Abstract
Transfusion of banked red blood cells (RBCs) has been associated with poor cardiovascular outcomes. Storage-induced alterations in RBC glycolytic flux, attenuated ATP export, and microvascular adhesion of transfused RBCs in vivo could contribute, but the underlying mechanisms have not been tested. We tested the novel hypothesis that improving deoxygenation-induced metabolic flux and the associated intracellular ATP generation in stored RBCs (sRBCs) results in an increased extracellular ATP export and suppresses microvascular adhesion of RBCs to endothelium in vivo following transfusion. We show deficient intracellular ATP production and ATP export by human sRBCs during deoxygenation (impairments ∼42% and 49%, respectively). sRBC pretreatment with a solution containing glycolytic intermediate/purine/phosphate precursors (i.e., “PIPA”) restored deoxygenation-induced intracellular ATP production and promoted extracellular ATP export (improvement ∼120% and 50%, respectively). In a nude mouse model of transfusion, adhesion of human RBCs to the microvasculature in vivo was examined. Only 2% of fresh RBCs (fRBCs) transfused adhered to the vascular wall, compared with 16% of sRBCs transfused. PIPA pretreatment of sRBCs significantly reduced adhesion to just 5%. In hypoxia, adhesion of sRBCs transfused was significantly augmented (up to 21%), but not following transfusion of fRBCs or PIPA-treated sRBCs (3.5% or 6%). Enhancing the capacity for deoxygenation-induced glycolytic flux within sRBCs increases their ability to generate intracellular ATP, improves the inducible export of extracellular anti-adhesive ATP, and consequently suppresses adhesion of stored, transfused RBCs to the vascular wall in vivo.
Keywords: erythrocytes, adenosine triphosphate, hypoxia, storage
red blood cell (rbc) transfusion is a common and sometimes lifesaving medical practice, but accumulating evidence questions both the criteria indicating a transfusion requirement and the relative benefits of transfusing RBCs later in their storage period (42-day maximum duration in the United States) (2, 5, 16–18, 20). Banking of RBCs elicits a “storage lesion” characterized by time-dependent metabolic and biochemical alterations including diminished intracellular ATP and 2,3-DPG, and a loss of bioactive nitric oxide (NO) derivatives (2, 30, 31, 33). Functionally, longer-banked RBCs are less deformable, promote hypoxic vasodilation poorly, and adhere excessively to blood vessel walls (2, 30, 31, 43). Such effects from RBC banking could contribute to the disappointing, adverse clinical outcomes (e.g., increased hospital days and duration of mechanical ventilation, multiple organ failure, transfusion-related lung injury, mortality) often associated with transfusion of longer-stored RBCs (20, 31, 32).
Remarkably, RBCs rely solely on glycolysis for intracellular ATP production, and hemoglobin (Hb) deoxygenation is a primary and potent activator of glycolytic flux within the RBC (4, 25, 39). Glycolytic enzymes normally bound to the RBC cytoplasmic domain of the membrane protein band 3 (cdb3; band 3 is also called anion exchanger-1) during R-state Hb conformation are liberated upon T-state Hb transition, thus shifting RBC metabolism from the pentose phosphate pathway (PPP; supporting generation of NADPH) toward the Embden Myerhof pathway (glycolytic production of NADH and ATP) (4, 23, 25). In this regard, coupling of RBC energy metabolism and O2-sensing is directly governed by O2-dependent regulation of binding of Hb by cdb3 (23, 25). Increasing intracellular ATP production, particularly in hypoxia, is essential for ion transport processes, phosphorylation reactions, and the production of reducing equivalents. These ATP-dependent events in turn regulate osmotic balance, cellular integrity, and redox reactions (9, 35, 39).
Over a decade of investigation now indicates that ATP is more than a mere intracellular energy molecule, but also undergoes regulated export to assist in controlling extracellular processes (3, 7). In RBCs, functional glycolysis and cdb3 activity are two key factors regulating the hypoxia-sensitive controlled export of ATP (3, 13). We recently demonstrated that stored RBCs (sRBCs) exhibit a marked impairment in deoxygenation-induced ATP export (43). Furthermore, we observed that extracellular ATP acts as an anti-adhesive molecule and that RBCs deficient in ATP export are excessively adherent to the vascular endothelium (43). Given the cumulative evidence demonstrating that glycolytic inhibition significantly blunts ATP export from fresh RBCs (fRBCs) (13) and that RBC band 3 content is lowered with RBC storage (22), we questioned whether banked RBCs are functionally defective in glycolysis-mediated ATP production in response to deoxygenation and whether restoration of ATP production and export might attenuate RBC adhesion after transfusion.
