Abstract
Reductions in arterial SIRT1 expression and activity with aging are linked to vascular endothelial dysfunction. We tested the hypothesis that the specific SIRT1 activator SRT1720 improves endothelial function [endothelium-dependent dilation (EDD)] in old mice. Young (4–9 mo) and old (29–32 mo) male B6D2F1 mice treated with SRT1720 (100 mg/kg body wt) or vehicle for 4 wk were studied with a group of young controls. Compared with the young controls, aortic SIRT1 expression and activity were reduced (P < 0.05) and EDD was impaired (83 ± 2 vs. 96 ± 1%; P < 0.01) in old vehicle-treated animals. SRT1720 normalized SIRT1 expression/activity in old mice and restored EDD (95 ± 1%) by enhancing cyclooxygenase (COX)-2-mediated dilation and protein expression in the absence of changes in nitric oxide bioavailability. Aortic superoxide production and expression of NADPH oxidase 4 (NOX4) were increased in old vehicle mice (P < 0.05), and ex vivo administration of the superoxide scavenger TEMPOL restored EDD in that group. SRT1720 normalized aortic superoxide production in old mice, without altering NOX4 and abolished the improvement in EDD with TEMPOL, while selectively increasing aortic antioxidant enzymes. Aortic nuclear factor-κB (NF-κB) activity and tumor necrosis factor-α (TNF-α) were increased in old vehicle mice (P < 0.05), whereas SRT1720 normalized NF-κB activation and reduced TNF-α in old animals. SIRT1 activation with SRT1720 ameliorates vascular endothelial dysfunction with aging in mice by enhancing COX-2 signaling and reducing oxidative stress and inflammation. Specific activation of SIRT1 is a promising therapeutic strategy for age-related endothelial dysfunction in humans.
Keywords: SIRT1, aging, COX-2 dilation, superoxide, vascular inflammation
despite recent declines in prevalence, cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in modern societies (37). Advancing age is the primary risk factor for CVD, and this is largely attributable to vascular pathology characterized in part by endothelial dysfunction (26). As such, endothelial dysfunction, most commonly assessed as impaired endothelium-dependent dilation (EDD), is an important therapeutic target for the prevention of age-associated CVD (26).
Impaired EDD with aging is mediated by reduced dilation to endothelium-derived dilators, most notably nitric oxide (NO) and vasodilatory prostanoids produced by cyclooxygenase (COX)-1 or COX-2 (40, 43, 45, 50). Impaired NO and prostanoid signaling in this setting is, in turn, associated with oxidative stress featuring increased vascular superoxide production, partially due to enhanced expression of the oxidant enzyme NADPH oxidase (NOX) (14, 21, 45). The oxidative stress stimulates a chronic, low-grade inflammation characterized by increases in inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), possibly mediated by enhanced activation of the proinflammatory transcription factor nuclear factor-κ B (NF-κB) (8, 12, 28). NF-κB activation is stimulated by an increase in intracellular reactive oxygen species (6) and can be regulated by posttranslational modifications, such as acetylation of the subunit p65 at lysine310 (5, 52).
Several novel cellular signaling pathways appear to modulate endothelial function with aging. SIRT1 is a member of the sirtuin family of enzymes associated with lifespan extension and other antiaging effects (4, 33, 34). SIRT1 is a nicotinamide adenine dinucleotide-dependent deacetylase (4), the expression of which decreases with advancing age in several tissues (13, 24, 47). We recently demonstrated that SIRT1 expression and activity decrease with age in the vasculature in both mice and humans, and this contributes to endothelial dysfunction (13, 36). This suggests that pharmacological activation of SIRT1 may hold therapeutic promise for treatment of age-related endothelial dysfunction. In this context, SRT1720, a specific small-molecule activator of SIRT1, exerts beneficial effects in rodent models of age-related metabolic diseases (15, 32) and increases lifespan in mice (33, 34).
In the present study, we hypothesized that a 4-wk treatment with SRT1720 would increase arterial SIRT1 expression and activity and improve vascular endothelial dysfunction as indicated by an increase in EDD in old mice. We also sought to gain insight into the vasodilatory pathways mediating this improvement, as well as any vascular antioxidant and/or anti-inflammatory effects of treatment.
METHODS
Ethical approval.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder and conformed to the Guide to the Care and Use of Laboratory Animals [National Institutes of Health (NIH) Publication No. 85-23, revised 1996].
Animals.
Young (4–9 mo) and old (29–32 mo) male B6D2F1 mice were obtained from the National Institute on Aging rodent colony and were fed normal rodent chow ad libitum. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12:12-h light-dark cycle. Young (n = 14–30 per group) and old (n = 34–35 per group) mice were treated with 100 mg/kg body wt SRT1720 (Sirtris, GlaxoSmithKline, Cambridge, MA) or vehicle (40% PEG-400/0.5% Tween-80/59.5% deionized water) for 4 wk via oral gavage (32) and were compared with a group of young nonvehicle treated (reference control, n = 38) mice that were studied over the same 4-wk period. Body weight was monitored weekly and at death.
Carotid artery vasodilatory responses.
EDD and endothelium-independent dilation (EID), a control measure of vascular smooth muscle responsiveness to NO, were determined ex vivo in isolated carotid arteries as previously described (36, 39). Briefly, mice were anesthetized using isoflurane and euthanized by exsanguination via cardiac puncture. The carotid arteries were carefully excised, cannulated onto glass micropipettes, and secured with nylon (11-0) suture in myograph chambers (DMT) containing buffered physiological saline solutions. The arteries were pressurized to 50 mmHg at 37°C and were allowed to equilibrate for 1 h. After submaximal preconstriction with phenylephrine (2 μM), increases in luminal diameter in response to acetylcholine (ACh; 1 × 10−9-1 × 10−4 M) were measured. To assess the contributions of specific vasodilatory enzymes to EDD, responses to ACh were repeated in the presence of the following inhibitors: the NO synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 0.1 mM, 30-min incubation), the nonspecific COX inhibitor indomethacin (0.01 mM, 60-min incubation) (46), and the COX-2 specific inhibitor N-(2-cyclohexyloxy-4-nitrophenyl)-methanesulfon-amide (NS-398; 10 μM, 30-min incubation) (46). Responses to ACh were also repeated in the presence of the superoxide scavenger TEMPOL (1 mM, 60-min incubation). NO-dependent dilation was determined from the maximal EDD in the absence or presence of l-NAME according to the following formula: NO-dependent dilation (%) = maximum dilationACh − maximal dilationACh + l-NAME. EID was determined by vasodilation in response to sodium nitroprusside (SNP; 1 × 10−10 to 1 × 10−4 M). Sensitivity to ACh was defined as the concentration that elicited 50% of the maximal response.
