Cioffi and colleagues (24) showed that sialic acids, negative charged carbohydrate moieties in complex sugars such as oligosaccharides and polysaccharides, within the glycocalyx, maintain endothelial barrier function by regulating cell-matrix and cell-cell interaction. They demonstrated that hydrolysis of sialic acid moieties using neuraminidase disrupted cell-cell and cell-matrix adhesions, suggesting that terminal sialic acids promote endothelial barrier integrity. Also, neuraminidase increased microvessel permeability in mice lungs. Similarly, Dull and colleagues (35–37) showed that degradation of heparan sulfate by heparanase in the perfused lungs increased microvessel permeability in response to pressure changes, secondary to induction of endothelial nitric oxide synthase (NOS) activity and actin cytoskeletal rearrangement. How can the above findings by Schmidt on the one side and Cioffi and Dull on the other then be reconciled? Is it because LPS induces heparanase activity in a tightly restricted spatiotemporal manner, limiting detection of endothelial barrier dysfunction?
Additionally, the above studies raise several novel questions that need to be addressed in favor of the idea that glycocalyx is an anti-inflammatory and barrier protective mechanism. 1) Are LPS-induced alterations in heparanase activity mediated through Toll-like receptors (TLR), and if so, which one(s)? 2) Does glycocalyx shedding alter endothelial cell-cell and cell-matrix attachment? 3) Are changes in nitric oxide (NO) levels, as seen by Dull and colleagues, linked to the posttranslational activation of heparanase? 4) Does shedding of the glycocalyx specifically expose endothelial cell surface receptors that maintain barrier homeostasis under basal conditions? Intravital two-photon microscopy and relevant transgenic mice models may prove useful to assess if the glycocalyx interacts with endothelial cell junctions through NO signaling or via the actin cytoskeleton, and whether these linkages play a role in regulating transendothelial leukocyte transmigration in real-time under physiological and pathological conditions.
Endothelial Scaffolds
The interaction between contiguous endothelial cells and their underlying matrix provides the endothelial monolayer with adhesive strength that resists separation of cells from the substratum, a hallmark of barrier disruption. Interendothelial junctions (IEJ) are composed of adherens junctions (AJs), tight junctions (TJs), and gap junctions (GJs), whereas integrin receptors link the cells with the matrix through focal adhesion proteins (87). In contrast to the key role of TJ in the epithelium, endothelial barrier function is predominantly regulated by AJs (48, 104). Recent studies published in the American Journal of Physiology - Lung Cellular and Molecular Physiology showed that focal adhesion kinase (FAK), IQRas GTPase-activating protein-1 (IQGAP-1), and AMP-activated protein kinase (AMPK) have the potential to interfere with barrier disruptive mechanisms, limiting lung injury. On the other hand, prehaptoglobin2, protein kinase C, ezrin, radixin, and moesin (ERM) proteins, or a disintegrin and metalloproteinase (ADAM) family member induces barrier disruption. Below, we compile these findings and pose fundamental questions that can be touted to analyze these signaling in a global manner.
FAK, a nonreceptor protein tyrosine kinase, was initially described as a regulator of cell-matrix attachment in endothelial cells (87). FAK is autophosphorylated on Tyr-397, but it can be further activated by tyrosine phosphorylation at several residues including Y576/577 residues in response to integrin activation induced by cell adhesion, antibody cross-linking, and permeability regulating mediators (124). FAK has a long list of interacting partners. Some of these proteins include the cytoplasmic domain of the β-subunit of integrins, band 4.1 containing proteins – ezrin, radixin, moesin-homology domain (FERM domain), N-WASP, pp60Src, and pp59fyn, growth factor receptor-bound protein 2 (Grb2), Arf (ADP ribosylation factor), GAP containing SH3, Ankyrin repeats and PH domain (ASAP1), and p130 Crk-associated substrate (p130Cas), vinculin, talin, paxillin, 190RhoGEF, and GTPase regulator associated with FAK (GRAF) (50, 87, 108, 139). Several studies showed that FAK through N-WASP and p120-catenin interacts with AJs and reanneals them by dampening endothelial contraction (65, 75, 88, 118). FAK also promotes endothelial cell survival during cytotoxic stress (10, 60). RhoA is the major regulator of actin-myosin-induced contraction in endothelial cells and thereby is a key determinant of increased endothelial permeability (20, 25, 56, 60, 66, 83, 111, 119, 153, 163). However, studies also showed that dominant negative FAK or a kinase dead FAK mutant prevented AJ disruption in response to VEGF or oxidants indicating that FAK in fact disrupts barrier function (19, 116, 144). Thus, Schmidt et al. (125) used mice where FAK deletion can be conditionally induced in an endothelial cell-specific manner to assess the role of FAK in regulating acute lung injury (ALI). These authors showed that onset of ALI by ip administration of lipopolysaccharide (LPS) or cecal ligation and puncture markedly decreased FAK expression in mice lungs. Conditional deletion of FAK increased transvascular albumin influx, edema, and neutrophil accumulation in the lung. Hyperactivation of RhoA due to increased p115RhoGEF binding with RhoA was shown to be responsible for these effects because activated RhoA suppressed Rac1 activity, which maintains AJs and inhibition of Rho kinase, a downstream effector of RhoA, reinstated normal endothelial barrier function in EC-FAK null mice.
IQGAP-1 can interact with several proteins including polymerized actin, activated small GTPases such as Rac and Cdc42, β-catenin, calmodulin, and microtubule-associated cytoplasmic linker integral protein-170 (87). Using IQGAP1 null mice, Bhattacharya and colleagues (12) showed that IQGAP1 is required for maintaining barrier function in endothelial cells. They demonstrated that endotoxin as well as live bacteria induced fulminant edema formation in IQGAP1 null mice. IQGAP1 functioned by using αvβ3-integrin as a new partner, which induces cortical actin formation thus strengthening AJs. Additionally, they showed that extracellular S1P, which ligates S1P receptor 1, recruits IQGAP1 at the AJs for inducing barrier enhancement by S1P. Previous studies showed that intracellular generation of S1P can also strengthen AJs by recruiting IQGAP1 and Rac1 to AJs (143). Similarly, Ang1 was shown to require IQGAP1 for inducing Rac1 activity and for promoting barrier defense (30). While these studies favor the idea that IQGAP1 induces AJs by promoting signaling through barrier protective agents such as Ang1 and S1P, depletion of IQGAP1 did not augment the barrier disruptive effect of thrombin in endothelial cells (30).
AMPK, a serine threonine kinase, regulates metabolic as well as anti-inflammatory functions (106, 126). AMPK was shown to suppress TLR4-dependent activation of nuclear factor NF-κB and the subsequent generation of cytokines (121, 137) and facilitated phagocytosis and clearance of apoptotic cells (107, 162), indicating that AMPK contributes to the resolution of inflammation. Creighton's laboratory showed that activation of AMPK with N1-(α-d-ribofuranosyl)-5-aminoimidizole-4-carboxamide (AICAR) or metformin, a clinically approved antidiabetic drug, resealed wounds in LPS-exposed rat pulmonary microvascular endothelial cells. AICAR interacts with the γ-subunit of AMPK, resulting in phosphorylation of the catalytic α-subunit, thereby inducing AMPK activity. In line with this notion, AICAR or metformin failed to induce barrier repair in AMPK-α1 depleted endothelial cells, indicating these effects required AMPK-α1. Also, AICAR and metformin both blocked LPS-induced increases in microvessel permeability and sepsis induced mortality (70).
Zonula occludens (ZO) proteins are family members of membrane-associated guanylate kinases (53), which form TJs and also allow TJs proteins to interact with AJ proteins such as α-catenin, GJ proteins such as connexin (Cx)-43, and actin polymerizing proteins such as vasodilator-stimulated phosphoprotein and spectrin (27, 40, 69, 98, 140). ZO-1 may thereby regulate endothelial permeability by serving as a scaffold for mediating the interaction of TJs with AJs and connexin. Prehaptoglobin2 has been identified as zonulin and is related to serine proteases (MASPs, C1qrs) that activate the complement system (120). A zonulin antagonist (AT-1001) in a dose-dependent manner, or zonulin neutralizing antibody, attenuated the intensity of ALI (as quantitated by albumin leak, neutrophil accumulation, and proinflammatory cytokines). Human immunodeficiency virus (HIV)-1 transgene expression also increased paracellular permeability of alveolar epithelial monolayers by decreasing the expression of ZO-1 expression. Interestingly, upregulating nuclear factor (erythroid-derived 2)-like 2 (Nrf2 or NFE2L2) (Nrf2) activity restored ZO1 expression normalizing barrier function. Nrf2 is a transcription factor constitutively expressed in all tissues and promotes cytoprotection by activating many proteins regulating metabolism of drugs and toxins, the protection against oxidative stresses and inflammation, the stability of proteins, and the removal of damaged proteins (149). However, whether AT-1001 or zonulin neutralizing antibody prevented ALI by augmenting Nrf2 activity remains unclear.