We performed a series of experiments to investigate 1) the capacity for sRBCs to increase glycolytic activity and ATP generation in hypoxia, 2) the impact of raising intracellular ATP content of banked RBCs on the controlled release of ATP from RBCs, and 3) how these interventions influence adhesion of transfused RBCs to the microvasculature in vivo. Accordingly, we first tested the hypothesis that banked RBCs are defective in the control of low O2-stimulated glycolysis (evidenced via intracellular ATP measures) and that RBC supplementation with an FDA-approved additive solution containing glycolytic intermediate/purine/phosphate precursors (i.e., pyruvate, inosine, inorganic phosphate, and adenine; here called “PIPA”) reverses this deficiency. Second, we tested the hypothesis that augmentation of hypoxia-mediated increases in intracellular ATP of banked RBCs is associated with an improvement in controlled, hypoxia-triggered ATP export. Third, we tested the hypothesis that banked human RBCs are markedly adhesive in vivo following transfusion and that pretransfusion treatment of RBCs with glycolytic intermediate/purine/phosphate precursors known to increase both intracellular ATP and the inducible extracellular release of ATP attenuates storage-mediated adhesion of transfused RBCs. To do so, we used an FDA-approved additive solution, PIPA, rich in RBC metabolic precursors and used a murine model of RBC transfusion to examine human RBC function in vivo.
METHODS
Collection, preparation, and treatment of RBCs.
With Institutional Review Board approval and after written informed consent was obtained, venous blood was obtained by venipuncture of the antecubital vein of healthy volunteers into Vacutainer tubes containing sodium heparin (158 USP units). fRBCs were isolated by centrifugation (500 g at 4°C for 10 min), and the plasma and buffy coat were removed. Packed RBCs were resuspended and washed three times in physiological salt solution (PSS; 4.7 mM KCl, 2.0 mM CaCl2, 1.2 mM MgSO4, 140.5 mM NaCl, 21.0 mM Tris-base, and 5.5 mM glucose with 0.5% BSA, pH adjusted to 7.4). This method of isolation yields a RBC suspension devoid of platelets and fewer than one leukocyte per 50 high-power fields (15, 37).
Stored, leukoreduced RBCs were obtained from banked RBC unit segments in their last week of storage (36–42 days; Duke Medical Center Transfusion Services). The additive solution used for sRBCs was most frequently additive solution-3, with the remainder in additive solution-1. Aliquots were washed three times in excess PSS and resuspended. To provide sRBCs with glycolytic substrate supplementation, a formula equivalent to Rejuvesol [here designated “PIPA”, and consisting of (in mmol/l) 100 sodium pyruvate, 99.9 inosine, 70.4 dibasic sodium phosphate, 29 monobasic sodium phosphate, and 5 adenine, pH 7.2] was generated according to published methods and incubated with packed sRBCs at a ratio of 1:4 for 60 min at 37°C (26). PIPA is believed to support glycolytic flux through 1) the conversion of exogenous inosine toward ribose-5-phosphate, which can then be converted to the key glycolytic intermediates, fructose-6-phosphate, and glyceraldehyde-3-phosphate, 2) production of NAD, which is essential to both inosine metabolism and the generation of ATP/2,3-DPG via production of 1,3-DPG, via conversion of pyruvate to lactate, and 3) the provision of exogenous adenine and inorganic phosphates as substrates for nucleotide synthesis (26, 27). Given the potential in vivo toxicity of certain agents within the PIPA formula, treated RBCs were again washed with PSS before study. All studies on isolated RBCs were performed on the day of acquisition to minimize any alterations associated with laboratory processing.
RBC deoxygenation and measurement of extracellular ATP.
The purpose of this procedure was to determine ATP export in response to RBC Hb deoxygenations. A 10% RBC suspension was placed in a rotating-bulb tonometer and warmed to 37°C (Eschweiler, Germany). To produce a normoxic isocapnic environment, RBCs were exposed to a blend of the gases 18% O2, 5.8% CO2, and balance N2. Progressive RBC deoxygenation was induced by lowering O2 to 5%, 2.5%, and 1%, maintaining a stable CO2 at 5.8% (balance N2). Gases were blended in a custom gas blender (MCQ Gas Blender Series 100; Italy) for 10 min, humidified, and introduced into the enclosed tonometer to produce graded degrees of RBC deoxygenation. Deoxygenation was confirmed by blood gas analysis (Siemens Rapid Point 405 Series Automatic Blood Gas System; Los Angeles, CA) (15).
