Abstract
Long QT syndrome type 3 (LQT3) is caused by mutations in the SCN5A-encoded Nav1.5 channel. LQT3 patients exhibit time of day-associated abnormal increases in their heart rate-corrected QT (QTc) intervals and risk for life-threatening episodes. This study determines the effects of uncoupling environmental time cues that entrain circadian rhythms (time of light and time of feeding) on heart rate and ventricular repolarization in wild-type (WT) or transgenic LQT3 mice (Scn5a+/ΔKPQ). We used an established light phase-restricted feeding paradigm that disrupts the alignment among the circadian rhythms in the central pacemaker of the suprachiasmatic nucleus and peripheral tissues including heart. Circadian analysis of the RR and QT intervals showed the Scn5a+/ΔKPQ mice had QT rhythms with larger amplitudes and 24-h midline means and a more pronounced slowing of the heart rate. For both WT and Scn5a+/ΔKPQ mice, light phase-restricted feeding shifted the RR and QT rhythms ∼12 h, increased their amplitudes greater than twofold, and raised the 24-h midline mean by ∼10%. In contrast to WT mice, the QTc interval in Scn5a+/ΔKPQ mice exhibited time-of-day prolongation that was flipped after light phase-restricted feeding. The time-of-day changes in the QTc intervals of Scn5a+/ΔKPQ mice were secondary to a steeper power relation between their QT and RR intervals. We conclude that uncoupling time of feeding from normal light cues can dramatically slow heart rate to unmask genotype-specific differences in the QT intervals and aggravate the LQT3-related phenotype.
Keywords: long QT syndrome, heart rate, feeding, circadian rhythms, SCN5A
congenital long QT syndrome (LQTS) is primarily an autosomal-dominant disease that delays ventricular repolarization and increases the risk for polymorphic ventricular tachycardia, which can cause syncope, seizures, and sudden cardiac arrest (26). The majority of LQTS cases are linked to mutations in three different cardiac ion channel genes: KCNQ1 (LQT1), KCNH2 (LQT2), or SCN5A (LQT3) (27). These three major types of LQTS show differences in disease penetrance, electrocardiographic characteristics, symptomatic risk, triggers for events, and responsiveness to treatment (23, 29). In particular, LQT3 is more lethal than LQT1 or LQT2, and LQT3 patients typically exhibit an abnormal increase in their heart rate-corrected QT (QTc) intervals and risk for life-threatening episodes at night (28, 30, 33).
Despite this strong time-of-day association between life-threatening events in the LQT3 phenotype, the role that manipulating circadian rhythms has on the expressivity of the LQT3 phenotype has not yet been investigated. Circadian rhythms are ≈24-h rhythms that are regulated at the cellular level by a transcription-translation feedback loop referred to as the molecular clock. The molecular clock mechanism is ubiquitous across all cell types, and so one of the challenges is in maintaining alignment of the clocks between the central and peripheral systems in the body. Historically, the clocks within the system were believed to be under strict neurohumoral control of the central clock in the suprachiasmatic nucleus (SCN). Recent research, however, has demonstrated that the alignment of the rhythms between the central clock in the SCN and peripheral tissues can be disrupted through offsetting the time of feeding to the light phase in nocturnal rodents (6, 24, 32, 36, 38). This desynchronization of tissue/organ circadian rhythms within the organism dramatically affects the timing of some well-known physiological variables (i.e., core body temperature) but does not alter the pattern of voluntary activity (i.e., wheel running) (6).
To begin to address the interaction between the circadian environment and the LQT3 phenotype, we used the LQT3 knock-in mouse model and mice heterozygous for the ΔKPQ mutation (Scn5a+/ΔKPQ) that shares many of the typical LQT3 features including longer QT intervals, ventricular and atrial arrhythmias, and sinus node dysfunction (episodes of bradycardia, sinus pauses, and longer sinus recovery times) (9, 11, 21, 37). Similar to LQT3 patients, the absolute incidence of spontaneous arrhythmias in these animals is extremely rare, but ventricular arrhythmias and sudden death can be unmasked by electrical stimulation or cholinergic-induced bradycardia (5, 9, 21). The purpose of this study is to determine if misalignment of the central and peripheral circadian rhythms are sufficient to induce changes in the daily fluctuations in heart rate and/or ventricular repolarization for wild-type (WT) and Scn5a+/ΔKPQ mice.
METHODS
ECG telemetry.