Arterial protein expression.
Aortas were used as a surrogate large elastic artery to provide sufficient tissue for Western blot analyses and cytokine levels. The use of the carotids for the functional measures of EDD, which included treatments with inhibitors of vasodilator pathways, precluded us from utilizing this tissue for the supporting biochemical measures, as they would not have reflected in vivo conditions. This approach has previously been used by our laboratory (14, 17, 18, 20, 27, 36, 39) and by those of other laboratories (2, 9, 11, 48). Fifteen micrograms of protein were loaded on 4–12% polyacrylamide gels, separated by electrophoresis, and transferred onto nitrocellulose membranes for Western blot analysis. Blots were stripped with Restore Western Blot Stripping Buffer (Pierce Biotechnology, Rockford, IL) and reprobed for glyceraldehyde-3-phosphate (GAPDH) and expression of additional proteins. Blots were developed using enhanced chemiluminescence (ECL; Pierce Biotechnology), and bands were visualized with a digital acquisition system (ChemiDoc-It; UVP, Upland, CA) as previously described (16, 20). Relative intensity of bands was quantified with ImageJ software (NIH) and normalized to GAPDH expression to account for differences in protein loading. These data are expressed relative to the mean of the young control group on a given blot. Representative Western blot bands for each experimental group were obtained from a single blot image for each protein; however, single bands are shown as the order of the experimental groups in the lanes of the polyacrylamide gels differed from the experimental group order that is depicted graphically. Antibodies for Western blot analysis included SIRT1 (1:1,000, 110 kDa; no. ab28170, Abcam, Cambridge, MA), p53 (1:1,000, 53 kDa; no. 2524, Cell Signaling, Danvers, MA), lysine379-acetyl-p53 (1:500, 53 kDa; no. 2570, Cell Signaling), GAPDH (1:1,000, 37 kDa; no. 2118, Cell Signaling), endothelial NOS (eNOS; 1:1,000, 140 kDa; no. 610296, BD Biosciences, San Jose, CA), COX-1 (1:100, 70 kDa; no. 160109, Cayman Chemical, Ann Arbor, MI), COX-2 (1:100, 72 kDa; no. sc-7951, Santa Cruz, Dallas, TX), NADPH oxidase 4 (NOX4; 1:650, 67 kDa; no. ab60940, Abcam), extracellular superoxide dismutase (ecSOD; 1:500; 31/35 kDa doublet; no. S4946, Sigma-Aldrich, St. Louis, MO), manganese superoxide dismutase (MnSOD; 1:2,000, 25 kDa; no. SOD-111, Stressgen, Ann Arbor, MI), copper zinc superoxide dismutase (CuZnSOD; 1:2,000, 19 kDa; no. ADI-SOD 101-E, Enzo Life Sciences, Famingdale, NY), catalase (1:2,500, 65 kDa; no. ab1877, Abcam), lysine310-acetyl NF-κB p65 (1:500, 65 kDa; no. 3045, Cell Signaling), and NF-κB p65 (1:1,000, 65 kDa; no. 3034, Cell Signaling). SIRT1 activity was assessed as the ratio of the known SIRT1 substrate lysine379-acetyl-p53 to total p53. Concentrations of the proinflammatory cytokines TNF-α and IFN-γ were determined in aortic whole cell lysates by multiplex ELISA (Searchlight Mouse Inflammatory Cytokine Kit; Aushon Biosystems, Billerica, MA) as previously described (36).
Arterial superoxide production.
Production of superoxide in two-millimeter aortic rings was measured by electron paramagnetic resonance (EPR) spectrometry using the spin probe 1-hydroxy-3-methoxycarbonly-2,2,5,5-tetramethylpyrrolidine (CMH; Alexis Biochemicals, San Diego, CA) as previously described (36, 39). Aortic rings were incubated for 60 min at 37°C in 200 μl of Krebs-HEPES buffer containing 0.55 mM CMH and analyzed immediately on an MS300 X-band EPR spectrometer (Magnettech, Berlin, Germany).
Statistics.
Results are presented as means ± SE. Statistical analysis was performed with SPSS 21.0 software (IBM, Somers, NY). For the ex vivo vasodilatory dose response, group differences were determined by repeated-measures ANOVA. For animal characteristics, protein expression, maximal dilation, sensitivity, superoxide production, and cytokine levels, comparisons between groups were made using either a one-way or two-way ANOVA. Least squares difference post hoc tests were used where appropriate. Significance was determined using P < 0.05.
RESULTS
Animal characteristics.
Animal characteristics of the groups are shown in Table 1. Body mass, heart mass, and carotid artery lumen maximal diameter were greater in the old mice compared with the young animals (P < 0.05). SRT1720 treatment had no effect on these variables.
Table 1.
Animal characteristics
| YC | OV | YS | OS | |
|---|---|---|---|---|
| Body mass, g | 32 ± 1 | 36 ± 1* | 31 ± 1 | 35 ± 1* |
| Heart mass, mg | 174 ± 7 | 233 ± 8* | 160 ± 4 | 218 ± 4* |
| Carotid artery lumen diameter, μm | 410 ± 3 | 434 ± 6* | 416 ± 4 | 434 ± 4* |
Values are means ± SE.
YC, young control mice; OV, old vehicle-control mice; YS, young SRT1720-treated mice; OS, old SRT1720-treated mice.
P < 0.05 vs. YC.
SIRT1 activation with SRT1720 restores EDD in old mice.