ATP was measured via luciferin-luciferase technique with light emission during the reaction detected by a luminometer. A sample of 10% Hct was diluted 250-fold, and a 200 μl RBC suspension (0.04% Hct) was injected into a cuvette containing 100 μl of 10 mg/ml crude firefly tail extract (Sigma) and 100 μl of 0.5 mg/mL D-luciferin (RPI) mixed in PBS. Extracellular ATP was normalized to a cell count of 4 × 108 cells/ml. For intracellular ATP measurements, a 50 μl sample of RBC suspension (10% Hct) was obtained, diluted 600-fold, lysed with water, and immediately analyzed for ATP and Hb. Intracellular ATP was normalized to [Hb] by spectrophotometry (BMG Labtech). A standard curve for ATP (Calbiochem) was obtained in the RBC suspension for each individual experiment. To confirm that ATP export was not due to hemolysis, RBC suspension aliquots acquired for ATP analysis were analyzed for cell free [Hb] similar to previous reports (15, 37, 43).
Mouse window-chamber surgery and model.
Mouse window-chamber surgery was performed to enable in vivo RBC and microvascular function assessment. Nude mice 9–12 wk of age were anesthetized with 100 mg/kg and 10 mg/kg ketamine/xylazine mixture by intraperitoneal injection and received surgical placement of a dorsal skin window chamber, as previously detailed (29, 42). Briefly, mice placed on a temperature-controlled mat were cleaned and disinfected with chlorhexidine solution and alcohol. Under a surgical hood, mice were placed on a sterile surgical stage, and a dorsal skin-fold was made by suturing the midline of the dorsum to a C clamp attached to a hanger. A 12-mm diameter flap of skin was dissected away from the surface of the dorsal skin flap, leaving a fascial plane with its associated vasculature. The opposite side of the skin flap of the mouse was left intact. After the skin-fold dissection, an apposed pair of titanium window frames were mounted and sutured to the skin flap. The circular incision was then aligned with the hole in the titanium frame, and a 12-mm glass cover was placed over the circular incision. Immediately upon completion of the surgery, each mouse received an injection of 0.05 mg/kg buprenorphine subcutaneously. Mice were studied 3–6 days after surgery to allow adequate wound healing.
RBC transfusion and oxygen manipulation protocol.
The purpose of this protocol was to examine in vivo the functional response of RBC-blood vessel interactions caused by the transfusion of human RBCs. Therefore, to prevent tissue rejection complications, nude mice were used as transfusion recipients as described (43). Mice were initially anesthetized with 4% isoflurane (inhaled) in an induction chamber. They were subsequently placed on a 37°C temperature-controlled heating pad, anesthetized with 2% inhaled isoflurane via nose cone breathing 100% O2, and monitored for breathing rate and response to toe pinch to assess the plane of anesthesia.
The transfusion and oxygen manipulation protocol is as follows. Ten minutes after completion of the study setup and confirmation of adequate anesthesia, the O2 concentration was changed to 21% and a tail vein catheter was inserted. After adequate catheter placement was confirmed, mice were administered a transfusion of either fresh, stored (36–42 days) RBC, or “stored + PIPA” RBCs over 60 s. Each mouse received a volume of 200 μl packed human RBCs, roughly equivalent to a 2-unit transfusion of RBCs or ∼10% blood volume (31). All transfused RBCs were pre-exposed to the cell-membrane fluorescent marker PKH67 to facilitate microscopic RBC tracking and delineation of the vasculature (see below). After in vivo image collection at 21% O2 and 30 min, inhaled O2 was switched to 10% for another 30 min while images were collected again. At the end of experiments, mice were euthanized and their spleen harvested and weighed.
Imaging, fluorescent labeling, and detection of transfused RBCs (in vivo adhesion).
Washed RBCs were labeled with the fluorescent green dye PKH67 (Sigma) as previously described (42, 43), followed by washing with PBS. RBCs were examined under a fluorescent microscope fitted with a GFP filter.