All animal procedures were conducted in compliance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee at University of Kentucky. WT mice or Scn5a+/ΔKPQ mice (kindly provided by Dr. Peter Carmeliet) (21) were housed in a 12-h:12-h light-dark cycle with ad libitum access to food and water. All the mice used in this study were male and between the ages of 12 and 14 wk. Mice were anesthetized with isoflurane, and telemetry transmitter units (PhysioTel ETA-F10; Data Sciences International) were implanted in the peritoneal cavity under aseptic conditions. The two ECG leads were secured near the apex of the heart and the right acromion. Mice were housed singly and allowed to recover for 2 wk. In vivo telemetry was used to measure core body temperature and ECGs. The method for RR interval analysis was done as previously described (25). We used two separate methods to assess the QT intervals. First, we manually measured the QT and preceding RR intervals to calculate QTc intervals and to examine the power of the QT and RR relation as previously described (17). Briefly, to obtain physiologically relevant values for the QTc, the observed R-R interval is expressed as a unitless multiple of 100 ms, which approaches the average R-R interval. This gives a normalized RR interval, i.e., RR100 = RR/100 ms. The value of the exponent y in the relationship QT = QTc × R − Ry100 was next assessed, where the QT interval is the observed QT (in ms); units for QTc are also milliseconds. Taking the natural logarithm of each side of this relationship gave ln(QT) = ln(QT) +yln(RR100). Thus the slope of the linear relationship between the log-transformed QT and RR100 defined the exponent to which the RR interval ratio should be raised to QTc. As a second method, we used an automated threshold approach so that we could compute an hourly average for the QT interval over several days (3, 13, 15, 22). Briefly, the ECG traces recorded for each hour were aligned to the peak of R wave to generate an average trace, the Q point was defined as the base of the QRS complex (where the slope of the profile changed from negative to positive), and the T point was defined to where the ECG returned 75% of the way from the minima of the T wave to the isoelectric level. The hourly averages for the RR and QT intervals for ∼3 days were then fit with the nonlinear sinusoidal model: Interval = A·cos[2π(t − τ)/T] + m, to calculate the period (T), the time between the peak amplitudes; phase (τ), timing of the peak rhythm in reference to the onset of the light phase [Zeitgeber time (ZT) = 0]; the amplitude (A), one-half of the peak to trough levels, and midline mean (m), a rhythm-adjusted mean halfway between the peak and trough amplitudes. Additionally, histograms were computed for RR intervals in bins of 5 ms similar to that previously reported (25). We also detected regions of bigeminy-like arrhythmia patterns by calculating the number of RR interval “flips” in one-min bins. A flip was defined as an alternating increase and decrease in the RR-interval > 80 ms. The threshold for characterizing a bigeminal arrhythmia was set at 80 flips per min.
Light phase-restricted feeding.
Several studies now show that feeding can act as a dominant environmental cue for setting the circadian rhythm of peripheral organ clock gene expression, metabolism and core body temperature (6, 24, 32, 36, 38). Mice are nocturnal and in ad libitum feeding conditions, they normally eat most of their caloric intake during the dark phase. We used an established light phase-restricted feeding protocol that limits food access to uncouple the circadian rhythms of the SCN and peripheral organs (4, 6). On the first day of the light phase-restricted feeding protocol, food was removed at 9 h after the beginning of the light cycle (ZT 9). On the next day, the presentation of the food was restricted for 7 h during the light phase between ZT 2–9. Importantly, once the mice adjusted to the light phase-restricted feeding protocol, they did not reduce caloric intake and the animals began to increase in weight during the second week similar to that previously reported (4). Studies from our laboratories show that the time-restricted, light-phase fed (tRF) mice consume approximately the same amount of food as ad libitum fed mice (2, 36). A 2-wk tRF acclimation period was used before ECG analysis to allow the peripheral circadian rhythms to achieve their new steady state.
Statistical analysis.
Results are reported as means ± SE unless noted. To determine if there was significant interaction between variables, an ANOVA or t-test was used when appropriate (PRISM, Mathworks). If a significant interaction was identified, then post hoc comparisons were performed using a Bonferonni correction or paired t-test when appropriate.
RESULTS
Light phase-restricted feeding alters the core body temperatures in WT and Scn5a+/ΔKPQ mice.
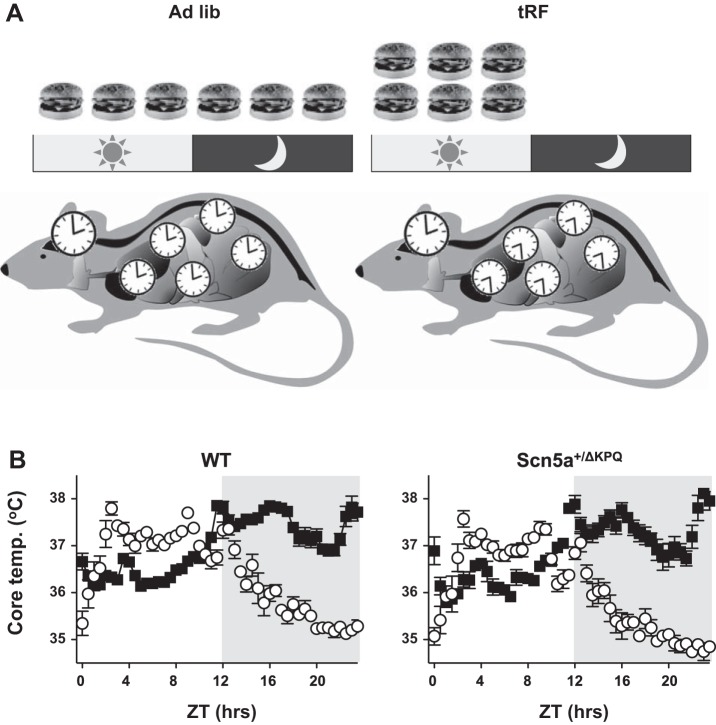
As nocturnal animals, mice consume ∼80% of their food during the dark (active) phase (1, 6). Time-restricted feeding to the light (inactive) phase shifts the circadian expression of timekeeping or clock genes and peripheral organ metabolism by ∼12 h, but it does not affect clock genes expression in the SCN (Fig. 1A) (4, 6). The desynchronization of the circadian oscillations between the SCN and peripheral tissues causes a dramatic change in the daily rhythm of the core body temperature: it lowers the trough, increases the amplitude, and shifts the phase by ∼12 h (6). We confirmed the effect that light-phase, time-restricted feeding has on the core body temperature in WT or Scn5a+/ΔKPQ mice before and after tRF conditions (Fig. 1B).