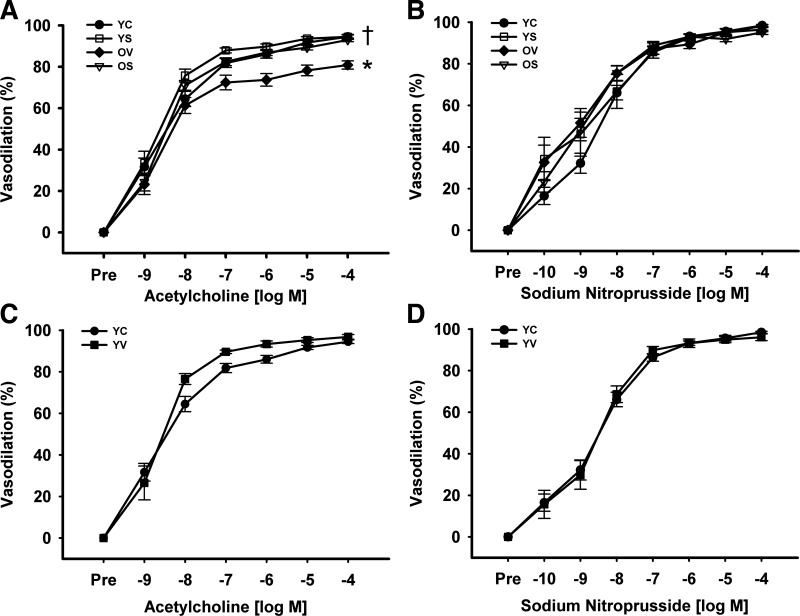
Compared with the young control mice, EDD was impaired in old vehicle-treated mice (83 ± 2 vs. 96 ± 1%; P < 0.01; Fig. 1A). SRT1720 administration restored EDD in old mice (95 ± 1%; P < 0.01) (Fig. 1A). SRT1720 treatment did not affect EDD in young animals (97 ± 1%; P > 0.05; Fig. 1A) compared with the young control mice. EID to SNP (Fig. 1B) and sensitivity to ACh (data not shown) were not different among these groups (all P > 0.05). Lastly, there were no differences in EDD (Fig. 1C), EID (Fig. 1D), or sensitivity to ACh (data not shown) in the young vehicle-treated and young (untreated) control groups (all P > 0.05). As such, young untreated animals were used as the reference control group for subsequent assessments.
Fig. 1.
Endothelium-dependent and endothelium-independent dilation. Dose responses to the endothelium-dependent dilator acetylcholine (ACh; A) and endothelium-independent dilator sodium nitroprusside (SNP; B) in young control (YC), young SRT1720-treated (YS), old vehicle-control (OV), and old SRT1720-treated (OS) mice. Dose responses to ACh (C) and SNP (D) in YC and young vehicle-treated (YV) mice. Values are means ± SE. ACh: YC, n = 38; OV, n = 17; YS, n = 24; OS, n = 28; YV, n = 11. SNP: YC, n = 23; OV, n = 11; YS, n = 13; OS, n = 17; YV, n = 6. Differences in dose-response curves were assessed with repeated-measures ANOVA. Least squares differences post hoc test was used to determine individual group differences to ACh. *P < 0.01 vs. YC; †P < 0.01 vs. OV.
SIRT1 activation with SRT1720 increases COX-2-mediated dilation in old mice.
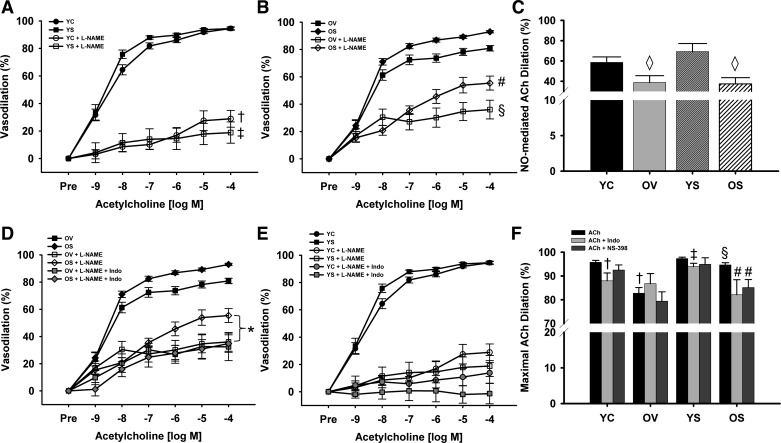
Blockade of NOS with the inhibitor l-NAME dramatically reduced dilation to ACh in all groups of mice (all P < 0.01; Fig. 2, A and B). NO-mediated dilation, i.e., the difference in dilation to ACh in the presence vs. absence of NOS inhibition with l-NAME, was reduced with age in old vehicle-treated mice compared with young control animals (39 ± 7 vs. 59 ± 5%; P < 0.05; Fig. 2C). However, SRT1720 treatment did not influence NO-mediated dilation in old (37 ± 6%; P < 0.05) or young (69 ± 8%; P > 0.05) mice (Fig. 2C). Together, these results indicated that SRT1720 ameliorated the age-related impairment in EDD through a dilator mechanism other than NO.
Fig. 2.