The protein kinase C (PKC) family consists of isoforms with different Ca2+ and phorbol ester requirements for activation: conventional (α, β, δ), novel (ϵ, γ), and atypical (ζ, υ) (85, 128). PKCα has a crucial role in mediating IEJ disassembly either by phosphorylating IEJ components or through inducing the RhoA pathway (76, 78, 96). Ca2+-independent isoforms, notably PKCζ, may also be important in IEJ disruption, which predominantly seems to require RhoA-induced signaling (91). PKCδ was shown to regulate focal adhesions and RhoA activity (57) as well as to induce NO generation via stimulating Akt activity (133). While some studies showed that PKCδ maintains basal barrier function, another showed that inhibition of PKCδ prevented phorbol ester-mediated barrier dysfunction (57). However, mice lacking PKCδ did not show any vascular leak in the lung under basal conditions and, intriguingly, resisted LPS-induced lung injury, indicating PKCδ also disrupt endothelial barrier function (22). Whether other PKC isoforms compensated for inducing barrier disruption in PKCδ null mice remains to be parsed out.
Adyshev and colleagues (3) showed that upon activation by thrombin receptors PKC also induces the phosphorylation of ERM proteins at canonical threonine residues (ezrin Thr567, radixin Thr564, moesin Thr558). Threonine phosphorylation within the COOH terminus unfolds the ERMs, leading to their activation and translocation to the periphery of endothelial cells (26). Activated ERMs cross-link actin and thereby play an important role in actin cytoskeletal remodeling. ERMs also act as a scaffold and interact with Rho-GDI-1, Gα13 protein, and FAK, which may modulate RhoA signaling and AJ function (26). Members of the stress-inducible small heat shock protein family (HSP) also regulate actin polymerization in vitro (164). Interestingly, depletion of moesin but not radixin markedly attenuated thrombin-induced increases in endothelial barrier permeability, cytoskeletal rearrangements, paracellular gap formation, and accumulation of phospho-myosin light chain. Inhibitors of Hsp90 also prevented LPS-induced lung endothelial barrier dysfunction by suppressing Src-mediated RhoA activity and signaling (18). In a recent study, Barabutis and coworkers (9) showed that LPS causes phosphorylation of HSP90 on the tyrosine Y309 residue in a Src-dependent manner. Expression of a nonphosphorylatable Hsp90 mutant reduced LPS-induced barrier dysfunction indicating HSP90 inhibition.
ADAM, single pass transmembrane glycoproteins, regulate several cellular functions in the lung vasculature and also regulate lung saccular formation by cleaving the ectodomains of cell surface protein and downstream signaling events (33, 154, 155). Several ADAMs such as ADAM 8, 9, 12, 15, and 19 have been shown to alter the expression of several vascular receptors and most of the time can cleave the same substrate (33). For example, ADAM 8, 10, 17, and 19 can cleave TNF-α. Loss of ADAM 17 in endothelial cells prevented inflammatory lung injury due to reduced shedding of JAM-A, a constituent of TJs (33). In contrast, leukocyte-expressed ADAM 10 but not ADAM 17 displays proinflammatory activities and may therefore serve as a target to limit inflammatory cell recruitment (114). Sun and colleagues (134) showed that ADAM 15 is activated during lung injury and disrupts endothelial barrier function and induces neutrophil infiltration. Therefore, LPS failed to induce inflammatory injury in ADAM 15 knockout mice. Rescuing ADAM 15 expression restored a leaky vascular phenotype and neutrophilic injury. However, the mechanistic basis of how LPS activated ADAM 15 and how ADAM 15 altered barrier function remains unclear. It is also unclear whether ADAM 15 acts, at least in part, via regulating ADAM 17 activity.
Transmembrane clusters of connexins form intercellular channels with a pore size of ∼2 nm diameter between two endothelial cells known as GJs (19, 54, 79). These channels mediate rapid propagation of transmembrane potential and second messengers (e.g., Ca2+, IP3) between cells (68, 74, 93). Endothelial cells express several connexins including Cx40 and Cx43, which may organize to form either homo- or heterohexameric pores (82). Endothelial-specific deletion of Cx43 caused hypotension resulting from a marked elevation of plasma NO level (81). While deletion of Cx37 or Cx40 in mice alone did not induce any apparent vascular effects, mice lacking both Cx37 and Cx40 died perinatally and demonstrated gross vascular abnormalities, most prominently hemorrhaging in several tissues (129). Cx43-mediated GJs form conduits for the spread of proinflammatory signals in the lung capillary bed (110). Parthasarthi (109) showed that in the lung microvasculature connexins transduce alveolar-capillary damage to distant sites as well as at the injury site. Using a micropuncture technique to locally instill hydrochloric acid along with rhodamine-dextran 70 kDa (RDx70) and FITC-dextran 20 kDa (FDx20) he showed that acid decreased the peak postwash fluorescence of FDx20 both in microvessels at the infusion site of alveoli and also those located >700 μm away. However, acid infusion did not have any effect on this ratio if high-molecular-weight FITC-dextran 70 kDa was infused. Inhibition of endothelial Cx43 function using Gap27 blunted the acid-induced decrease in FDx20 ratio not only in microvessels away from the site of injury, but also in those abutting directly injured alveoli, which is known to regulate Cx function (87). Whether acid-induced connexin phosphorylation was a factor in transmitting the signal to the distant sites and whether it required alteration in AJ and TJ function remain to be elucidated.
The studies outlined above highlight that FAK, IQGAP, and AMPK1 induce reannealing of endothelial junctions but also identify several gaps in our understanding of barrier protective and barrier disruptive mechanisms. 1) Do FAK, IQGAP, and AMPK1 act synergistically or in parallel to maintain barrier function? 2) How is FAK, IQGAP, and AMPK1 function and/or expression altered during injury? 3) Is Rac1 a common effector of FAK, IQGAP, and AMPK1? 4) Can AICAR and metformin induce IQGAP and FAK activity to maintain endothelial barrier function? 5) Can dietary supplementation with sulforaphane, a naturally occurring isothiocyanate that inhibits cellular apoptosis and oxidative stress in part through activating Nfr2 (38), augment the function of FAK, IQGAP, and AMPK1? 6) Do barrier disruptive signals, such as Hsp70 and moesin, interfere with Rac1 induction of actin remodeling by FAK and IQGAP? Or do they rather counteract FAK inhibition of RhoA activity and if so, how? 7) Do ADAM 15 and PKC disrupt barrier integrity by interfering with these barrier stabilizing mechanisms?
MEDIATORS REGULATING ENDOTHELIAL BARRIER FUNCTION
While traumatic, infectious, or inflammatory insults have long been recognized as primary triggers of lung vascular barrier failure, the rise of new chemical and biological challenges such as the current Ebola epidemic has broadened the spectrum of potential causes. Below, we therefore discuss triggers and mediators that can cause barrier failure, as well as novel mediators and drugs that can enhance barrier maintenance and/or restoration.
Regulation of Endothelial Barrier by Mechanical Stretch
Mechanical forces imposed by circulating blood and respiratory cycles activate numerous signals including stretch-activated ion channels and calcium influx, secretory group V phospholipase A2 (gVPLA2), small GTPases, Rho kinase, selectin expression, mitogen-activated kinases, and FAK (2, 4, 14, 43, 89, 94, 159), which may impact lung vascular barrier function. Critically ill patients with ALI are supported by mechanical ventilation with tidal volumes that typically range from 6 to 12 ml/kg body wt. However, it has become apparent that high-tidal-volume ventilator support leads to ventilator-induced lung injury, multiorgan dysfunction, and early mortality (158). Birukokva and coworkers (15) showed that mechanical stretch in the phase of on-going endothelial barrier injury induces RhoA signaling via interleukin-6 receptors that otherwise induce Jak and p38 MAP kinase and NF-κB signaling. NO, induced during pressure-induced shedding of glycocalyx by heparanase, is known to cause nitration of proteins including RhoA, which activates RhoA signaling (72, 117). Thus a number of questions remain unanswered. Does activation of heparanase induce RhoA GEFs to induce RhoA signaling? How does RhoA and p38 kinase signaling overlap? How do alterations in heparan sulfates influence the activity of endothelial mechanical sensors such as transient receptor potential vanilloid 4 (TRPV4) and platelet endothelial cell adhesion molecule? How does heparan sulfate communicate with sialic acid linkages during static vs. mechanical perturbations to maintain cell-cell and cell-matrix interaction and thereby endothelial permeability? Is restoration of heparan sulfates and sialic acid a viable means to limit ALI?