Fig. 1.
Light-phase, time-restricted feeding (tRF) shifts the peak of the core body temperature (Temp) to the light phase. A: depiction showing how light phase-restricted feeding selectively influences the timing of peripheral organ metabolism (clocks) relative to the light-dark cycle and the timing of circadian oscillations in the suprachiasmatic nucleus. B: core body temperature data plotted as the half-hour mean for ad libitum (Ad Lib)-fed (■) and tRF conditions (○) for wild-type (WT; left) and Scn5a+/ΔKPQ (right) mice (n = 6 animals each). Zeitgeber time (ZT) = 0 is the onset of the light phase and the shaded region signifies the dark phase.
Uncoupling time of feeding from normal light cues dramatically alters the circadian rhythm in RR and QT intervals.
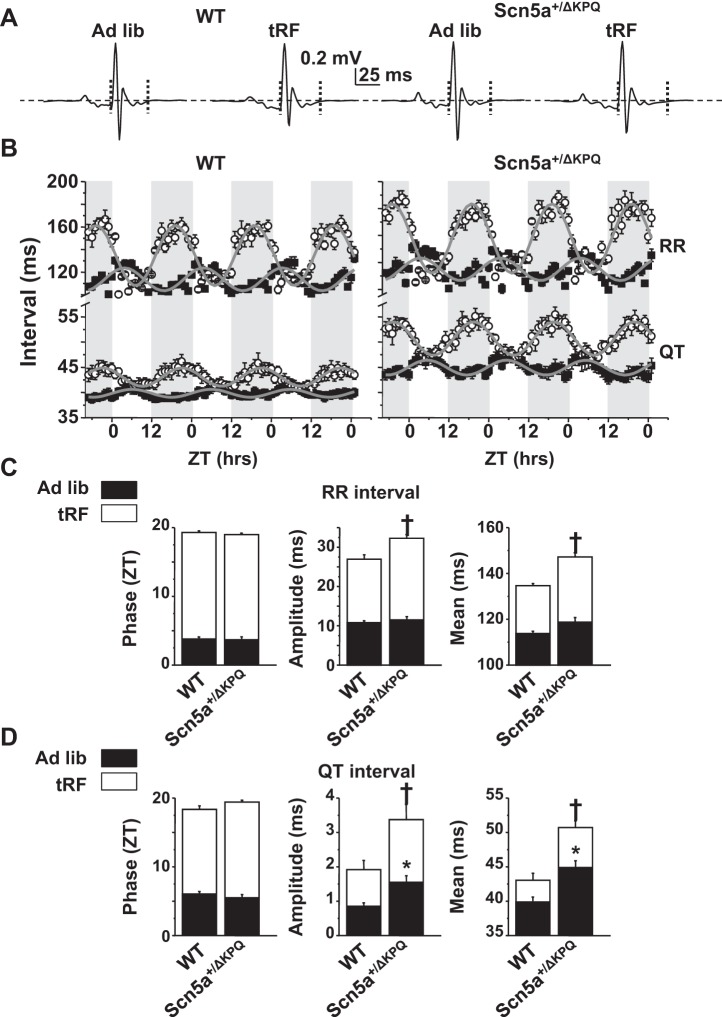
The effect that light phase-restricted feeding has on the circadian rhythms in heart rate and ventricular repolarization has not yet been studied. We quantified the circadian rhythms of the RR and QT intervals (RR and QT rhythms, respectively) in WT and Scn5a+/ΔKPQ mice by plotting hourly RR and QT intervals as a function of ZT for ∼3 days (ZT = 0 is the start of the light phase) (Fig. 2, A and B) and fit the data with a sinusoidal model to calculate the period, phase, amplitude, and midline means (Fig. 2, C and D). As expected for mice housed in 12-h:12-h light-dark cycle conditions, the RR and QT rhythms followed an ∼24-h period in all the conditions tested. In ad libitum-fed conditions, the peak of the RR and QT rhythms for both WT and Scn5a+/ΔKPQ mice occurred between ZT 4 and 6 h, and the daily amplitude and 24-h midline means of the QT rhythms were larger in the Scn5a+/ΔKPQ mice (Fig. 2D). We next quantified the RR and QT rhythms after tRF conditions. Similar to changes in body temperatures (Fig. 1B), the phase of the RR and QT rhythms in WT and Scn5a+/ΔKPQ mice shifted ∼12 h (Fig. 2, B–D). Moreover, the amplitudes and 24-h midline means of the RR and QT rhythms were larger. Interestingly, tRF conditions unmasked a genotype-specific difference in the RR rhythms. When compared with those of tRF WT mice, the RR rhythms of the tRF Scn5a+/ΔKPQ mice had a larger amplitude and 24-h midline mean (Fig. 2C). The genotype-specific difference in the QT rhythms seen in ad libitum conditions persisted in tRF conditions (Fig. 2D). Together, these data demonstrate that 1) when compared with WT mice, the Scn5a+/ΔKPQ mice had altered QT rhythms in ad libitum conditions, 2) light phase-restricted feeding altered the RR and QT rhythms in both WT and Scn5a+/ΔKPQ mice similar to body temperature, and 3) light phase-restricted feeding unmasked a difference in the RR rhythms of Scn5a+/ΔKPQ mice.