Nitric oxide (NO)-, cyclooxygenase (COX)-, and COX-2-dependent modulation of endothelium-dependent dilation (EDD). EDD to ACh in the absence or presence of NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME) in YC and YS mice (A) and OV and OS mice (B). C: NO-mediated ACh dilation (maximal dilation to ACh − maximal dilation to ACh + l-NAME) in YC, OV, YS, and OS mice. EDD to ACh in the absence or presence of l-NAME and l-NAME plus the COX inhibitor indomethacin (Indo) in OV and OS mice (D) and in YC and YS mice (E). F: maximal EDD to ACh in the absence or presence of Indo or the COX-2 specific inhibitor N-(2-cyclohexyloxy-4-nitrophenyl)-methanesulfon-amide (NS-398) in YC, OV, YS, and OS mice. Values are means ± SE. ACh + l-NAME: YC, n = 12; OV, n = 11; YS, n = 14; OS, n = 14. NO-mediated ACh dilation: YC, n = 12; OV, n = 10; YS, n = 12; OS, n = 12. ACh + l-NAME + Indo: YC, n = 8; OV, n = 7; YS, n = 8; OS, n = 7. ACh + Indo: YC, n = 11; OV, n = 6; YS, n = 8; OS, n = 9. ACh + NS-398: YC, n = 12; OV, n = 7; YS, n = 7; OS, n = 13. Differences in dose-response curves were assessed with repeated-measures ANOVA. Differences in NO-mediated ACh maximal dilation and in maximal dilation to ACh with or without Indo and NS-398 were determined via one-way ANOVA and least squares differences post hoc test. †P < 0.05 vs. YC ACh; ‡P < 0.05 YS ACh; §P < 0.05 vs. OV ACh; #P < 0.05 vs. OS ACh; ◇P < 0.05 vs. YC NO-mediated ACh dilation. *P < 0.05 vs. OS ACh + l-NAME.
Accordingly, dilation to ACh next was repeated in the presence of both l-NAME and the nonspecific COX inhibitor indomethacin. Combined NOS and COX inhibition selectively reduced dilation in old mice treated with SRT1720 compared with NOS inhibition alone (35 ± 6 vs. 55 ± 5%; P < 0.05; Fig. 2D) but did not affect dilation in old vehicle-treated animals (32 ± 10 vs. 36 ± 7%; Fig. 2D) or young mice (Fig. 2E; all P > 0.05). These observations suggested that the improvement in EDD in old mice treated with SRT1720 was mediated by increases in the production of COX vasodilators.
To confirm SRT1720 treatment restored EDD in old mice via enhanced COX vasodilator production, dilation to ACh with indomethacin was repeated without NOS inhibition. Inhibition of COX vasodilators did not change maximal dilation in old vehicle-treated animals (87 ± 4%; P > 0.05; Fig. 2F) but reduced maximal EDD in old SRT1720-treated mice compared with dilation to ACh alone (82 ± 6 vs. 95 ± 1%; P < 0.01; Fig. 2F), resulting in similar dilation to that observed in the old vehicle-treated animals. These findings indicate an age-related loss of COX-mediated dilation, which is restored with SRT1720 treatment.
To determine the specific COX isoform mediating the increase in EDD in old mice treated with SRT1720, dilation to ACh was repeated in the presence of the COX-2-specific inhibitor NS-398. COX-2 inhibition selectively reduced maximal dilation to ACh in old mice treated with SRT1720 (85 ± 3 vs. 95 ± 1%; P < 0.01; Fig. 2F), thus abolishing treatment differences in the old animals. There were no effects on the young animals. These results demonstrate that treatment with SRT1720 improves EDD in old mice via increased production of COX-2 vasodilators.
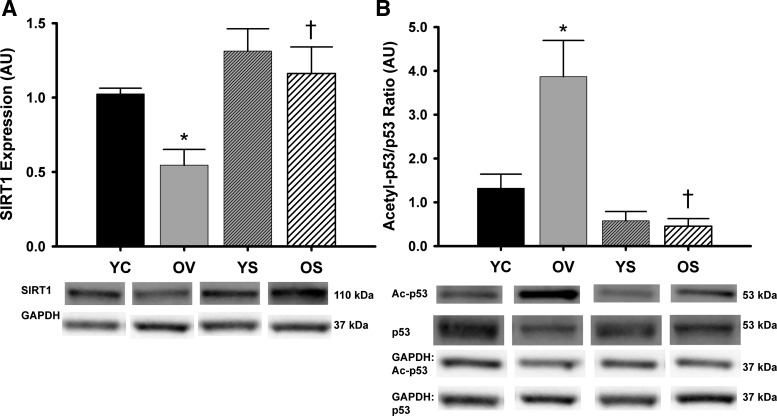
SRT1720 restores aortic SIRT1 expression and activity in old mice.
SIRT1 protein expression was reduced 47% in aorta of old vehicle-treated mice compared with young control animals (P < 0.05; Fig. 3A). SRT1720 treatment restored SIRT1 expression in old mice but had no effect in young animals (Fig. 3A). Moreover, the expression ratio of the known SIRT1 substrate acetyl-p53 to total p53 was 193% greater in aorta of old vehicle-treated mice compared with young control animals (P < 0.05), indicating reduced SIRT1 activity (Fig. 3B). This age-related increase in the acetyl-p53-to-total p53 ratio was reversed in SRT1720-treated old mice, indicating enhanced aortic SIRT1 activity with SRT1720 treatment (Fig. 3B).
Fig. 3.
Arterial SIRT1 expression and activity. Aortic protein expression of SIRT1 (A) and ratio of acetylated-p53 to total p53 (B) in YC, OV, YS, and OS mice. Data are expressed relative to GAPDH and normalized to YC mean value. Individual representative Western blot bands from a single image are at bottom. AU, arbitrary units. Values are means ± SE. SIRT1: YC, n = 15; OV, n = 11; YS, n = 14; OS, n = 14. Acetyl-p53/p53: YC, n = 7; OV, n = 8; YS, n = 5; OS, n = 5. Differences in protein expression were determined with one-way ANOVA and least squares differences post hoc test. *P < 0.05 vs. YC; †P < 0.05 vs. OV.
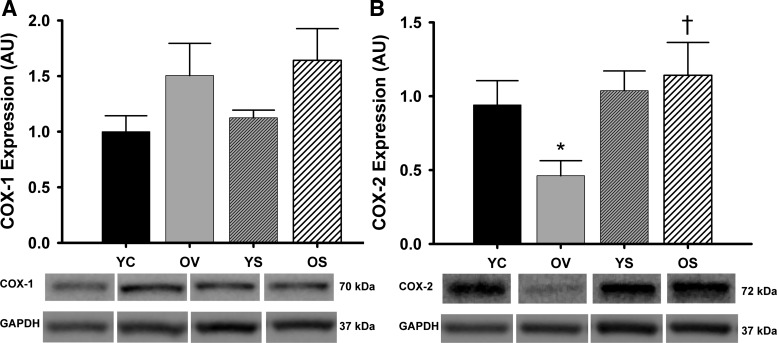
SRT1720 restores arterial COX-2 protein expression in old mice.