Through the dorsal skin window, fluorescence microscopy was performed for visualization of PKH-green labeled transfused human RBCs. Images were collected on an inverted microscope (Zeiss) using a 5× objective over a 2.2 × 2.2 mm field of view, and acquired statically at 15 min posttransfusion during 21% O2 exposure. This imaging sequence was repeated when the FiO2 level was changed to 10%. Datasets were collected over 10 s with continuous imaging (exposure time = 30 ms). Images were stored digitally on a computer and analyzed offline for measurement of RBC adhesion.
To confirm the effects of hypoxia, images were acquired using a halogen brightfield source to acquire transmission images of the window chamber. A wavelength-tunable filter allowed for sequential collection of images over a wavelength range from 520 to 620 nm in 10-nm increments. A least-squares fit of the intensity across all wavelengths allowed for calculation of the optical absorption indicating the relative concentrations of oxy- and deoxyhemoglobin, thus yielding the Hb O2 saturation throughout the window-chamber vasculature (28).
Measurement of in vivo RBC adhesion was performed by examining video image sequences collected at 5× magnification. RBC adherence was considered as any completely stationary, tagged (human) cell attached to the vascular wall for the entire duration of video sequence (323 frames over 10 s). The percentage adhesion was defined as the estimated number of nonmoving human RBCs/total number of human RBCs in an image × 100. Total human RBC number was estimated by way of an initial determination of pixel area for a single cell and applied to the total region of interest area for all adherent RBCs. The total number of labeled RBCs for a given frame within an image sequence was determined by measuring pixel counts at a given fluorescent threshold intensity. All image processing was performed with ImageJ software.
Statistics.
All values are reported as means ± SE. Unless otherwise noted, analyses were performed using SigmaPlot (Systat Software, San Jose, CA). Specific hypothesis testing within each condition over time was performed using repeated-measures ANOVA. Data comparison at specific time points between conditions was made with unpaired t-tests, and the values within each condition were made with paired t-tests. Significance was set at P < 0.05.
RESULTS
RBC production of intracellular ATP during deoxygenation.
We first examined the impact of graded Hb deoxygenation on glycolytic flux in human RBCs. Intracellular ATP of fresh RBCs increased in dose-dependent fashion from 2.75 ± 0.12 μmol/g Hb during normoxia to 3.49 ± 0.13, 4.53 ± 0.18, and 5.35 ± 0.25 μmol/g Hb with progressively lower O2 levels (Fig. 1A). Consistent with previous reports (26, 33), intracellular ATP content was significantly lower in sRBCs versus fRBCs in normoxia (1.73 ± 0.06 vs. 2.75 ± 0.13 μmol/g Hb), and our present data further indicate a markedly reduced intracellular ATP content in sRBCs (vs. fRBCs) at each PO2 studied (Fig. 1A; 2.02 ± 0.05, 2.63 ± 0.08, and 3.09 ± 0.07 μmol/g Hb). When the depressed normoxic intracellular ATP baseline is accounted for, the relative changes (increases) in ATP of sRBCs with progressive hypoxia were similar (Fig. 1B), suggesting a significant defect in glycolytic flux in response to deoxygenation within stored RBCs. Supplementation with PIPA completely rescued the intracellular ATP content of sRBCs in normoxia (3.60 ± 0.28 μmol/g Hb), yielding levels slightly higher than those observed in fRBCs. In addition, PIPA treatment of sRBCs also improved ATP generation during Hb deoxygenation (Fig. 1A; 4.70 ± 0.30, 5.72 ± 0.44, and 6.58 ± 0.68 μmol/g Hb). Accordingly, the increase in intracellular ATP production to Hb deoxygenation within sRBCs was significantly blunted compared with fresh RBCs, but ATP production was intact in sRBCs pretreated with PIPA (Fig. 1B).
Fig. 1.