Fig. 2.
Light phase-restricted feeding alters the circadian rhythm of RR and QT intervals in WT and Scn5a+/ΔKPQ mice. A: ECG traces averaged over 1 h for ad libitum-fed or tRF WT and Scn5a+/ΔKPQ mice. Horizontal dashed lines are the zero potential line, and vertical dotted lines denote the averaged trace's QT interval. B: hourly averages in the RR or QT intervals for WT (left) or Scn5a+/ΔKPQ (right) mice in ad libitum-fed (■) or after tRF conditions (○). Averages are plotted as a function of ZT over ∼3 days. Shaded regions denote the dark phases. The gray line is the sinusoidal fit to the data. C: mean phases, amplitudes, and 24-h midline means for the RR. D: QT intervals from the sinusoidal fits to the individual data from WT or Scn5a+/ΔKPQ mice in ad libitum (black bars) or after tRF conditions (white bars). Not denoted is the finding that compared with ad libitum-fed conditions, tRF conditions increased the phase, amplitude, and midline means for both WT and Scn5a+/ΔKPQ mice (n = 6 animals each, P < 0.05). Significance denoted for WT vs. Scn5a+/ΔKPQ mice in ad libitum-fed conditions (*P < 0.05) or tRF (†P < 0.05) conditions.
Light phase-restricted feeding exaggerates a genotype-specific difference in the heart rates of Scn5a+/ΔKPQ mice.
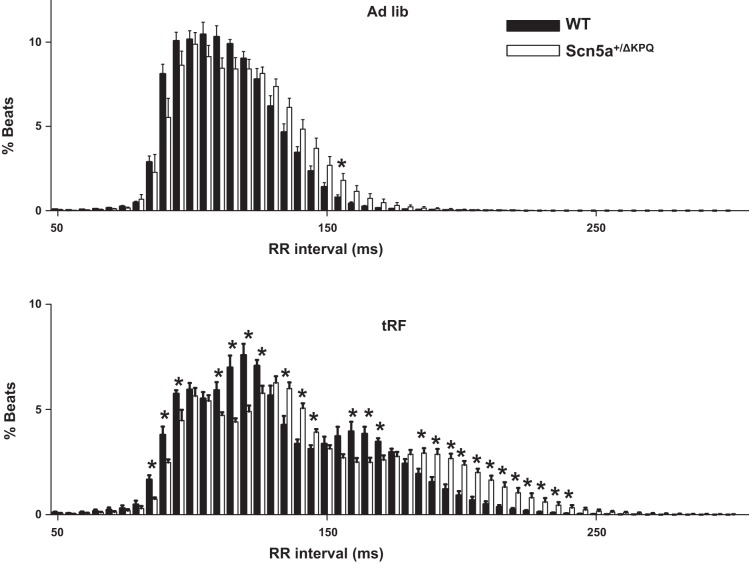
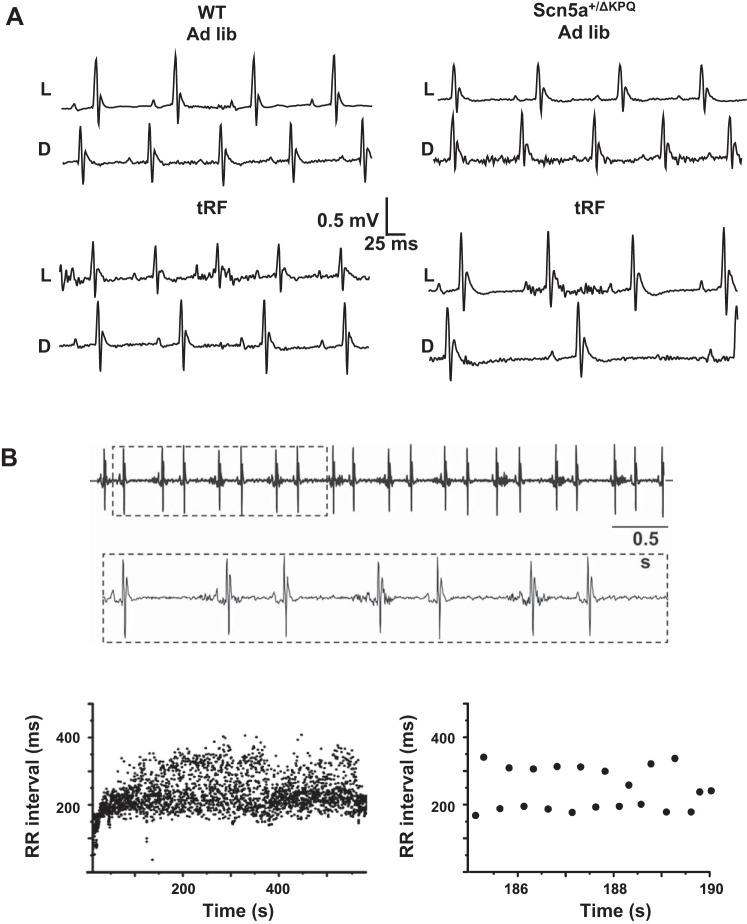
To better determine how tRF differentially affected the heart rate of WT and Scn5a+/ΔKPQ mice, we calculated the relative percentage of beats with RR intervals that occurred between 50–300 ms in 5-ms bins (Fig. 3). In ad libitum-fed conditions, there was only a negligible difference between WT and Scn5a+/ΔKPQ mice. However, when compared with tRF WT mice, tRF Scn5a+/ΔKPQ mice showed a large increase in the percentage of beats with RR intervals > 200 ms. Close examination of the ECG records suggested that the tRF Scn5a+/ΔKPQ mice had a disproportionate number of pauses (Fig. 4A). Moreover, in one of the tRF Scn5a+/ΔKPQ mice, we observed four discrete episodes of bigeminal-like arrhythmias lasting at least 1 min over the 3-day recording period (Fig. 4B). All of these were likely caused by premature atrial contractions (PACs) originating near the sinoatrial node, because the morphologies of the P waves within each couplet were not visibly different and there were no obvious P waves during the compensatory pauses (suggesting the sinus node had been reset). These arrhythmias were not observed in WT mice (ad libitum fed or tRF) or the ad libitum-fed Scn5a+/ΔKPQ mice.