Aortic protein expression of COX-1 was unchanged with age or SRT1720 treatment (P > 0.05; Fig. 4A). However, COX-2 protein expression was reduced by 50% in old mice treated with vehicle compared with young control mice (P = 0.05; Fig. 4B). SIRT1 activation with SRT1720 treatment normalized COX-2 expression in old mice but had no effect in young mice (Fig. 4B). Aortic protein expression of eNOS was unchanged with age or SRT1720-treatment (P > 0.05) (data not shown). These results show arterial COX-2 protein expression is reduced with aging but is restored in old mice with SRT1720 treatment.
Fig. 4.
Aortic expression of COX enzymes. Aortic protein expression of COX-1 (A) and COX-2 (B) in YC, OV, YS, and OS mice. Data are expressed relative to GAPDH and normalized to YC mean value. Individual representative Western blot bands from a single image are at bottom. Values are means ± SE. COX-1: YC, n = 7; OV, n = 8; YS, n = 11; OS, n = 9. COX-2: YC, n = 6; OV, n = 6; YS, n = 8; OS, n = 6. Differences in protein expression were determined with one-way ANOVA. Least squares differences post hoc test was used to determine individual group differences to COX-2. *P = 0.05 vs. YC; †P < 0.05 vs. OV.
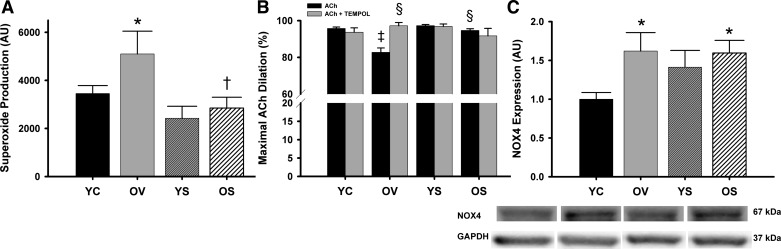
SRT1720 normalizes vascular superoxide and superoxide suppression of EDD without altering NOX4 protein expression and selectively increases antioxidant enzymes in old mice.
Aortic superoxide production was 46% greater in the old mice treated with vehicle compared with young control mice (P < 0.05), and SRT1720 treatment in old mice restored superoxide to levels comparable with young animals (Fig. 5A). Consistent with this, incubation with TEMPOL, a superoxide scavenger, restored EDD in carotid arteries from old vehicle-treated mice (83 ± 3 vs. 97 ± 1%; P < 0.01) without affecting EDD in the other groups (all P > 0.05; Fig. 5B). However, aortic expression of the oxidant enzyme NOX4 was increased with age (P < 0.05) but was not altered with SRT1720 treatment (P > 0.05; Fig. 5C). Together, these observations indicate that SRT1720 treatment ameliorates the increase in arterial superoxide production with aging and its suppression of endothelial function in old animals in a manner independent of changes in NOX4 protein expression.
Fig. 5.
Arterial superoxide production, superoxide-dependent modulation of endothelium-dependent dilation, and oxidant enzyme expression. A: mean electron paramagnetic resonance (EPR) signal of aortic rings from YC, OV, YS, and OS mice. B: maximal dilation of carotid arteries to ACh and to ACh + TEMPOL, a superoxide scavenger, in YC, OV, YS, and OS mice. C: aortic protein expression of NADPH oxidase 4 (NOX4) in YC, OV, YS, and OS mice. Western blot data are expressed relative to GAPDH and normalized to YC mean value. Individual representative Western blot bands from a single image are at bottom. Values are means ± SE. EPR: YC, n = 16; OV, n = 5; YS, n = 7; OS, n = 7. ACh + TEMPOL: YC, n = 8; OV, n = 6; YS, n = 7; OS, n = 8. NOX4: YC, n = 9; OV, n = 8; YS, n = 7; OS, n = 7. Group n for ACh are listed in Fig. 1. Differences in EPR and maximal dilation in the presence or absence of TEMPOL were determined with one-way ANOVA and least squares differences post hoc test. Differences in NOX4 protein expression were determined with one-way ANOVA (P = 0.06) and two-way ANOVA (treatment × age). *P < 0.05 vs. YC; †P < 0.05 vs. OV; ‡P < 0.05 vs. YC ACh; §P < 0.05 vs. OV ACh.
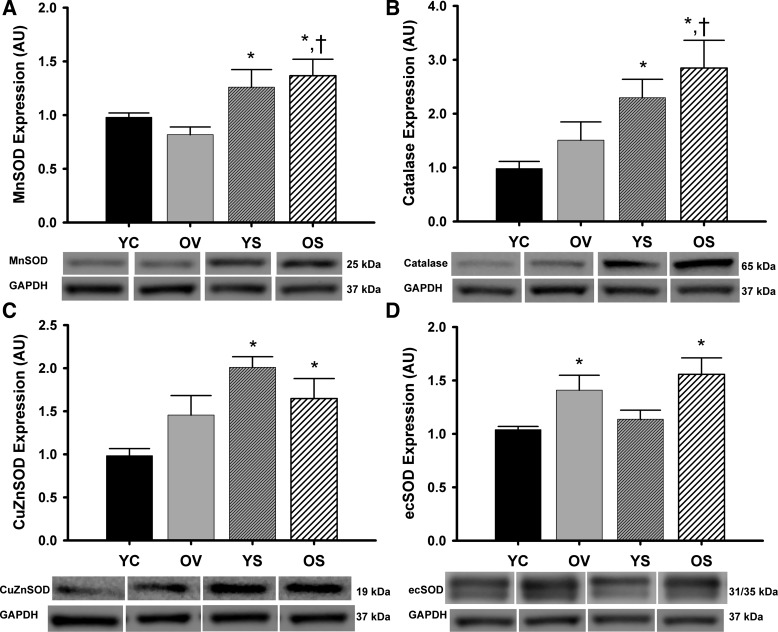
Aortic expression of the antioxidant enzymes SOD and catalase was either unchanged or increased in old vehicle-treated mice compared with young controls (Fig. 6, A-D). In old mice, SRT1720 treatment increased MnSOD by 67% and catalase by 89% compared with vehicle treatment (both P < 0.05; Fig. 6, A and B) and increased expression of MnSOD, CuZnSOD, ecSOD, and catalase above levels observed in young control mice (all P < 0.05; Fig. 6, A–D). In general, SRT1720 also increased antioxidant expression in young mice. These data suggest that SIRT1 selectively enhances arterial antioxidant enzyme expression in both old and young mice.