Elevation in arterial carbon dioxide tension (acidosis) during protective lung ventilation reduces ischemic lung injury and preserves lung compliance, but it may induce immunosuppression and worsen infection in sepsis (115, 158). For this and other reasons, buffering acidosis with tris-hydroxymethyl aminomethane but not sodium bicarbonate is recommended in patients with hemodynamic instability. Sayner and colleagues (101, 123) showed that bicarbonate in a dose-dependent manner decreased resistance across the pulmonary microvascular endothelial cell monolayer. Furthermore, perfusion of mice lungs ex vivo with bicarbonate increased vascular permeability while increasing osmolality had no effect. These authors showed that bicarbonate can stimulate mammalian soluble adenylyl cyclase (AC) isoform 10 to generate cytosolic cAMP. While transmembrane AC activity is pulmonary endothelial barrier protective, cytosolic AC activity of bacterial toxins is endothelial barrier disruptive, leading to pulmonary edema (123). Whether bicarbonate may concomitantly compromise barrier integrity and promote lung edema formation by altering glycocalyx components or RhoA signaling remains to be addressed.
Mediators Disrupting the Endothelial Barrier
Chlorine gas (Cl2) is a reactive gas that is considered a chemical threat agent, but exposure may equally result from chemical disasters, such as railway spills, or passive exposure, such as inhalation of disinfectants. Upon inhalation, Cl2 gas causes ALI and occasionally chronic airway fibrosis (92). According to the Office of The Surgeon General, United States Army, Cl2 gas exposure in World War I was also frequently followed by bacterial superinfections, suggesting a potentially increased susceptibility to opportunistic infections postexposure (142). Indeed, as recently demonstrated by Gessner and colleagues (49), mice exposed to Cl2 demonstrate a >500-fold higher fungal burden following experimental inoculation with Aspergillus fumigatus. This increased susceptibility to opportunistic pathogens is associated with an increased permeability to plasma proteins, as evidenced by increased albumin and IgG levels in the bronchoalveolar lavage fluid, thus establishing a potentially vicious circle of endothelial barrier failure, leukocyte emigration, and microbial infection. It is important to note that opportunistic infections in this study were associated with enhanced recruitment of inflammatory cells into the lung, but the cellular infiltration failed to generate microbicidal superoxide. This finding highlights the dual role of oxidative stress in lung infection and vascular barrier function, in that reactive oxygen species (ROS) are essential to prevent alveolar pathogen invasion yet concomitantly themselves promote lung vascular inflammation and barrier failure. A recent study by Menden and colleagues (90) highlights this idea: These authors show that bacterial LPS induced an increase in endothelial ICAM-1 expression that was mediated by superoxide formation from NADPH oxidase (NOX) 2, subsequent phosphorylation of transforming growth factor-β-associated kinase 1 and inhibitor of κ-B kinase-β, and ultimately, activation of NF-κB. Increased susceptibility to oxidative stress also underlies alveolo-capillary barrier failure in HIV-1 infection, a finding that is of particular relevance in view of the fact that pulmonary complications constitute the leading cause of death in HIV-1-infected individuals. In HIV-1 transducing transgenic rats, Fan and colleagues (39) show that HIV-1-related viral proteins downregulate Nrf2, the key transcription factor that regulates a number of genes that comprise the antioxidant response element, thus effectively impairing lung antioxidant defenses and barrier function. It is noteworthy that the detrimental effects of oxidative stress on endothelial barrier function are further amplified by its concomitant effects on alveolar fluid clearance, an active epithelial mechanism that maintains alveolar fluid homeostasis via an ion transport-driven absorption of fluid from the distal air spaces. In a variety of experimental or clinical conditions associated with impaired lung barrier function and increased oxidative stress such as e.g., circulatory shock or bacteremia, alveolar fluid clearance is significantly impaired, thus contributing to impaired gas exchange and presumably, higher mortality (63, 160). It could therefore be speculated that ROS may play a critical role in inhibiting alveolar fluid clearance; however, it turns out that in vivo, ROS have the capacity to stimulate lung fluid clearance by increasing the activity of the epithelial sodium channel ENaC, the main driving force for alveolar fluid absorption (55). The opposing effects of ROS on invading pathogens, barrier integrity and alveolar fluid absorption, give rise to a characteristic U-shaped curve indicating that ROS level have to be uniquely titrated in dose, time, and space to prevent detrimental effects. Despite documented effectiveness of antioxidants such as N-acetylcysteine (NAC) to confer protection against ALI and lung edema in e.g., preclinical models of sepsis (17), the Janus-faced role of ROS in lung injury and barrier function has likely been the main reason why NAC has failed to improve outcome of acute respiratory distress syndrome (ARDS) patients in clinical trials (32). A recent preclinical study has now proposed an alternative and potentially, more promising antioxidative strategy by vitamin C supplementation (44). Ascorbic acid had previously been shown to reduce macromolecular permeability in cultured human umbilical vein endothelial cells (HUVECs) (145). Fisher and colleagues (44) now show that parenteral infusion of ascorbic acid in mice with experimentally induced abdominal peritonitis increases survival and reduces histological signs of lung injury, the expression of proinflammatory cytokines, and the extravasation of fluid and macromolecules in the lung. Notably, these effects were accompanied by an increased expression of key molecules involved in alveolar fluid absorption such as ENaC, the cystic fibrosis transmembrane conductance regulator CFTR, and the Na+-K+-ATPase and consequently, by an augmentation in alveolar fluid clearance. The molecular mechanisms that determine why inhibition of ROS production from NOX2 (55) attenuates fluid clearance in naïve mice, while the antioxidant ascorbic acid promotes it in lung injury (44) remain to be determined but likely reflect again the U-shaped homeostatic regulation that is characteristic for ROS. On this background, efforts to utilize nonselective antioxidants or inhibitors of ROS production seem hopelessly blunt and likely to fail unless we succeed in realizing and controlling both temporal and spatial targeting of these drugs.
In experimental lung injury and its clinical correlate, the ARDS, lung vascular barrier failure, the invasion of immune cells, and the activation of inflammatory signaling pathways typically go hand in hand. The relevance of immune cells as important regulators of lung vascular barrier function is highlighted by the recent observation that inhibition of leukocyte-endothelial interaction in lung capillaries and venules by immunoneutralization of the adhesion molecule CD162 not only reduces the accumulation of neutrophils but also attenuates lung edema in murine models of lung injury that was triggered by intravenous injection of streptococcal M1 protein (161). Not only inflammatory cells, but also microparticles released from neutrophils, platelets, or lymphocytes, can increase endothelial permeability and trigger lung vascular barrier failure. Such microparticles are extracellular vesicles of 50 nm up to 1 μm in size with a lipid bilayer that can act as intercellular carriers for multiple membrane and cytosolic proteins, organelles, lipids, and RNA from their respective parental cells to various target cells (86). As such, microparticles can shuttle, e.g., short-lived barrier-disruptive lipid mediators such as thromboxane A2 or platelet-activating factor to endothelial cells and, thus, promote barrier failure (86). Conversely, endothelial activation and expression of adhesion molecules are essential prerequisites for neutrophil invasion and emigration. For example, endothelial knock-down or inhibition of calpains, a family of Ca2+-dependent, nonlysosomal cysteine proteases, reduces endothelial NO synthase (NOS3)-mediated NO production, subsequent phosphorylation of ICAM-1, and thereby, neutrophil recruitment as well as lung edema and protein extravasation (80). Similarly, deficiency in the polymodal cation channel TRPV4, which is highly expressed in pulmonary endothelial (95) and smooth muscle cells (29, 157) but seemingly absent in neutrophils (8), prevented both edema formation and neutrophil invasion in murine models of chlorine gas- and acid aspiration-induced lung injury (8). However, in conditions where stimuli and/or inhibitors act in parallel on both endothelial and immune cells, cause and effect in the interaction between these cell types are often hard to differentiate. For example, the prehaptoglobin zonulin acts both as a modulator of tight junctions and as an activator of the complement system. In a recent study, Rittirsch and colleagues (120) could show that a zonulin antagonist or a zonulin neutralizing antibody both effectively attenuate albumin leak and neutrophil accumulation in two different models of ALI; however, given the duality of zonulin's action, it is virtually impossible to dissect the individual roles of immune and endothelial cells in such an in vivo scenario. An abundant literature has documented that triggers such as thrombin, H2O2, or LPS can increase endothelial permeability in vitro in the absence of immune cells. Recent data by Leonard and colleagues (80) demonstrate that the underlying dynamic regulation of the actin cytoskeleton not only mediates the endothelial permeability response to thrombin but also modulates in turn proinflammatory signaling pathways including NF-κB. Modulation of actin filament stability through phosphorylation or dephosphorylation of the actin binding protein cofilin regulates not only endothelial barrier properties but also NF-κB activity and expression of its target genes such as ICAM-1. While these data provide compelling evidence for a mechanism by which endothelial barrier leak may precede the recruitment of immune cells, the situation is somewhat more complex in the in vivo scenario, where for reasons that are so far poorly understood many of the above-mentioned triggers of endothelial permeability in vitro fail to cause vascular leak in the absence of immune cells (141). Our understanding of the intricate interplay between immune and endothelial cells in the regulation of both neutrophil extravasation and endothelial barrier regulation is rudimentary at best, and we currently lack any approach that would allow us to target one yet not the other in an in vivo setting. We are in dire need, however, of such strategies, as they would potentially enable us to protect the lung vascular barrier while simultaneously allowing for extravasation of immunocompetent cells into the alveolar and interstitial space to fight off invading pathogens.