Fig. 3.
Time-restricted feeding unmasks slower RR intervals in the Scn5a+/ΔKPQ mice. Histogram plots for the distribution of RR intervals from WT (black bars) or Scn5a+/ΔKPQ (white bars) mice in ad libitum-fed (top) or tRF conditions (bottom). The RR intervals are separated into 5-ms bins (*P < 0.05; n = 6 animal each).
Fig. 4.
Light phase-restricted feeding unmasks ECG differences between WT and Scn5a+/ΔKPQ mice. A: representative ECG traces recorded in WT (left) and Scn5a+/ΔKPQ (right) mice during both light (L) and dark (D) phase in ad libitum (top 2 traces)- or tRF-feeding (bottom 2 traces) conditions. B: small portion of an atrial bigeminy arrhythmia recorded from an Scn5a+/ΔKPQ mouse. Inset: boxed ECG region at a higher resolution. Graphs show beat-to-beat changes in the RR interval measured during on entire episode of a bigeminal arrhythmia (left) or an amplified portion (right) to highlight the alteration in the RR intervals.
The time-of-day change in the QTc interval of Scn5a+/ΔKPQ mice is due to a steeper QT-RR100 relation.
We next examined the impact that light phase-restricted feeding had on ventricular repolarization by calculating the QTc intervals measured from ad libitum-fed or tRF WT and Scn5a+/ΔKPQ mice during the middle of the light or dark phase (ZT = 5 and 17), respectively. The QTc formula we used was a modified version of Bazett's formula developed for mice by Mitchell and colleagues (17): [QTc = QT/(RR100)0.5]. In ad libitum-fed or tRF conditions, the mean QTc intervals measured from WT mice did not show any differences. However, the QTc intervals from ad libitum-fed Scn5a+/ΔKPQ mice showed a time-of-day prolongation during the light phase, and this phenomenon was flipped in the tRF Scn5a+/ΔKPQ mice (Table 1). These data demonstrate that there is a time-of-day prolongation in the QTc interval of the Scn5a+/ΔKPQ mice, and the daily prolongation in the QTc interval tracks with time of day that the heart rate is slowest (not light cues) (Fig. 2B).
Table 1.
Scn5a+/ΔKPQ mice show a time-of-day prolongation in their QTc interval that is flipped after light phase-restricted feeding
| Genotype | Ad Libitum QTc Light | Ad Libitum QTc Dark | tRF QTc Light | tRF QT Dark | n |
|---|---|---|---|---|---|
| WT | 41.3 ± 1.4 | 42.0 ± 0.4 | 44.7 ± 0.8 | 45.5 ± 0.9 | 6 |
| Scn5a+/ΔKPQ | 48.3 ± 1.1* | 43.1 ± 1.2† | 50.0 ± 1.3* | 55.9 ± 1.0*† | 7 |
Values are means ± SE; n, number of mice. Shown are the average heart rate-corrected QT (QTc) intervals calculated using the correction formula: QTc = QT/(RR100)0.5 [see Mitchell et al. (17)]. The QTc intervals were calculated from ECG traces recorded during the hours that corresponded to Zeitgeber time (ZT) =5 (light) and ZT =17 (dark) in ad libitum-fed or tRF conditions.
P < 0.05, wild-type (WT) vs. Scn5a+/ΔKPQ;
P < 0.05, light vs. dark.