Fig. 6.
Arterial antioxidant enzymes. Aortic protein expression of manganese superoxide dismutase (MnSOD; A), catalase (B), copper zinc superoxide dismutase (CuZnSOD; C), and extracellular superoxide dismutase (ecSOD; D) from YC, OV, YS, and OS mice. Data are expressed relative to GAPDH and normalized to YC mean value. Individual representative Western blot bands from a single image are at bottom. Values are means ± SE. MnSOD: YC, n = 16; OV, n = 13; YS, n = 15; OS, n = 15. Catalase: YC, n = 11; OV, n = 10; YS, n = 12; OS, n = 13. CuZnSOD: YC, n = 11; OV, n = 9; YS, n = 10; OS, n = 10. ecSOD: YC, n = 13; OV, n = 11; YS, n = 15; OS, n = 13. Differences in protein expression were determined with one-way ANOVA and least squares differences post hoc test. *P < 0.05 vs. YC; †P < 0.05 vs. OV.
SIRT1 activation with SRT1720 reduces arterial inflammation in old mice.
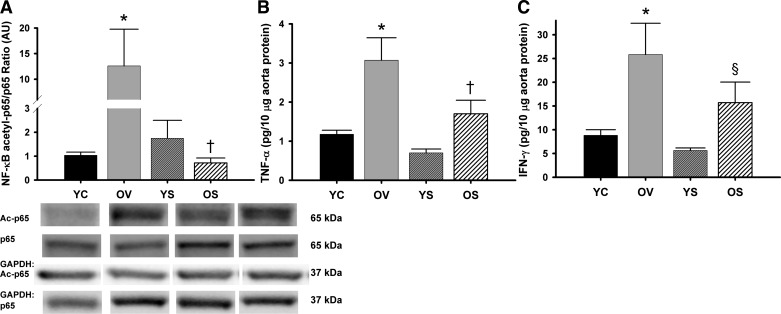
NF-κB activation, assessed by the NF-κB acetyl-p65-to-total p65 ratio, was increased ∼12-fold in aorta from the old vehicle-treated mice compared with the young controls (P < 0.05; Fig. 7A). Treatment with SRT1720 completely reversed the activation of NF-κB in old mice (Fig. 7A). Expression of the inflammatory cytokines TNF-α and IFN-γ were elevated 161% and 193%, respectively, in aorta of old vehicle-treated compared with young control animals (P < 0.05; Fig. 7, B and C). In older animals, SRT1720 activation decreased the expression of TNF-α by 45% (P < 0.05; Fig. 7B) and tended to reduce levels of IFN-γ by 39% (P = 0.1; Fig. 7C). SRT1720 treatment had no effect on NF-κB activation or inflammatory cytokine expression in young mice (Fig. 7, A–C). These data indicate that 4 wk of SIRT1 activation with SRT1720 ameliorates age-related increases in arterial NF-κB activation and reduces expression of inflammatory cytokines in aorta of old mice.
Fig. 7.
Arterial inflammation. Aortic protein expression of ratio of nuclear factor-κB (NF-κB) subunit acetyl-p65 to total p65 (A), tumor necrosis factor-α (TNF-α; B), and interferon-γ (IFN-γ; C) in YC, OV, YS, and OS mice. Western data are expressed relative to GAPDH and normalized to YC mean value. Individual representative Western blot bands from a single image are at bottom. Values are mean ± SE. NF-κB acetyl-p65/p65: YC, n = 6; OV, n = 6; YS, n = 8; OS, n = 8. TNF-α: YC, n = 4; OV, n = 5; YS, n = 3; OS, n = 6. IFN-γ: YC, n = 4; OV, n = 5; YS, n = 3; OS, n = 6. Differences in protein expression and cytokine levels were determined with one-way ANOVA and least squares differences post hoc test. *P < 0.05 vs. YC; †P < 0.05 vs. OV; §P = 0.1 vs. OV.
DISCUSSION
The key novel findings from the present study are that direct activation of SIRT1 with SRT1720 for 4 wk ameliorates age-related vascular endothelial dysfunction via enhanced COX-2 expression and vasodilator production and restores arterial SIRT1 activity and expression in old mice. SIRT1 activation by SRT1720 also normalizes vascular superoxide and its suppression of endothelial function in old animals and stimulates expression of arterial antioxidant enzymes. Moreover, SRT1720 treatment reverses age-associated arterial NF-κB activation and reduces inflammatory cytokines in old mice. To our knowledge, this is the first study to show the efficacy of direct SIRT1 activation for improving vascular dysfunction and reducing arterial oxidative stress and inflammation with aging. These preclinical findings provide evidence for the translational potential of direct SIRT1 activation as a strategy to reduce the risk of age-related CVD in humans.
Vascular endothelial dysfunction, NO, and COX-2.
The present findings of age-related reductions in vascular endothelial function, as indicated by impaired EDD, are consistent with previous findings from our laboratory (28, 36) and others (35, 45). Recently, we showed that ex vivo inhibition of SIRT1 in femoral arteries abolished age-related differences in vascular endothelial function (13), and previously it was reported that endothelial-specific expression of a dominant negative SIRT1 mutant impairs EDD (30). The present results extend the previous findings by demonstrating that activation of SIRT1 with SRT1720 restores EDD in old mice to levels similar to young animals.