Strategies to Enhance Lung Endothelial Barrier Function
Regardless of the emerging challenge to counterbalance barrier protection versus antimicrobial defense in the alveolar compartment, a series of novel experimental strategies and therapeutic targets have recently emerged that present promising perspectives as potential stabilizers of the lung endothelial barrier. Aside from the classic mediators S1P and angiopoietin-1, the neurotransmitter dopamine has recently emerged as a putative barrier stabilizer. Dopamine had previously been shown to inhibit barrier disruptive effects of VEGF in HUVECs by inhibiting the phosphorylation of VE-cadherin and ZO-1 (13). Recently, Vohra and coworkers (150) used this strategy now as a pretreatment in a murine model of LPS-induced lung injury and could demonstrate that dopamine increased survival and decreased lung edema, protein extravasation, and pulmonary infiltration of neutrophils. Similar to previous reports in HUVECs, these effects seem to be mediated via the VEGF-VEGF receptor axis, in that dopamine pretreatment reduced circulating VEGF levels in serum and attenuated phosphorylation of the VEGF receptor 2. As dopamine also increases transepithelial fluid flux in type II pneumocytes via translocation of the basolateral Na+-K+-ATPase in a MAPK/ERK-dependent manner (58), dopamine treatment may counteract lung edema formation at both the vascular endothelial and alveolar epithelial level. Like dopamine, the arachidonate metabolite prostaglandin E2 (PGE2) had previously been reported to prevent increases in endothelial permeability in vitro through its ability to activate Rac in a cAMP/PKA-dependent manner (16, 41). However, as PGE2 signals through any of four different receptors (EP1–EP4), which all belong to the family of G protein-coupled receptors, the biological effects of PGE2 become heavily dependent on relative expression of EP receptor subtypes. In isolated lungs, PGE2 can in fact contribute to barrier failure via activation of EP3, but not EP1, EP2, or EP4 receptors (51). Conversely, selective activation of EP4 in human pulmonary microvascular endothelial cells stimulates the production of the proinflammatory chemokine IL-8 in a p38-dependent manner (7), a mechanism that may contribute to neutrophil recruitment and secondary endothelial barrier failure in ARDS. In line with this notion, nimesulide, an inhibitor of prostaglandin H synthase-2, which is upstream of PGE2 synthesis, was recently shown to inhibit LPS-induced increases in both PGE2 and fetal lung IL-8 mRNA (151). Thus, while prostanoids such as PGE2 present interesting candidates for barrier modulation, these findings underline the need for agonists and/or antagonists that act more specifically on individual receptors. A similar case for higher therapeutic receptor specificity can be built for S1P, which signals through its receptors S1P1–S1P5, of which S1P1–S1P3 and potentially S1P4 are expressed in the pulmonary vasculature. S1P, by acting through its receptor S1P1, has long been recognized for its barrier-stabilizing effects that involve activation of Rac and result in a rearrangement of the endothelial cytoskeleton to form strong cortical actin rings in the cell periphery and enhanced cell-to-cell and cell-to-matrix tethering dynamics (1). However, activation of S1P2 increases endothelial leak in cultured HUVECs and in isolated perfused lungs stimulated with hydrogen peroxide (122), and single nucleotide polymorphisms (SNPs) in S1P3 were recently found to be associated with an increased risk for sepsis-associated ARDS (135). Conversely to these dichotomous effects of PGE2 and S1P and therefore, of particular therapeutic interest, the effects of adenosine in the lung seem rather uniformly beneficial, at least in the setting of ARDS. Adenosine acts through four receptors, adenosine receptors A1, A2A, A2B, and A3, of which A3 is least expressed in endothelial cells (61). Ngamsri et al. (99) had previously shown A1AR to regulate neutrophil influx and microvascular permeability in lung injury, in that A1AR-deficient mice had increased, while mice treated with a specific A1AR agonist had decreased lung inflammation and edema following LPS challenge. More recently, Konrad et al. (77), from the same laboratory of Jörg Reutershan, showed similar effects for the A2b receptor, in that A2b-deficient mice showed more LPS-induced neutrophil infiltration and microvascular leakage, while a specific A2b receptor agonist decreased these effects. Generation of chimeric mice further revealed that A2b receptor expression on hematopoietic cells was critical for polymorphonuclear leukocyte migration, while LPS-induced microvascular leakage was under the control of A2b on both hematopoietic and nonhematopoietic cells. Activation of the A2a receptor furthermore accelerates the resolution of alveolar edema, an effect that could be blocked by inhibition of epithelial ENaC, but not CFTR channels (46). Hence, adenosine as well as single, dual, or triple adenosine receptor agonists may indeed present a powerful new avenue for the treatment of ALI and lung endothelial barrier failure. However, in view of recent findings that sustained elevations of adenosine levels may contribute to cigarette smoke-induced lung endothelial apoptosis and emphysema formation (83), such interventions should probably be restricted to the acute exudative phase in lung edema.
Another promising strategy for endothelial barrier enhancement builds on the well-recognized barrier-stabilizing effects of subplasma membrane cAMP generated by transmembrane adenylate cyclase (131). In a murine model of ALI secondary to polymicrobial sepsis, Oishi and colleagues (103) tested the effectiveness of two different approaches to increase endothelial cAMP levels, either by enhancing cAMP generation by the water-soluble adenylate cyclase stimulator colforsin or by attenuating cAMP degradation by the specific phosphodiesterase III inhibitor, olprinone. Treatment with both agents prevented the onset of ALI through a mechanism that involved activation of Akt, but not the cyclic AMP response element binding protein CREB, as inhibition of the Akt upstream regulator phosphatidylinositol 3-kinase, yet not a CREB decoy oligodeoxynucleotide, blocked the effects of both compounds. Finally, gaseous transmitters and inhaled gases have received great interest as potential therapeutic avenues to alleviate lung injury and restore endothelial barrier function. While sobering results from a series of clinical trials failed to show significant improvements in clinical outcome in ARDS patients treated with inhaled NO, preclinical evidence is accumulating for potential protective roles of carbon monoxide, hydrogen sulfide, or hydrogen gas. In a recent study, Fernandez-Gonzalez and colleagues (42) could now show that constitutive lung-specific overexpression of the carbon monoxide-generating enzyme heme oxygenase 1 (HO-1) confers vasculoprotective effects in a murine model of hyperoxia-induced bronchopulmonary dysplasia, in that pulmonary inflammation, arterial remodeling, and pulmonary edema were markedly improved. In adult lungs, Kawamura and colleagues (73) found the detrimental effects of hyperoxia on lung injury, inflammation, and edema to be markedly alleviated in animals inhaling 2% hydrogen, and this effect was associated with an increased expression of HO-1. Notably, protection by hydrogen gas inhalation was alleviated in mice deficient in the main transcription factor for antioxidative genes, Nrf2, which regulates HO-1 expression, suggesting that hydrogen gas alleviates hyperoxic lung injury through induction of Nrf2-dependent genes, such as HO-1.