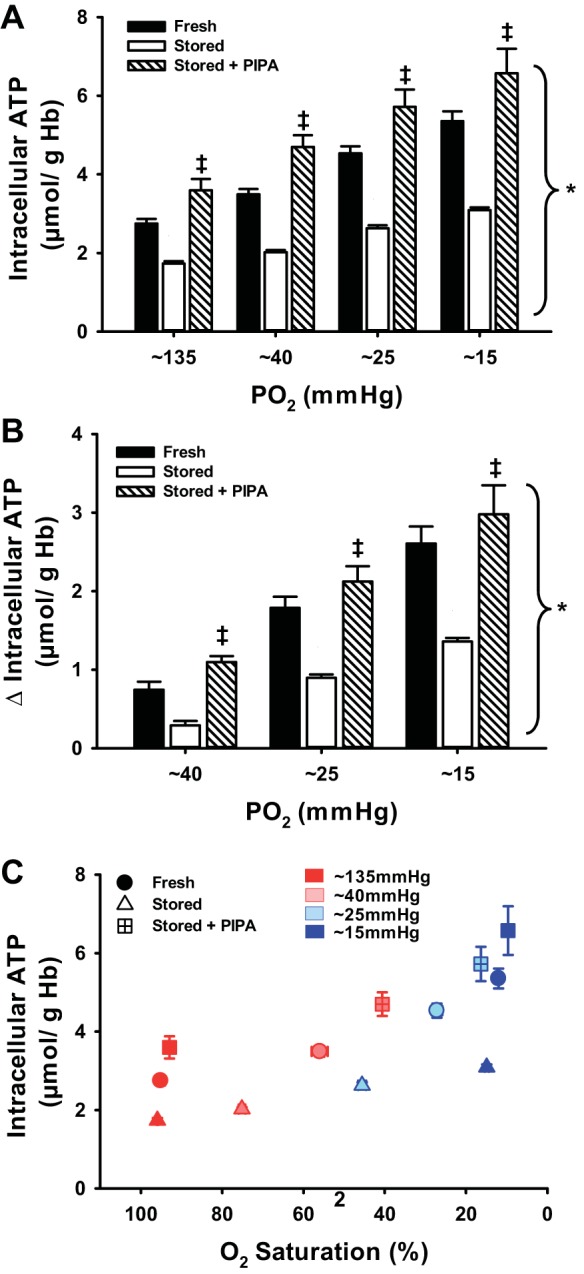
Intracellular ATP and graded red blood cell (RBC) deoxygenation. Deoxygenation evoked significant and progressive increases in intracellular ATP, an index of glycolysis, in fresh RBCs (fRBCs) but not stored RBCs (sRBCs). Pretreatment of stored RBCs with pyruvate, inosine, inorganic phosphate, and adenine (PIPA) to stimulate glycolysis completely restored deoxygenation-induced increases in intracellular ATP (A). The change in intracellular ATP to hypoxia was significantly impaired in sRBCs vs. fRBCs, but sRBCs pretreated with PIPA had greater ATP production than untreated sRBCS (B). C: relation between intracellular ATP and O2 saturation across hypoxia levels within each RBC group. *P < 0.05 vs. 135 mmHg within condition; ‡P < 0.05 vs. stored for a given PO2.
Export of ATP from RBCs in response to deoxygenation.
To determine whether RBC storage-induced deficits in ATP export could be ameliorated by treatment with PIPA, we measured extracellular ATP in the suspension of PIPA-treated stored RBCs during progressive deoxygenation. First, we demonstrated that fresh RBCs release ATP in a dose-dependent fashion to deoxygenation, which is associated with increases in intracellular ATP concentration (r2 = 0.94; Fig. 3B). We show that deoxygenation-induced ATP release from fRBCs was significantly greater at PO2s of 40, 25, and 15 mmHg compared with 135 mmHg (9.09 ± 0.49, 10.13 ± 0.51, and 11.21 ± 0.48 vs. 7.55 ± 0.28 nmol/4 × 108 RBC, P < 0.05; Fig. 2, A–C). Basal ATP export from stored RBCs (4.8 5 ± 0.33 nmol/4 × 108 RBC) was lower than that from fRBCs and only significantly increased with deoxygenation at the lowest (∼15 mmHg) PO2. Furthermore, the absolute amount of ATP released was still significantly lower than that from fRBCs (5.01 ± 0.36, 5.06 ± 0.39, 5.50 ± 0.43 nmol/4 × 108 RBC for 45, 25, and 15 mmHg, respectively; Fig. 2A). Deoxygenation of sRBCs pretreated with PIPA evoked significant increases in ATP export at both the moderate and severe hypoxia levels compared with untreated sRBCs (Δ2.22 ± 0.57 and 3.93 ± 0.67 vs. Δ0.21 ± 0.17 and 0.65 ± 0.16 nmoles/4 × 108 RBC; Fig. 2, B and C). Importantly, hemolysis did not differ between groups and/or within conditions, nor was it directionally related to an increase in ATP export, consistent with evidence for genuine ATP export in response to deoxygenation (3, 7, 13, 15, 24, 36, 38, 41, 43). The degree of hemolysis for each condition is as follows in the order from normoxia through lowest level of hypoxia: fRBCs, 0.041 ± 0.014%, 0.026 ± 0.007%, 0.04 ± 0.009%, and 0.036 ± 0.006%; sRBCs, 0.044 ± 0.012%, 0.042 ± 0012%, 0.046 ± 0.013%, and 0.042 ± 0.006%; PIPA-sRBCs, 0.086 ± 0.035%, 0.10 ± 0.044%, 0.07 ± 0.023%, and 0.07 ± 0.019%.