The present study found impaired NO-mediated dilation with age, consistent with our previous findings and those of others (21, 35, 36, 39, 45). SIRT1 has been reported to increase NO production via direct deacetylation and activation of eNOS (13, 30) and to enhance EDD, at least under some conditions (30). However, the normalization of EDD by SIRT1 activation with SRT1720 in old mice in our study was not mediated by increased NO bioavailability nor associated with changes in eNOS. We suspect the lack of improvement in NO-mediated dilation in the current study may be due to the allosteric activation of SIRT1 with SRT1720, which strongly depends on substrate structure (10, 23), indicating SRT1720 may not enhance SIRT1-mediated deacetylation, and hence activation, of eNOS to increase NO production.
Rather, our results indicate that SIRT1 activation with SRT1720 improves EDD in old mice by enhancing the production of COX-2 vasodilators. Endothelial COX-2 production of vasodilators can act as a compensatory mechanism to maintain EDD in settings of impaired NO-mediated dilation (19, 44). The current study provides the first evidence that enhanced COX-2 vasodilation via SIRT1 activation can restore EDD in a chronic state of endothelial dysfunction. Consistent with the improvement in vasodilatory function, we found an age-related decrease in arterial COX-2 protein, which was restored with SRT1720 treatment, whereas there was no change in COX-1 with age or treatment. This is in agreement with the results of a recent study showing that SRT1720 protects against apoptosis in response to kidney injury by induction of renal COX-2 expression via SIRT1 transcriptional control of COX-2 through the COX2 gene promoter (22).
Arterial SIRT1 expression and activity.
The present results are consistent with our previous observations and those of others showing reduced vascular SIRT1 expression and/or activity with age in rodents (13, 36, 47) and humans (13, 24). The current study extends these findings by establishing that SIRT1 activation with SRT1720 normalizes protein expression and activity of SIRT1 in arteries of aged mice to those of young mice. SIRT1 is known to exert positive transcriptional regulation of itself via enhanced deacetylation and activity of transcription factors resulting in elevated SIRT1 protein expression (31, 51). SRT1720 has previously been shown to increase SIRT1 expression in liver and brown adipose tissue of mice on a high-fat diet (15).
Arterial superoxide and expression of oxidant and antioxidant enzymes.
Using direct measurements via EPR spectroscopy, we have previously shown increased superoxide production in aorta with aging in mice (36, 39) and demonstrated that this is associated with superoxide-mediated impairment of EDD (36, 39). Here we show for the first time that SIRT1 activation by SRT1720 normalizes aortic superoxide production in old mice and abolishes superoxide-induced impairment of EDD in old animals. These observations are consistent with a previous report of reductions in superoxide production in coronary endothelial cells in vitro after treatment with the nonspecific SIRT1 activator resveratrol (49).
The current results also demonstrate an increase in aortic levels of the oxidant enzyme NOX4 with aging, in agreement with previous work in rats (29). Resveratrol has been shown to reduce NOX4 expression in cultured endothelial cells (42), and further SIRT1 inhibition has also been shown to increase NOX4 levels in aortic rings (55). However, we did not find a change in aortic NOX4 expression with SIRT1 activation with SRT1720, indicating reductions in NOX4 levels did not contribute to the decrease in arterial superoxide in old mice treated with SRT1720. The discrepancy between our results and those found in vitro in endothelial cells with resveratrol may be due to the well-known pleiotropic effects mediated by resveratrol vs. the use of SRT1720 (41).
In the present study, we also found that SIRT1 activation with SRT1720 selectively increased arterial expression of key antioxidant enzymes in old, as well as young, mice. Specifically, aortic MnSOD, CuZnSOD, and catalase were upregulated with SRT1720 treatment in old and/or young animals, whereas ecSOD was unchanged. There was a tendency for some antioxidant enzymes to be greater with aging per se, possibly as an attempt to defend against chronic oxidative stress. Our results here are in agreement with earlier findings that activation of SIRT1 induces expression of MnSOD and catalase (1, 49) and suggest that upregulation of antioxidant enzymes may contribute to reductions in superoxide-mediated suppression of EDD with SRT1720 treatment.
Arterial inflammation.
We have previously reported vascular NF-κB activation accompanied by increased levels of proinflammatory cytokines with aging in both humans (12) and mice (28). Consistent with this, in the current study we found an increase in aortic NF-κB activation in old mice, associated with elevated expression of the inflammatory cytokines TNF-α and IFN-γ. Most importantly, we extend these previous findings by demonstrating that SIRT1 activation with SRT1720 completely reverses NF-κB activation in old animals and that this is associated with decreased expression of TNF-α and a trend towards reduced levels of IFN-γ. These observations are in agreement with previous reports showing that SIRT1 inhibits NF-κB transcriptional activity via deacetylation of the p65 subunit (52) and, conversely, that inhibition of SIRT1 increases acetylation of p65, resulting in enhanced NF-κB activation (53). Moreover, SRT1720 administration per se reduces acetylation of NF-κB p65 in vitro (54) and in vivo (53) and decreases gene expression of proinflammatory cytokines in liver (33, 53) and heart (33). To our knowledge, the present findings are the first evidence demonstrating SIRT1 activation with SRT1720 reduces NF-κB activation and partially normalizes age-associated increases in inflammatory cytokines in vascular tissue.
Experimental considerations.
We wish to emphasize several experimental considerations. We did not directly measure prostanoids associated with COX vasodilatory activity. These molecules have very short half-lives (25, 38), and their metabolites are present in very small amounts in vivo (3). We studied different arteries for vasodilation and supporting biochemical measures. Both carotids were utilized for assessment of vascular endothelial function, and this included the addition of inhibitors to the vessel bath to assess the influence of vasodilatory enzymes and superoxide on vascular function. The aorta, another large elastic artery, was used to assess protein expression and superoxide production as described previously by our laboratory (14, 17, 18, 20, 27, 36, 39) and others (2, 9, 11, 48). Finally, we did not perform extensive biochemical assessments of eNOS, including expression of acetylated-eNOS, because the improvement in EDD with SRT1720 treatment was not mediated by increases in NO bioavailability.
Summary and conclusions.