CONCLUSION
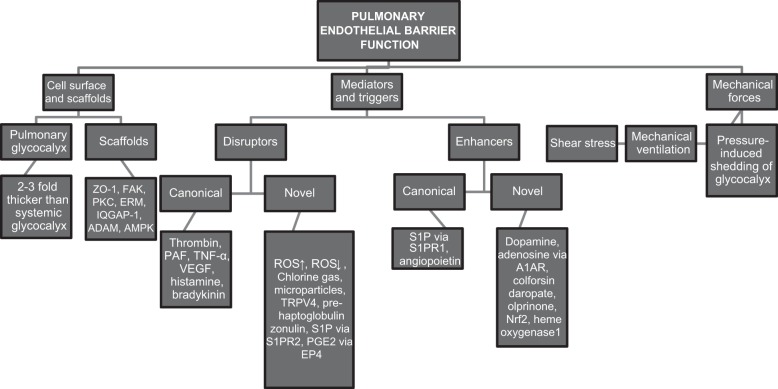
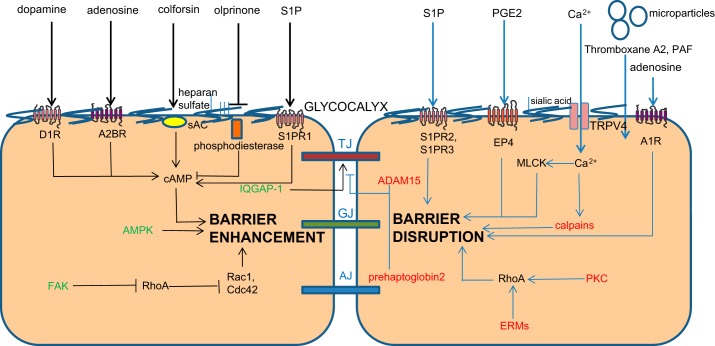
As summarized in Figs. 1 and 2, great strides have been made in our understanding of signaling mechanisms and mediators that regulate endothelial permeability. However, the emerging overall picture of endothelial barrier regulation has several fundamental gaps that need to be filled systematically to foster the development of new therapeutic targets and drugs for the treatment of ARDS. A combinatorial, parallel strategy at the cellular, tissue, and organ level using key barrier-disrupting and barrier-stabilizing mediators that induce acute or chronic changes in barrier properties may inform us better about “signaling nodes” that can be targeted than single hit studies in individual models (Figs. 1 and 2). These nodes can then be verified by integration of novel technologies such as intravital microscopy in conditional mice along with gene editing tools and epigenetics assessment. These types of integrative studies can be expected to significantly advance our understanding of the mechanisms regulating microvessel endothelial permeability in the normal state and during inflammation. For example, suppression of RhoA emerges as a central player in various scenarios of endothelial barrier disruption. The real challenge that emerges is whether we will be able to identify strategies to target, e.g., RhoA in the endothelium only to such a degree that it prevents disruption of AJs following injury, without interfering with basal barrier formation and functions including the emigration of immune cells to sites of injury and inflammation? Similarly, can we modulate ROS production in a way that we maintain cellular and physiological homeostasis at the alveolo-capillary membrane yet prevent the detrimental effects of excessively high (or low) ROS levels? And along these lines, how safe are barrier-protective strategies such as metformin, AICAR, sulforaphane, dopamine, adenosine, colforsine, or olprinone in scenarios of, e.g., pathogen invasion? The regulation of endothelial permeability and lung vascular barrier homeostasis turns out to be much more complex than often anticipated, and, as a result, so is the development of novel and safe therapeutic strategies.
Fig. 1.
Critical determinants of pulmonary endothelial barrier function, both previously established and newly identified, as outlined in this perspective. ZO-1, zona occludens protein 1; FAK, focal adhesion kinase; PKC, protein kinase C; ERM, ezrin radixin and moesin proteins; IQGAP-1, IQRas GTPase-activating protein 1; ADAM, a disintegrin and metalloproteinase; AMPK, adenosine monophosphate-activated protein kinase; PAF, platelet-activating factor; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; ROS, reactive oxygen species; Nrf2, nuclear factor (erythroid-derived)-like 2; TRPV4, transient receptor potential vanilloid 4; S1P, sphingosine 1 phosphate; S1PR1, S1P receptor 1; S1PR2, S1P receptor 2; PGE2, prostaglandin E2.
Fig. 2.
Schematic representation of endothelial barrier function, including interendothelial junctions, novel mediators and intracellular signaling molecules. sAC, soluble adenylyl cyclase; D1R, D1 dopamine receptor; A2BR, adenosine A2b receptor; A1R, adenosine A1 receptor; S1PR3, S1P receptor 3, MLCK, myosin light chain kinase; cAMP, cyclic adenosine monophosphate. For other abbreviations see Fig. 1 legend.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-71794, HL-84153, and HL-060678 to D. Mehta and Canadian Institutes of Health Research Grants 93584, 114925, 123417; the Canadian Blood Services Grant CIHR-TRA201403-WK-325399; the German Research Foundation Grant KU1218/4, KU1218/5, and KU1218/7; and the Heart & Stroke Foundation of Canada Grant G-13-0002755 to W. M. Kuebler.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.M., K.R., and W.M.K. drafted manuscript; D.M., K.R., and W.M.K. edited and revised manuscript; D.M., K.R., and W.M.K. approved final version of manuscript.
REFERENCES
- 1.Abbasi T, Garcia JG. Sphingolipids in lung endothelial biology and regulation of vascular integrity. Hand Exp Pharmacol 216: 201–226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdulnour REE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE, Kayyali US, Garcia JGN, Tuder RM, Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291: L345–L353, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Adyshev DM, Dudek SM, Moldobaeva N, Kim K, Ma SF, Kasa A, Garcia JGN, Verin AD. Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am J Physiol Lung Cell Mol Physiol 305: L240–L255, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali MH, Mungai PT, Schumacker PT. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol 291: L38–L45, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol Lung Cell Mol Physiol 277: L1057–L1065, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol 148: 203–216, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aso H, Ito S, Mori A, Morioka M, Suganuma N, Kondo M, Hasegawa Y. Prostaglandin E2 enhances interleukin-8 production via EP4 receptor in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 302: L266–L273, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thornloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barabutis N, Handa V, Dimitropoulou C, Rafikov R, Snead C, Kumar S, Joshi A, Thangjam G, Fulton D, Black SM, Patel V, Catravas JD. LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am J Physiol Lung Cell Mol Physiol 304: L883–L893, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellas RE, Harrington EO, Sheahan KL, Newton J, Marcus C, Rounds S. FAK blunts adenosine-homocysteine-induced endothelial cell apoptosis: requirement for PI 3-kinase. Am J Physiol Lung Cell Mol Physiol 282: L1135–L1142, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 12: 1286–1293, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A, Burlingame AL, Matthay MA, Sheppard D. IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia. Am J Physiol Lung Cell Mol Physiol 303: L12–L19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya R, Sinha S, Yang SP, Patra C, Dutta S, Wang E, Mukhopadhyay D. The neurotransmitter dopamine modulates vascular permeability in the endothelium. J Mol Signal 3: 14, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukova AA, Tian Y, Meliton A, Leff A, Wu T, Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol 302: L965–L975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA-and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos R, Shimizu MHM, Volpini RA, de Bragança AC, Andrade L, dos Santos FDTQ, Seguro AC. N-acetylcysteine prevents pulmonary edema and acute kidney injury in rats with sepsis submitted to mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 302: L640–L650, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD. Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol 294: L755–L763, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S, Baeter S, Bhattacharya J. Endothelial and epithelial signaling in the lung. Am J Physiol Lung Cell Mol Physiol 293: L517–L519, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Pendyala S, Natarajan V, Garcia JGN, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol 295: L575–L583, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. VEGF-induced vascular permeability is mediated by FAK. Dev Cell 22: 146–157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chichger H, Grinnell KL, Casserly B, Chung CS, Braza J, Lomas-Neira J, Ayala A, Rounds S, Klinger JR, Harrington EO. Genetic disruption of protein kinase Cδ reduces endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 303: L880–L888, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung HT, Pae HO, Cha YN. Role of heme oxygenase-1 in vascular disease. Curr Pharm Des 14: 422–428, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Cioffi DL, Pandey S, Alvarez DF, Cioffi EA. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am J Physiol Lung Cell Mol Physiol 302: L1067–L1077, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288: L294–L306, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Clucas J, Valderrama F. ERM proteins in cancer progression. J Cell Sci 127: 267–275, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 16: 583–585, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Cordingley JJ, Keogh BF. The pulmonary physician in critical care. 8: Ventilatory management of ALI/ARDS. Thorax 57: 729–734, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahan D, Ducret T, Quignard JF, Marthan R, Savineau JP, Estève E. Implication of the ryanodine receptor in TRPV4-induced calcium response in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 303: L824–L833, 2012. [DOI] [PubMed] [Google Scholar]