Fig. 3.
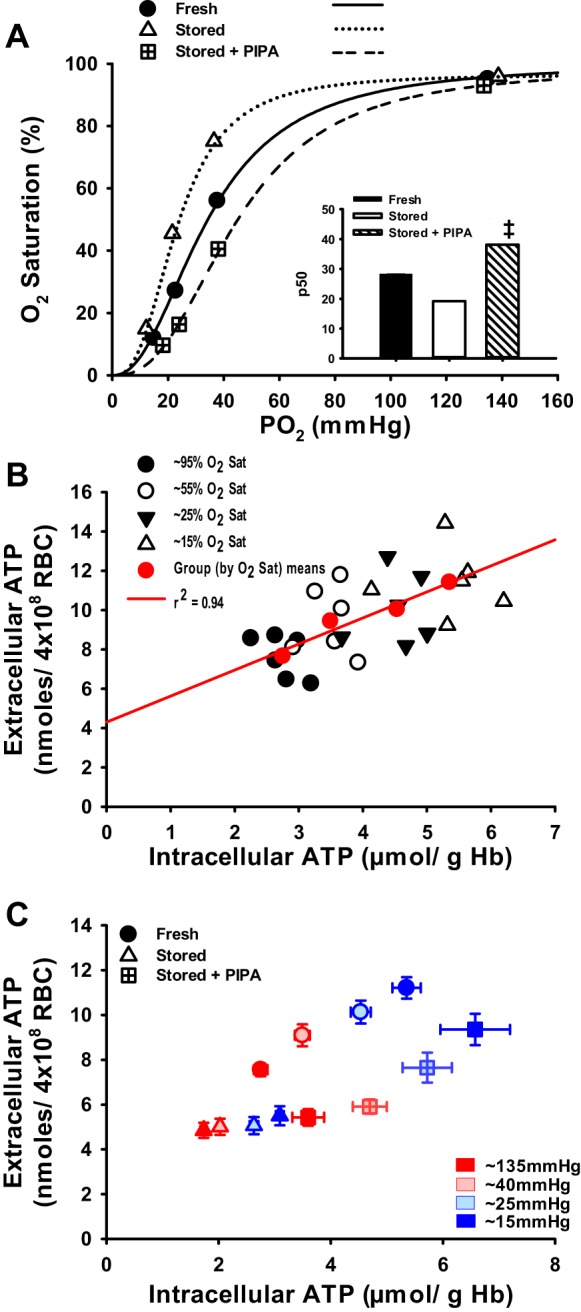
Hb O2 affinity and the relation between intracellular ATP content and ATP export during RBC deoxygenation. Hb O2 affinity is greater in human sRBCs vs. fRBCs (left-shifted curve and lower P50), and PIPA pretreatment of sRBCs attenuated Hb O2 affinity (right-shifted curve and higher P50; A). Extracellular ATP released from fresh RBCs in response to deoxygenation is strongly associated with intracellular ATP production to low O2 (B). Extracellular ATP is related to intracellular ATP during hypoxia for all RBC groups. C: Although sRBCs treated with PIPA had a rescued capacity to export ATP in response to hypoxia, lower absolute levels of ATP were still present compared with those of fRBCs, indicating secondary impairment beyond solely intracellular ATP concentration. ‡P < 0.05 vs. stored for a given PO2.
Fig. 2.