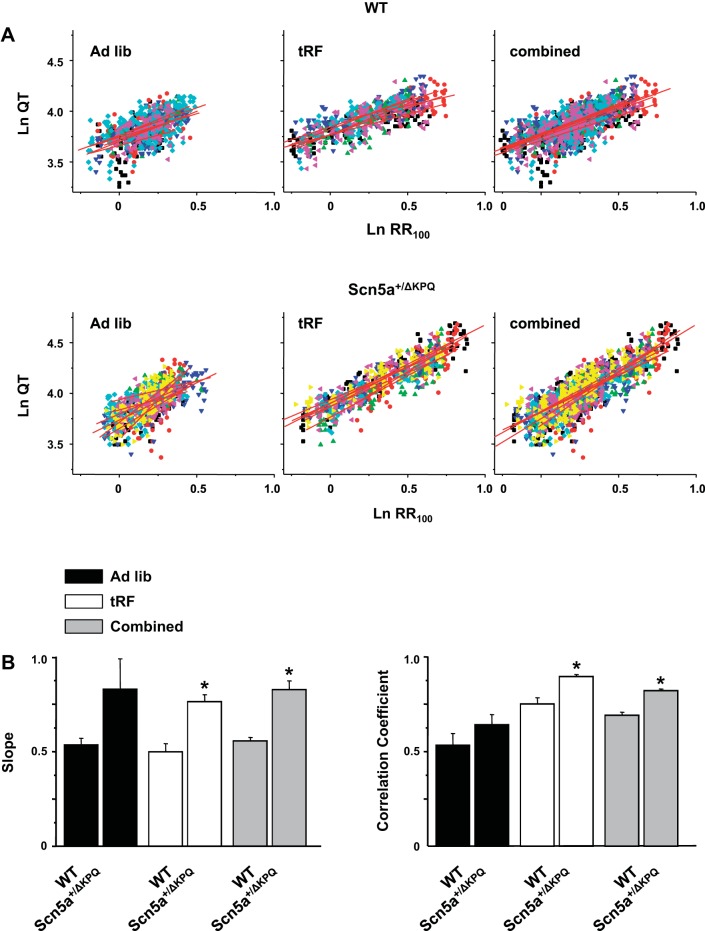
Since the QTc interval gets disproportionately longer in Scn5a+/ΔKPQ mice during the time of day when the heart rates are slowest, we compared the power of the QT and RR relation for WT and Scn5a+/ΔKPQ mice in ad libitum-fed or tRF conditions (Fig. 5A). Interestingly, the larger range of RR intervals caused by light phase-restricted feeding allowed us to better define the QT-RR100 relations as assessed by the improvement in the correlation coefficients (Fig. 5B). The WT mice had QT-RR100 relations with slopes of ∼0.5, similar to what was previously shown by Mitchell and colleagues (17) (Fig. 5B). In contrast, the slopes of the QT-RR100 relations for Scn5a+/ΔKPQ mice was ∼0.8. If we further adjusted the QT correction formula to account for the steeper QT-RR100 power relation in Scn5a+/ΔKPQ mice [QTc = QT/(RR100)0.8], then the time-of-day change in the QTc intervals disappears (data not shown). Therefore, the increase in the QTc interval of the Scn5a+/ΔKPQ mice at slow heart rates (Table 1) is secondary to the inability of Bazett's formula to normalize for the steeper QT-RR100 relation. Overall, these data demonstrate that uncoupling time of feeding from normal light cues slows basal heart rates to unmask genotype-specific differences in the QT-RR100 relations that aggravate the LQT3-related phenotype.
Fig. 5.
The power of the QT-RR100 relation is steeper for Scn5a+/ΔKPQ mice. A: power of the relation between the QT and RR intervals for the WT (top) or Scn5a+/ΔKPQ (bottom) mice calculated in ad libitum-fed (left), tRF (middle), or combined ad libitum-fed and tRF (right) conditions. Each animal is highlighted as a different colored symbol, and red line shows the corresponding fits (n = 6 to 7 for WT and Scn5a+/ΔKPQ, respectively). B: mean slopes (left) and correlation coefficients (right) for the ad libitum-fed, tRF, or combined conditions (*P < 0.05 for WT vs. Scn5a+/ΔKPQ).
DISCUSSION
This is the first study to investigate the role that desynchronizing the circadian alignment has on heart rate, ventricular repolarization, and LQT3 expressivity in transgenic mice. We found that light phase-restricted feeding shifted the phase, amplified the oscillations, and increased the 24-h midline mean of the RR and QT rhythms. Light phase-restricted feeding caused a more pronounced slowing of the heart rates in Scn5a+/ΔKPQ mice and flipped the time-of-day prolongation in the QTc interval not seen in WT mice. The light phase-restricted feeding sufficiently slowed the WT and Scn5a+/ΔKPQ mouse heart rates to amplify differences in the QT as defined by the different slopes of the QT-RR100 relations. Together, the data demonstrate that uncoupling light and feeding cues that entrain circadian rhythms slow heart rate to intensify the LQT3-related phenotype in Scn5a+/ΔKPQ mice.
Previous studies.
Classically, the hierarchical organization of the tissue clocks within an integrated system was believed to be dominantly controlled via the SCN, which is entrained by the daily light-dark cycle (31). However, recent studies have demonstrated that feeding nocturnal mice exclusively during the light phase shifts many of the peripheral tissue clocks, including the heart, independent of the central clock in the SCN (4, 6). Thus time of feeding acts as a dominant environmental time cue over light for the timing of peripheral circadian rhythms. Studies show that even though light phase-restricted feeding in mice does not alter the phase of clock gene expression in the SCN or voluntary cage activity, it results in significant changes in core body temperature (6). We now report analogous changes in heart rate and ventricular repolarization. These are the first data to show that the circadian rhythm in heart rate and ventricular repolarization can be uncoupled from the light-dark cycle. Thus the significant shift and changes in core body temperature, heart rate, and ventricular repolarization argues that the circadian rhythms of these physiological variables are not dominantly controlled via the SCN to autonomic nervous system regulation but rather influenced by the metabolic rate of peripheral organs (4).