- 30.David S, Ghosh CC, Mukherjee A, Parikh SM. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol 31: 2643–2652, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desjardins C, Duling BR. Heparinase treatment suggest a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol Heart Circ Physiol 258: H647–H654, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Domenighetti G, Suter PM, Schaller MD, Ritz R, Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care 12: 177–182, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Dreymueller D, Martin C, Kogel T, Pruessmeyer J, Hess FM, Horiuchi K, Uhlig S, Ludwig A. Lung endothelial ADAM17 regulates the acute inflammatory response to lipopolysaccharide. EMBO Mol Med 4: 412–423, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117: 231–41, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dull RO, Cluff M, Kingston J, Hill D, Chen H, Hoehne S, Malleske DT, Kaur R. Lung heparin sulfates modulate K(fc) during increased vascular pressure: evidence for glycocalyx-mediated mechanotransduction. Am J Physiol Lung Cell Mol Physiol 302: L816–L828, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, Garcia JGN. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol 285: L986–L995, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Dull RO, Mecham I, McJames S. Heparan sulfates mediate pressure-induced increase in lung endothelial hydraulic conductivity via nitric oxide/reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 292: L816–L828, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Elhalem E, Recio R, Werner S, Lieder F, Calderón-Montaño JM, López-Lázaro M, Fernández I, Khiar N. Sulforaphane homologues: Enantiodivergent synthesis of both enantiomers, activation of the Nrf2 transcription factor and selective cytotoxic activity. Eur J Med Chem 87C: 552–563, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Fan X, Staitieh BS, Jensen JS, Mould KJ, Greenberg JA, Joshi PC, Koval M, Guidot DM. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am J Physiol Lung Cell Mol Physiol 305: L267–L277, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Farmer PJ, Bernier SG, Lepage A, Guillemette G, Regoli D, Sirois P. Permeability of endothelial monolayers to albumin is increased by bradykinin and inhibited by prostaglandins. Am J Physiol Lung Cell Mol Physiol 280: L732–L738, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Gonzalez A, Alex Mitsialis S, Liu X, Kourembanas S. Vasculoprotective effects of heme oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 302: L775–L784, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finigan JH, Boueiz A, Wilkinson E, Damico R, Skirball J, Pae HH, Damarla M, Hasan E, Pearse DB, Reddy SP, Grigoryev DN, Cheadle C, Esmon CT, Garcia JGN, Hassoun PM. Activated protein C protects against ventilator-induced pulmonary capillary leak. Am J Physiol Lung Cell Mol Physiol 296: L1002–L1011, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol 303: L20–L32, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Folkesson HG, Kuzenko SR, Lipson DA, Matthay MA, Simmons MA. The adenosine 2A receptor agonist GW328267C improves lung function after acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 303: L259–L271, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao X, Kouklis P, Xu N, Minshall RD, Sandoval R, Vogel SM, Malik AB. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol 279: L1218–L1225, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Gessner MA, Doran SF, Yu Z, Dunaway CW, Matalon S, Steele C. Chlorine gas exposure increases susceptibility to invasive lung fungal infection. Am J Physiol Lung Cell Mol Physiol 304: L765–L773, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girault JA, Labesse G, Mornon JP, Callebaut I. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem Sci 24: 54–57, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Göggel R, Hoffman S, Nüsing R, Narumiya S, Uhlig S. Platelet-activating factor-induced pulmonary edema is partly mediated by prostaglandin E(2), E-prostanoid 3-receptors, and potassium channels. Am J Respir Crit Care Med 166: 657–662, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 3: 88ps25, 2011. [DOI] [PubMed] [Google Scholar]
- 53.González-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11: 315–324, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4: 285–294, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Goodson P, Kumar A, Jain L, Kundu K, Murthy N, Koval M, Helms MN. NADPH oxidase regulates alveolar epithelial sodium channel activity and lung fluid balance in vivo via O−2 signaling. Am J Physiol Lung Cell Mol Physiol 302: L410–L419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grinnell KL, Casserly B, Harrington EO. Role of protein tyrosine phosphatase SHP2 in barrier function of pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 298: L361–L370, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grinnell KL, Harrington EO. Interplay between FAK, PKCδ, and p190RhoGAP in the regulation of endothelial barrier function. Microvasc Res 83: 12–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guerrero C, Lecuona E, Pesce L, Ridge KM, Sznajder JI. Dopamine regulates Na-K-ATPase in alveolar epithelial cells via MAPK-ERK-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol 281: L79–L85, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Haldenby KA, Chappell DC, Winlove CP, Parker KH, Firth JA. Focal and regional variations in the composition of the glycocalyx of large vessel endothelium. J Vasc Res 31: 2–9, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Harrington EO, Newton J, Morin N, Rounds S. Barrier dysfunction and RhoA activation are blunted by homocysteine and adenosine in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 287: L1091–L1097, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Hassanian SM, Dinarvand P, Rezaie AR. Adenosine regulates the proinflammatory signaling function of thrombin in endothelial cells. J Cell Physiol 229: 1292–1300, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heffner JE. The story of oxygen. Respir Care 58: 18–31, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Herrero R, Tanino M, Smith LS, Kajikawa O, Wong VA, Mongovin S, Matute-Bello G, Martin TR. The Fas/FasL pathway impairs the alveolar fluid clearance in mouse lungs. Am J Physiol Lung Cell Mol Physiol 305: L377–L388, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herold S, Gabrielli NM, Vadasz I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 305: L665–L681, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem 281: 2296–2305, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCalpha and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol 289: L176–L185, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol 278: H1177–H1185, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol 291: L596–L601, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol 121: 491–502, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol 305: L844–L855, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Job KM, Dull RO, Hlady V. Use of reflectance interference contrast microscopy to characterize the endothelial glycocalyx stiffness. Am J Physiol Lung Cell Mol Physiol 302: L1242–L1249, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi AD, Dimitropoulou C, Thangjam G, Snead C, Feldman S, Barabutis N, Fulton D, Hou Y, Kumar S, Patel V, Gorshkov B, Verin AD, Black SM, Catravas JD. Heat shock protein 90 inhibitors prevent LPS-induced endothelial barrier dysfunction by disrupting RhoA signaling. Am J Respir Cell Mol Biol 50: 170–179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawamura T, Wakabayashi N, Shigemura N, Huang CS, Masutani K, Tanaka Y, Noda K, Peng X, Takahashi T, Billiar TR, Okumara M, Toyoda Y, Kensler TW, Nakao A. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol 304: L646–L656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kiefmann R, Islam MN, Lindert J, Parthasarathi K, Bhattacharya J. Paracrine purinergic signaling determines lung endothelial nitric oxide production. Am J Physiol Lung Cell Mol Physiol 296: L901–L910, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knezevic N, Tauseef M, Thennes T, Mehta D. The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J Exp Med 206: 2761–2777, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolosova IA, Ma SF, Adyshev DM, Wang P, Ohba M, Natarajan V, Garcia JGN, Verin AD. Role of CPI-17 in the regulation of endothelial cytoskeleton. Am J Physiol Lung Cell Mol Physiol 287: L970–L980, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Konrad FM, Witte E, Vollmer I, Stark S, Reutershan J. Adenosine receptor A2b on hematopoietic cells mediates LPS-induced migration of PMNs into the lung interstitium. Am J Physiol Lung Cell Mol Physiol 303: L425–L438, 2012. [DOI] [PubMed] [Google Scholar]