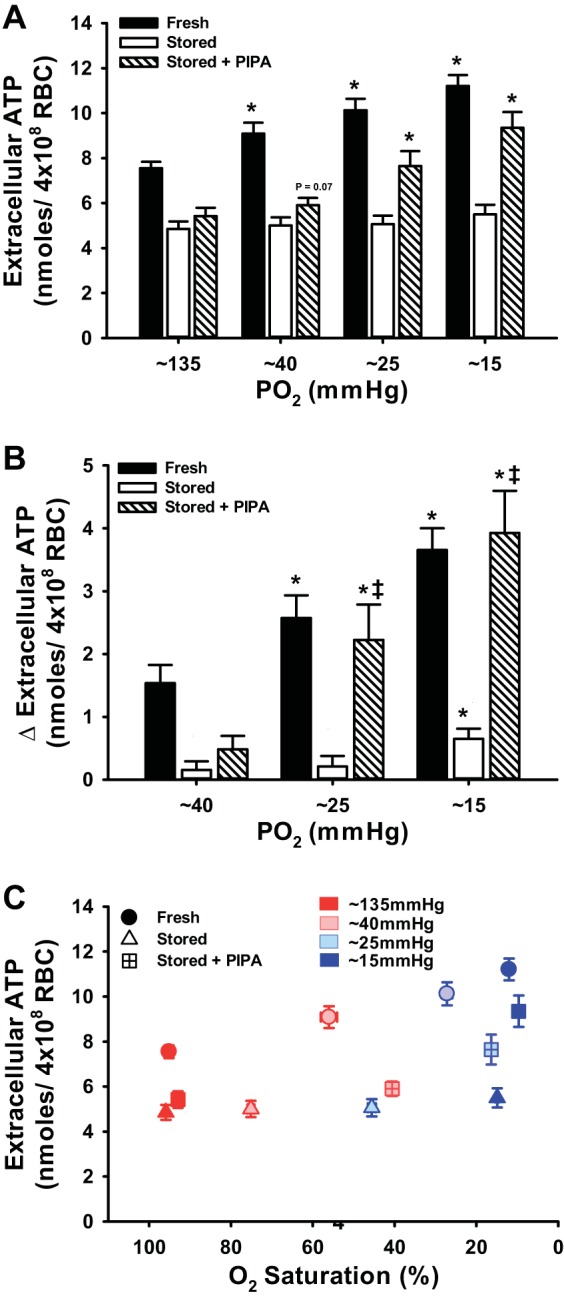
Intracellular ATP content and deoxygenation-mediated ATP export. Low O2 exposure elicited export of ATP from fRBCs, but not from sRBCs; however, PIPA pretreatment of sRBCs augmented ATP export at the lowest levels of PO2 (A). ATP export at a given level of hypoxia was blunted in stored (vs. fresh) RBCs, but pretreatment of sRBCs with PIPA significantly increased ATP export at the lowest PO2s (B). C: relation between extracellular ATP and O2 saturation across hypoxia levels within each RBC group. Note that impaired ATP export from sRBCs is independent of the elevated Hb O2 affinity and that improved ATP export from PIPA pretreatment is independent of reduced Hb O2 affinity. *P < 0.05 vs. 135 mmHg within condition; ‡P < 0.05 vs. untreated stored for a given PO2.
O2 affinity of fRBCs, sRBCs, and PIPA-pretreated RBCs.
By study design, a graded deoxygenation protocol allowed assessment of RBC Hb O2 affinity through the creation of standard O2-dissociation curves and P50 calculation. fRBCs had a P50 of 28.3 ± 0.3 mmHg, whereas sRBC O2 affinity was significantly higher at 19.2 ± 0.2 mmHg, with a leftward shift of the O2 equilibrium curve (OEC; Fig. 3A). As anticipated, PIPA greatly shifted the OEC further to the right, therefore significantly increasing the P50 of sRBCs to 38.1 ± 0.4 mmHg and demonstrating a genuine increase in RBC glycolytic flux with consequent production of the allosteric modulators ATP and 2,3 DPG. Such changes in P50 (lower in sRBCs and higher in PIPA-treated sRBCs) are consistent with previous observations (2, 26, 27).
In vivo adhesion of sRBCs and the impact of PIPA treatment.
Inhalation of 10% FiO2 markedly lowered the blood O2 content as compared with 21% FiO2 in nude mice (Fig. 4). Fresh human RBCs were not substantially adherent to the microvasculature under normoxic or hypoxic stimuli (1.6 ± 0.7 and 3.5 ± 1.5%), but human RBCs within the last week of storage (36–42 days) were significantly adherent, unlike fRBCs during both normoxia and hypoxia (15.6 ± 2.3 and 21.4 ± 2.8%; Fig. 5A). PIPA-treated sRBCs (also 36–42 days stored) were significantly less adherent than untreated sRBCs (normoxia: 4.5 ± 0.7, hypoxia: 5.6 ± 1.0%; Fig. 5A). The increase in in vivo percent RBC adhesion from normoxia to hypoxia was significantly greater for sRBCs versus fRBCs (Δ5.8 ± 0.7 vs. 1.8 ± 0.9%), and this hypoxia-induced augmentation was blunted in PIPA-pretreated sRBCs (Δ1.1 ± 1.6%; Fig. 5B). To examine circulatory clearance of transfused RBCs by the recipient, spleen weight was examined. Recipient spleens were significantly heavier after transfusion with sRBCs than fRBCs (0.10 ± 0.01 vs. 0.19 ± 0.03 g). Spleens from mice transfused with PIPA-preteated sRBCs were not significantly heavier than those transfused with fRBCs, and were slightly less heavy than spleens from recipients receiving untreated stored RBCs (0.13 ± 0.01 g; P = 0.08; Fig. 6).