Similar to what has been shown in LQT3 patients, we found that the Scn5a+/ΔKPQ mice showed a time-of-day prolongation in their QTc intervals (using the modified Bazett equation) (30). The time-of-day prolongation in the QTc interval tracked with the daily trough in heart rate and flipped from the light to the dark phase after light phase-restricted feeding. We found that the QT-RR100 relation was steeper in the Scn5a+/ΔKPQ mice, which is consistent with what has been seen in ungenotyped LQTS patients (16) and the observation that LQT3 patients a show a rate-dependent prolongation in their QT interval at slower heart rates (28). The QT interval is often seen as a correlate with the duration of the ventricular action potential, and several groups show action potentials recorded from Scn5a+/ΔKPQ mouse cardiomyocytes (atrial or ventricular) exhibit a greater prolongation at slower cycle lengths than their WT counterparts (9, 11, 21). This phenomenon is secondary to the dysfunctional molecular impact that LQT3 mutations have on the Scn5a-encoded Nav1.5 channel. Most LQT3-linked mutations (including Scn5a-ΔKPQ) disrupt Nav1.5 inactivation gating to cause an increase in the sustained or late Na+ current (INa,L) (34), and Nagatomo and colleagues (20) showed that the size of the INa,L becomes progressively larger in cells paced at slower cycle lengths. Therefore, the steeper QT-RR100 relation in the Scn5a+/ΔKPQ mice is likely secondary to a disproportionate increase in the amplitude of the INa,L at slower heart rates.
Implications.
An emergent area of clinical research is focusing on circadian rhythms and cardiovascular disease (14). For example, it is well recognized that the incidence of several adverse cardiovascular events, like sudden cardiac death (18, 35) and myocardial infarction (19), peak in the early morning hours. Recently, clinicians have begun to monitor daily rhythms in heart rate and blood pressure as an index for abnormal autonomic function (14). A blunting in the daily rhythms of the heart rate and blood pressure are useful prognostic indicators for patients with hypertension, myocardial infarction, or diabetes (7, 8, 10). Our data suggest that individual monitoring of daily heart rate rhythms in LQT3 patients might help identify/predict times of the time of day when a patient has the greatest risk for arrhythmias. Whether or not this daily biofeedback would allow LQT3 patients to tailor the timing of medications or alter their eating and sleeping patterns to help regulate heart rate and minimize the risk for LQT3-related symptoms warrants further investigation.
Study limitations.
Even though light phase-restricted feeding unmasked genotype-specific changes in the RR and QT intervals between WT and Scn5a+/ΔKPQ mice, we only observed an overt atrial arrhythmia phenotype in one of the tRF Scn5a+/ΔKPQ mice, and we did not observe any obvious ventricular tachycardia phenotypes. This is not surprising since we monitored the mice for a total of ∼6 days, and the absolute incidence of LQT3-related life-threatening tachyarrhythmia is extremely low in mice and people in basal conditions (9, 12, 23). An animal model that shows lots of arrhythmias under basal conditions would be inconsistent with what is most often seen in LQTS patients. Regardless, we must be cautious when extrapolating data obtained in mice to people.
In summary, this study introduces a novel and important concept that circadian uncoupling has a meaningful effect on heart rate and the LQT3 phenotype in Scn5a+/ΔKPQ mice. These changes are secondary to a slowing of the heart rate that uncover genotype-specific differences in the QT-RR100 relations. Future studies that explore additional circadian time cues or drugs that influence the heart rate and/or the QT-RR100 relation, might identify ways to minimize the risk for LQT3-related phenotypes. Whether or not similar mechanisms exist in LQT3 patients warrants further investigation.
GRANTS
This work was supported by the University of Kentucky undergraduate summer research and creativity fellowship (to C. L. Manning) and National Institutes of Health Grants RC1ES018636 and AR55246 (to K. A. Esser) and R01 HL087039 (to B. P. Delisle).
DISCLOSURES
B. P. Delisle has a research contract with Gilead Scientific.
AUTHOR CONTRIBUTIONS
E.A.S., K.A.E., and B.P.D. conception and design of research; E.A.S., D.E.B., C.L.M., Y.Z., and B.P.D. analyzed data; E.A.S., A.J.M., A.R.P., C.S.E., K.A.E., and B.P.D. interpreted results of experiments; E.A.S., C.L.M., and B.P.D. prepared figures; E.A.S. and B.P.D. drafted manuscript; E.A.S., D.E.B., A.J.M., A.R.P., C.S.E., K.A.E., and B.P.D. edited and revised manuscript; E.A.S., D.E.B., Y.Z., A.J.M., A.R.P., C.S.E., K.A.E., and B.P.D. approved final version of manuscript; B.P.D. performed experiments.
ACKNOWLEDGMENTS
We acknowledge support from the University of Kentucky Center for Muscle Biology, Dr. Peter Carmeliet (VIB, Flanders, Belgium) for providing the Scn5a+/ΔKPQ mice, and Dr. Jonathan Makielski (University of Wisconsin-Madison) for assistance in the development of this project.