- 78.Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol 285: L434–L442, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Kumar NM, Gilula NB. The gap junction communication channel. Cell 84: 381–388, 1996. [DOI] [PubMed] [Google Scholar]
- 80.Leonard A, Marando C, Rahman A, Fazal F. Thrombin selectively engages LIM kinase 1 and slingshot-1L phosphatase to regulate NF-κB activation and endothelial cell inflammation. Am J Physiol Lung Cell Mol Physiol 305: L651–L664, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci USA 98: 9989–9994, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol Heart Circ Physiol 268: H729–H739, 1995. [DOI] [PubMed] [Google Scholar]
- 83.Lu Q, Sakhatskyy P, Newton J, Shamirian P, Hsiao V, Curren S, Gabino Miranda GA, PEdroza M, Blackburn MR, Rounds S. Sustained adenosine exposure causes lung endothelial apoptosis: a possible contributor to cigarette smoke-induced endothelial apoptosis and lung injury. Am J Physiol Lung Cell Mol Physiol 304: L361–L370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 25: 1773–1783, 1966. [PubMed] [Google Scholar]
- 85.Lum H, Podolski JL, Gurnack ME, Schulz IT, Huang F, Holian O. Protein phosphatase 2B inhibitor potentiates endothelial PKC activity and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 281: L546–L555, 2001. [DOI] [PubMed] [Google Scholar]
- 86.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol 303: L364–L381, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–236, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol 539: 779–789, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meliton AY, Muñoz NM, Meliton LN, Birukova AA, Leff AR, Birukov KG. Mechanical induction of group V phospholipase A2 causes lung inflammation and acute lung injury. Am J Physiol Lung Cell Mol Physiol 304: L689–L700, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Menden H, Tate E, Hogg N, Sampath V. LPS-mediated endothelial activation in pulmonary endothelial cells: role of Nox2-dependent IKK-β phosphorylation. Am J Physiol Lung Cell Mol Physiol 304: L445–L455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minshall RD, Vandenbroucke EE, Holinstat M, Place AT, Tiruppathi C, Vogel SM, van Nieuw Amerongen GP, Mehta D, Malik AB. Role of protein kinase Czeta in thrombin-induced RhoA activation and inter-endothelial gap formation of human dermal microvessel endothelial cell monolayers. Microvasc Res 80: 240–249, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mo Y, Chen J, Schlueter CF, Hoyle GW. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L92–L102, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moerenhout M, Himpens B, Vereecke J. Intercellular communication upon mechanical stimulation of CPAE-endothelial cells is mediated by nucleotides. Cell Calcium 29: 125–136, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Moldobaeva A, Jenkins J, Wagner E. Effects of distension on airway inflammation and venular P-selectin expression. Am J Physiol Lung Cell Mol Physiol 295: L941–L948, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morty RE, Kuebler WM. TRPV4: an exciting new target to promote alveolo-capillary barrier function. Am J Physiol Lung Cell Mol Physiol 304: L92–L102, 2013. [DOI] [PubMed] [Google Scholar]
- 96.Moy AB, Blackwell K, Wang N, Haxhinasto K, Kasiske MK, Bodmer J, Reyes G, English A. Phorbol ester-mediated pulmonary artery endothelial barrier dysfunction through regulation of actin cytoskeletal mechanics. Am J Physiol Lung Cell Mol Physiol 287: L153–L167, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol 283: H1282–H1291, 2002. [DOI] [PubMed] [Google Scholar]
- 98.Müller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem 280: 3747–3756, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Ngamsri KC, Wagner R, Vollmer I, Starks S, Reutershan J. Adenosine receptor A1 regulates polymorphonuclear cell trafficking and microvascular permeability in lipopolysaccharide-induced lung injury. J Immunol 185: 4374–4384, 2010. [DOI] [PubMed] [Google Scholar]
- 100.Nickles HT, Kuebler WM. Take my breath away: perivascular fluid cuffs impair lung mechanics. Crit Care Med 38: 1494–1496, 2010. [DOI] [PubMed] [Google Scholar]
- 101.Obiako B, Calchary W, Xu N, Kunstadt R, Richardson B, Nix J, Sayner SL. Bicarbonate disruption of the pulmonary endothelial barrier via activation of endogenous soluble adenylyl cyclase, isoform 10. Am J Physiol Lung Cell Mol Physiol 305: L185–L192, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O'Callaghan R, Job KM, Dull RO, Hlady V. Stiffness and heterogeneity of the pulmonary endothelial glycocalyx measured by atomic force microscopy. Am J Physiol Lung Cell Mol Physiol 301: L353–L360, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oishi H, Takano KI, Tomita K, Takebe M, Yokoo H, Yamazaki M, Hattori Y. Olprinone and colforsin daropate alleviate septic lung inflammation and apoptosis through CREB-independent activation of the Akt pathway. Am J Physiol Lung Cell Mol Physiol 303: L130–L140, 2012. [DOI] [PubMed] [Google Scholar]
- 104.Orrington-Myers J, Gao X, Kouklis P, Broman M, Rahman A, Vogel SM, Malik AB. Regulation of lung neutrophil recruitment by VE-cadherin. Am J Physiol Lung Cell Mol Physiol 291: L764–L771, 2006. [DOI] [PubMed] [Google Scholar]
- 105.Ozdulger A, Cinel I, Koksel O, Cinel L, Avlan D, Unlu A, Oral U. The protective effect of N-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock 19: 366–372. [DOI] [PubMed] [Google Scholar]
- 106.Park DW, Jiang S, Liu Y, Siegal GP, Inoki K, Abraham E, Zmijewski JW. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages, and enhances severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 10.1152/ajplung.00165.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park DW, Jiang S, Tadie JM, Stigler WS, Gao Y, Deshane J, Abraham E, Zmijewski JW. Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol Med 19: 387–398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 116: 1409–1416, 2003. [DOI] [PubMed] [Google Scholar]
- 109.Parthasarathi K. Endothelial connexin43 mediates acid-induced increases in pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol 303: L33–L42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 116: 2193–2200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pirot N, Delpech H, Deleuze V, Dohet C, Courtade-Saïdi M, Basset-Léobon C, Chalhoub E, Mathieu D, Pinet V. Lung endothelial barrier disruption in Lyl1-deficient mice. Am J Physiol Lung Cell Mol Physiol 306: L775–L785, 2014. [DOI] [PubMed] [Google Scholar]
- 112.Pries AR, Kuebler WM. Normal endothelium. Hand Exp Pharmacol 176: 1–40, 2006. [DOI] [PubMed] [Google Scholar]
- 113.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflügers Arch 440: 653–666, 2000. [DOI] [PubMed] [Google Scholar]
- 114.Pruessmeyer J, Hess FM, Alert H, Groth E, Pasqualon T, Schwarz N, Nyamoya S, Kollert J, van der Vorst E, Donners M, Martin C, Uhlig S, Saftig P, Dreymueller D, Ludwig A. Leukocytes require ADAM10 but not ADAM17 for their migration and inflammatory recruitment into the alveolar space. Blood 123: 4077–4088, 2014. [DOI] [PubMed] [Google Scholar]
- 115.Pugin J, Dunn-Siegrist I, Dufour J, Tissières P, Charles PE, Comte R. Cyclic stretch of human lung cells induces an acidification and promotes bacterial growth. Am J Respir Cell Mol Biol 38: 362–370, 2008. [DOI] [PubMed] [Google Scholar]
- 116.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 284: L972–L980, 2003. [DOI] [PubMed] [Google Scholar]
- 117.Rafikov R, Dimitropoulou C, Aggarwal S, Kangath A, Gross C, Pardo D, Sharma S, Jezierska-Drutel A, Patel V, Snead C, Lucas R, Verin A, Fulton D, Catravas JD, Black SM. Lipopolysaccharide-induced lung injury involves the nitration-mediated activation of RhoA. J Biol Chem 289: 4710–4722, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajput C, Kini V, Smith M, Yazbeck P, Chavez A, Schmidt T, Zhang W, Knezevic N, Komarova Y, Mehta D. Neural Wiskott-Aldrich syndrome protein (N-WASP)-mediated p120-catenin interaction with Arp2-Actin complex stabilizes endothelial adherens junctions. J Biol Chem 288: 4241–4250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ramchandran R, Mehta D, Vogel SM, Mirza MK, Kouklis P, Malik AB. Critical role of Cdc42 in mediating endothelial barrier protection in vivo. Am J Physiol Lung Cell Mol Physiol 295: L363–L369, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rittirsch D, Filerl MA, Nadeau BA, Day DE, Huber-Lang MS, Grailer JJ, Zetounne FS, Andjelkovic AV, Fasano A, Ward PA. Zonulin as prehaptoglobin2 regulates lung permeability and activates the complement system. Am J Physiol Lung Cell Mol Physiol 304: L863–L872, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Russe OQ, Möser CV, Kynast KL, King TS, Olbrich K, Grösch S, Geisslinger G, Niederberger E. LPS inhibits caspase 3-dependent apoptosis in RAW264.7 macrophages induced by the AMPK activator AICAR. Biochem Biophys Res Commun 447: 520–525, 2014. [DOI] [PubMed] [Google Scholar]