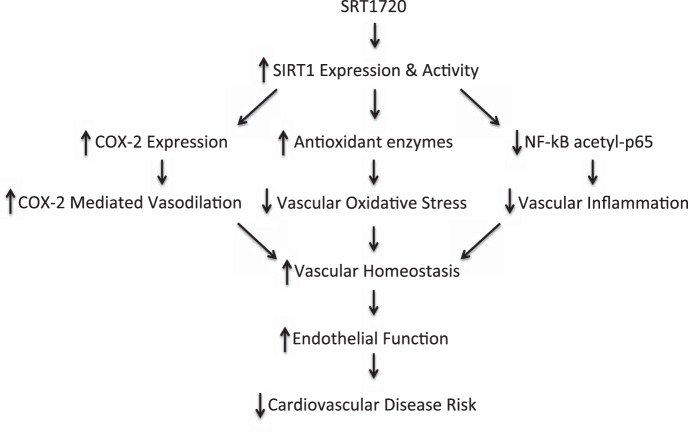
The results of the current study are the first to demonstrate that SIRT1 activation with SRT1720 ameliorates vascular endothelial dysfunction with aging via enhanced production of COX-2 vasodilators associated with upregulation of COX-2 protein and normalizes arterial SIRT1 expression and activity. The restoration of endothelial function in old animals with SRT1720 is associated with normalization of arterial superoxide production and reversal of superoxide-mediated suppression of EDD, accompanied by selective improvements in antioxidant enzyme expression. We further show that SIRT1 activation with SRT1720 reverses arterial NF-κB activation and mitigates vascular inflammation in old mice (Fig. 8).
Fig. 8.
Working hypothesis. In old mice, treatment with SRT1720 increases aortic SIRT1 activity and expression and this results in increased arterial COX-2 protein and enhanced COX-2 mediated vasodilation, as well as an upregulation of aortic antioxidant enzymes and reduced vascular oxidative stress, and a decrease in NF-κB acetyl-p65 levels and a reduction in arterial inflammation. These changes restore vascular homeostasis and ameliorate the age-related decline in endothelial function, which may reduce the risk of cardiovascular disease.
Previous reports of the beneficial effects of increased SIRT1 activity on the vasculature have utilized genetic manipulation (30, 56) or nonspecific activators such as resveratrol (7). The novel results of the present study provide the first direct support for the potential of therapies aimed at specific SIRT1 activation to reverse key features of arterial aging with the promise of reducing the risk of age-associated CVD in humans.
GRANTS
This work was supported by National Institute on Aging Grants AG-013038, AG-040297, AG-029337, AG-000279, and AG-045339.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.B.G., A.J.D., and D.R.S. conception and design of research; L.B.G., H.M.P., C.M.H., and A.L.S. performed experiments; L.B.G. analyzed data; L.B.G., A.J.D., and D.R.S. interpreted results of experiments; L.B.G. prepared figures; L.B.G. drafted manuscript; L.B.G., A.J.D., A.L.S., and D.R.S. edited and revised manuscript; L.B.G., A.J.D., H.M.P., C.M.H., A.L.S., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
All experiments were performed at the University of Colorado Boulder. We thank Shin-ichiro Imai for critical review of the manuscript and Melanie Zigler and Brooke Lawson for technical assistance.
REFERENCES
- 1.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Cawello W, Schweer H, Muller R, Bonn R, Seyberth HW. Metabolism and pharmacokinetics of prostaglandin E1 administered by intravenous infusion in human subjects. Eur J Clin Pharmacol 46: 275–277, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25: 138–145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 25: 7966–7975, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal 8: 572–581, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J 17: 1183–1185, 2003. [DOI] [PubMed] [Google Scholar]
- 9.d'Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension 49: 1142–1148, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem 285: 32695–32703, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res 91: 938–944, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589: 4545–4554, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8: 347–358, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gendron ME, Thorin-Trescases N, Villeneuve L, Thorin E. Aging associated with mild dyslipidemia reveals that COX-2 preserves dilation despite endothelial dysfunction. Am J Physiol Heart Circ Physiol 292: H451–H458, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001. [DOI] [PubMed] [Google Scholar]
- 22.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120: 1056–1068, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, ESY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339: 1216–1219, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao CL, Chen LK, Chang YL, Yung MC, Hsu CC, Chen YC, Lo WL, Chen SJ, Ku HH, Hwang SJ. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb 17: 970–979, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Kawabe J, Ushikubi F, Hasebe N. Prostacyclin in vascular diseases. Recent insights and future perspectives. Circulation 74: 836–843, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64: 9–20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci 66: 409–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Smith A, Hagen TM, Frei B. Vascular oxidative stress and inflammation increase with age: ameliorating effects of alpha-lipoic acid supplementation. Ann NY Acad Sci 1203: 151–159, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Luscher B, Larsson LG, Hermeking H. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc Natl Acad Sci USA 109: E187–196, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci Rep 1: 70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6: 836–843, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125: e2–e220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rolland PH, Jouve R, Pellegrin E, Mercier C, Serradimigni A. Alteration in prostacyclin and prostaglandin E2 production. Correlation with changes in human aortic atherosclerotic disease. Arteriosclerosis 4: 70–78, 1984. [DOI] [PubMed] [Google Scholar]
- 39.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci 102: 595–600, 2002. [PubMed] [Google Scholar]
- 41.Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci 67: 158–167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol 60, Suppl 4: 111–116, 2009. [PubMed] [Google Scholar]
- 43.Spier SA, Delp MD, Stallone JN, Dominguez JM, 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am J Physiol Heart Circ Physiol 291: H1429–H1435, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol 285: H2255–H2263, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J 27: 4332–4342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodman CR, Price EM, Laughlin MH. Selected Contribution: aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol 95: 2164–2170, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem 286: 5289–5299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, Considine T, Riera T, Suri V, White B, Ellis JL, Vlasuk GP, Loh C. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS One 7: e46364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29: 1363–1374, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C, Bandyopadhyay G, Olefsky JM. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab 298: E419–E428, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, Romero M, Gomez-Guzman M, Ungvary Z, Perez-Vizcaino F, Jimenez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 85: 1288–1296, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 80: 191–199, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]