REFERENCES
- 1.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17: 2100–2102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32: 435–443, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batchvarov V, Yi G, Guo X, Savelieva I, Camm AJ, Malik M. QT interval and QT dispersion measured with the threshold method depend on threshold level. Pacing Clin Electrophysiol 21: 2372–2375, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes (Lond) 37: 843–852, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvillo L, Spazzolini C, Vullo E, Insolia R, Crotti L, Schwartz PJ. Propranolol prevents life-threatening arrhythmias in LQT3 transgenic mice: implications for the clinical management of LQT3 patients. Heart Rhythm 11: 126–132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, Vinyoles E, Pallares V, Sarria A, Aranda P, Ruilope LM; Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension 53: 466–472, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Eguchi K, Ishikawa J, Hoshide S, Pickering TG, Schwartz JE, Shimada K, Kario K. Night time blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am J Hypertens 22: 46–51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabritz L, Damke D, Emmerich M, Kaufmann SG, Theis K, Blana A, Fortmuller L, Laakmann S, Hermann S, Aleynichenko E, Steinfurt J, Volkery D, Riemann B, Kirchhefer U, Franz MR, Breithardt G, Carmeliet E, Schafers M, Maier SK, Carmeliet P, Kirchhof P. Autonomic modulation and antiarrhythmic therapy in a model of long QT syndrome type 3. Cardiovasc Res 87: 60–72, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Bombelli M, Cuspidi C, Facchetti R, Bolla G, Mancia G. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension 52: 925–931, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Lemoine MD, Duverger JE, Naud P, Chartier D, Qi XY, Comtois P, Fabritz L, Kirchhof P, Nattel S. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res 92: 67–74, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Liu JF, Moss AJ, Jons C, Benhorin J, Schwartz PJ, Spazzolini C, Crotti L, Ackerman MJ, McNitt S, Robinson JL, Qi M, Goldenberg I, Zareba W. Mutation-specific risk in two genetic forms of type 3 long QT syndrome. Am J Cardiol 105: 210–213, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol 36: 1749–1766, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Martino TA, Sole MJ. Molecular time: an often overlooked dimension to cardiovascular disease. Circ Res 105: 1047–1061, 2009. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin NB, Campbell RW, Murray A. Comparison of automatic QT measurement techniques in the normal 12 lead electrocardiogram. Br Heart J 74: 84–89, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merri M, Moss AJ, Benhorin J, Locati EH, Alberti M, Badilini F. Relation between ventricular repolarization duration and cardiac cycle length during 24-hour Holter recordings. Findings in normal patients and patients with long QT syndrome. Circulation 85: 1816–1821, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol Heart Circ Physiol 274: H747–H751, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation 75: 131–138, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, Sobel BE, Willerson JT, Braunwald E; the MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313: 1315–1322, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Nagatomo T, January CT, Ye B, Abe H, Nakashima Y, Makielski JC. Rate-dependent QT shortening mechanism for the LQT3 deltaKPQ mutant. Cardiovasc Res 54: 624–629, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos MA, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med 7: 1021–1027, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Qiuzhen X, Reddy S. New algorithms for QT dispersion analysis. In: Computers in Cardiology 1996. Indianapolis: IEEE, 1997, p. 293–296. [Google Scholar]
- 23.Ruan Y, Liu N, Napolitano C, Priori SG. Therapeutic strategies for long-QT syndrome: does the molecular substrate matter? Circ Arrhythm Electrophysiol 1: 290–297, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms 18: 250–260, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Schroder EA, Lefta M, Zhang X, Bartos DC, Feng HZ, Zhao Y, Patwardhan A, Jin JP, Esser KA, Delisle BP. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am J Physiol Cell Physiol 304: C954–C965, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J 34: 3109–3116, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz PJ, Ackerman MJ, George AL, Jr, Wilde AA. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol 62: 169–180, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation 92: 3381–3386, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 103: 89–95, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Stramba-Badiale M, Priori SG, Napolitano C, Locati EH, Vinolas X, Haverkamp W, Schulze-Bahr E, Goulene K, Schwartz PJ. Gene-specific differences in the circadian variation of ventricular repolarization in the long QT syndrome: a key to sudden death during sleep? Ital Heart J 1: 323–328, 2000. [PubMed] [Google Scholar]
- 31.Takahashi JS. Molecular neurobiology and genetics of circadian rhythms in mammals. Annu Rev Neurosci 18: 531–553, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JRB, Bray MS, Young ME. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem 285: 2918–2929, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van den Berg MP, Haaksma J, Veeger NJ, Wilde AA. Diurnal variation of ventricular repolarization in a large family with LQT3-Brugada syndrome characterized by nocturnal sudden death. Heart Rhythm 3: 290–295, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80: 805–811, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol 60: 801–806, 1987. [DOI] [PubMed] [Google Scholar]
- 36.Wolff G, Esser K. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc 44: 1663–1670, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Zhang Y, Zhang X, Cheng L, Lammers WJ, Grace AA, Fraser JA, Zhang H, Huang CL, Lei M. Altered sinoatrial node function and intra-atrial conduction in murine gain-of-function Scn5a+/ΔKPQ hearts suggest an overlap syndrome. Am J Physiol Heart Circ Physiol 302: H1510–H1523, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu T, ZhuGe F, Sun L, Ni Y, Fu O, Gao G, Chen J, Kato H, Fu Z. Enhanced effect of daytime-restricted feeding on the circadian rhythm of streptozotocin-induced type 2 diabetic rats. Am J Physiol Endocrinol Metab 302: E1027–E1035, 2012. [DOI] [PubMed] [Google Scholar]