- 122.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27: 1312–1318, 2007. [DOI] [PubMed] [Google Scholar]
- 123.Sayner SL, Balczon R, Frank DW, Cooper DMF, Stevens T. Filamin A is a phosphorylation target of membrane but not cytosolic adenylyl cyclase activity. Am J Physiol Lung Cell Mol Physiol 301: L117–L124, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 14: 92–101, 2004. [DOI] [PubMed] [Google Scholar]
- 125.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18: 1217–1223, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi X, Sun H, Zhou D, Xi H, Shan L. Arctigenin attenuates lipopolysaccharide-induced acute lung injury in rats. Inflammation (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 127.Shimoda M, Hashimoto G, Mochizuki S, Ikeda E, Nagai N, Ishida S, Okada Y. Binding of ADAM28 to P-selectin glycoprotein ligand-1 enhances P-selectin-mediated leukocyte adhesion to endothelial cells. J Biol Chem 282: 25864–25874, 2007. [DOI] [PubMed] [Google Scholar]
- 128.Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol 284: L435–L451, 2003. [DOI] [PubMed] [Google Scholar]
- 129.Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol 251: 206–220, 2002. [DOI] [PubMed] [Google Scholar]
- 130.Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can prove albumin dynamics inside lung endothelial glycocalyx. Am J Physiol Lung Cell Mol Physiol 293: L328–L335, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol Lung Cell Mol Physiol 277: L119–L126, 1999. [DOI] [PubMed] [Google Scholar]
- 132.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest 138: 179–187, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sud N, Wedgwood S, Black SM. Protein kinase Cdelta regulates endothelial nitric oxide synthase expression via Akt activation and nitric oxide generation. Am J Physiol Lung Cell Mol Physiol 294: L582–L591, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 134.Sun C, Beard RS, McLean DL, Rigor RR, Konia T, Wu MH, Yuan SY. ADAM15 deficiency attenuates pulmonary hyperpermeability and acute lung injury in lipopolysaccharide-treated mice. Am J Physiol Lung Cell Mol Physiol 304: L135–L142, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun X, Ma SF, Wade MS, Acosta-Herrera M, Villar J, Pino-Yanes M, Zhou T, Liu Belvitch P, Moitra J, Han YJ, Machado R, Noth I, Natarajan V, Dudek SM, Jacobson JR, Flores C, Garcia JG. Functional promoter variants in sphingosine 1-phosphate receptor 3 associate with susceptibility to sepsis-associated acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 305: L467–L477, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tabuchi A, Mertens M, Kuppe H, Pries AR, Kuebler WM. Intravital microscopy of the murine pulmonary microcirculation. J Appl Physiol 104: 338–346, 2008. [DOI] [PubMed] [Google Scholar]
- 137.Tadie JM, Bae HB, Deshane JS, Bell CP, Lazarowski ER, Chaplin DD, Thannickal VJ, Abraham E, Zmijewski JW. Toll-like receptor 4 engagement inhibits adenosine 5′-monophosphate-activated protein kinase activation through a high mobility group box 1 protein-dependent mechanism. Mol Med 18: 659–668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med 209: 1953–1968, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thennes T, Mehta D. Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvasc Res 83: 31–44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273: 12725–12731, 1998. [DOI] [PubMed] [Google Scholar]
- 141.Uhlig S, Yang Y, Waade J, Wittenberg C, Babendreyer A, Kuebler WM. Differential regulation of lung endothelial permeability in vitro and in situ. Cell Physiol Biochem 34: 1–19, 2014. [DOI] [PubMed] [Google Scholar]
- 142.Urbanetti JS. Toxic inhalation injury. In: Medical Aspects of Chemical and Biological Warfare, edited by Sidell FR, Takafuji ET, Franz DR. Washington, DC: The Office of the Surgeon General, TMM Publications, Borden Institute, 1997. [Google Scholar]
- 143.Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JGN, Natarajan V. Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways. Am J Physiol Lung Cell Mol Physiol 300: L840–L850, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Usatyuk PV, Natarajan V. Regulation of reactive oxygen species-induced endothelial cell-cell and cell-matrix contacts by focal adhesion kinase and adherens junction proteins. Am J Physiol Lung Cell Mol Physiol 289: L999–L1010, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Utoguchi N, Ikeda K, Saeki K, Oka N, Mizuguchi H, Kubo K, Mayumi T. Ascorbic acid stimulates barrier function of cultured endothelial cell monolayer. J Cell Physiol 163: 393–399, 1995. [DOI] [PubMed] [Google Scholar]
- 146.Van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol 285: H2848–H2856, 2003. [DOI] [PubMed] [Google Scholar]
- 147.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996. [DOI] [PubMed] [Google Scholar]
- 148.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol 278: H285–H289, 2000. [DOI] [PubMed] [Google Scholar]
- 149.Voelkel NF, Bogaard HJ, Al Husseini A, Farkas L, Gomez-Arroyo J, Natarajan R. Antioxidants for the treatment of patients with severe angioproliferative pulmonary hypertension? Antioxid Redox Signal 18: 1810–1817, 2013. [DOI] [PubMed] [Google Scholar]
- 150.Vohra PK, Hoeppner LH, Sagar G, Dutta SK, Misra S, Hubmayr RD, Mukhopadhyay D. Dopamine inhibits pulmonary edema through the VEGF-VEGFR2 axis in a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 302: L185–L192, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Westover AJ, Hooper SB, Wallace MJ, Moss TJ. Prostaglandins mediate the fetal pulmonary response to intrauterine inflammation. Am J Physiol Lung Cell Mol Physiol 302: L664–L678, 2012. [DOI] [PubMed] [Google Scholar]
- 152.Westphalen K, Monma E, Islam MN, Bhattacharya J. Acid contact in the rodent pulmonary alveolus causes proinflammatory signaling by membrane pore formation. Am J Physiol Lung Cell Mol Physiol 303: L107–L116, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wojciak-Stothard B, Tsang LYF, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005. [DOI] [PubMed] [Google Scholar]
- 154.Wyatt TA, Poole JA, Nordgren TM, DeVasure JM, Heires AJ, Bailey KL, Romberger DJ. cAMP-dependent protein kinase activation decreases cytokine release in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 307: L643–L651, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu W, Liu C, Kaartinen V, Chen H, Lu CH, Zhang W, Luo Y, Shi W. TACE in perinatal mouse lung epithelial cells promotes lung saccular formation. Am J Physiol Lung Cell Mol Physiol 305: L953–L963, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamanaka K, Hatano E, Narita M, Kitamura K, Yanagida A, Asechi H, Uemoto S. Olprinone attenuates excessive shear stress through up-regulation of endothelial nitric oxide synthase in a rat excessive hepatectomy model. Liver Transplant 17: 60–69, 2011. [DOI] [PubMed] [Google Scholar]
- 157.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L555–L568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang Y, Schmidt EP. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue barriers 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zebda N, Dubrovskyi O, Birukov KG. Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res 83: 71–81, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeyed YF, Bastrache JA, Matthay MA, Ware LB. The severity of shock is associated with impaired rates of net alveolar fluid clearance in clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol 303: L550–L555, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang S, Song L, Wang Y, Herwald H, Thorlacius H. Targeting CD162 protects against streptococcal M1 protein-evoked neutrophil recruitment and lung injury. Am J Physiol Lung Cell Mol Physiol 305: L756–L763, 2013. [DOI] [PubMed] [Google Scholar]
- 162.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L497–L504, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao YD, Ohkawara H, Vogel SM, Malik AB, Zhao YY. Bone marrow-derived progenitor cells prevent thrombin-induced increase in lung vascular permeability. Am J Physiol Lung Cell Mol Physiol 298: L36–L44, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zilaee M, Ferns GAA, Ghayour-Mobarhan M. Heat shock proteins and cardiovascular disease. Adv Clin Chem 64: 73–115, 2014. [DOI] [PubMed] [Google Scholar]