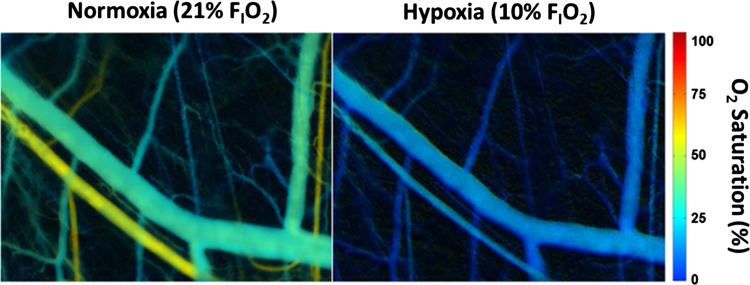
Fig. 4.
Representative images mapping in vivo oxygenation during RBC transfusion experiments. The microvascular network of nude mice following transfusion of human RBCs was imaged via brightfield microscopy with a tunable wavelength filter. Hb O2 saturation was calculated by measuring the absorption of light over the visible spectrum and calculating the amount of oxy- and deoxyHb. Left: nasal inhalation of 21% O2; right: inhalation of 10% O2 by the same mouse. Color scaling on the far right demonstrates the percent Hb O2 saturation of blood within the microvascular network. HbO2 saturations <75% during normoxia are anticipated given O2 distribution in the microcirculation, anesthesia effects, and the high p50 of mouse RBCs (8, 14, 34). FiO2, inspired O2 fraction.
Fig. 5.
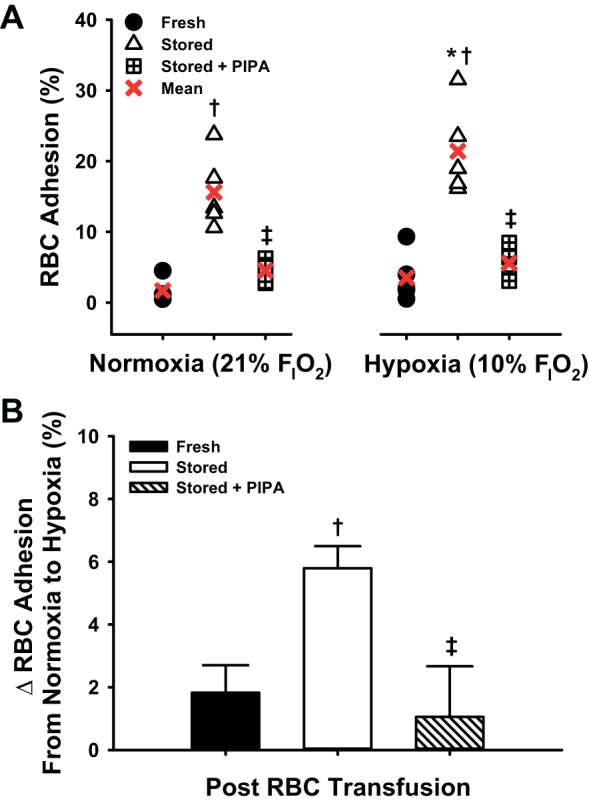
Adhesion of human RBCs to microvasculature in vivo: impact of storage and oxygenation. Transfused human sRBCs were significantly more adhesive than fRBCs within mouse recipients during both systemic normoxia and hypoxia. Pretreatment of sRBCs with PIPA attenuated RBC adhesion independent of blood oxygenation (A). Hypoxia itself significantly augmented RBC adhesion following transfusion of sRBCs compared with transfusion of fRBCs. This response was abrogated following pretreatment of sRBCs with PIPA (B). *P < 0.05 vs. normoxia within RBC condition; †P < 0.05 vs. fresh for a given O2 condition; ‡P < 0.05 vs. stored for a given O2 condition.
Fig. 6.
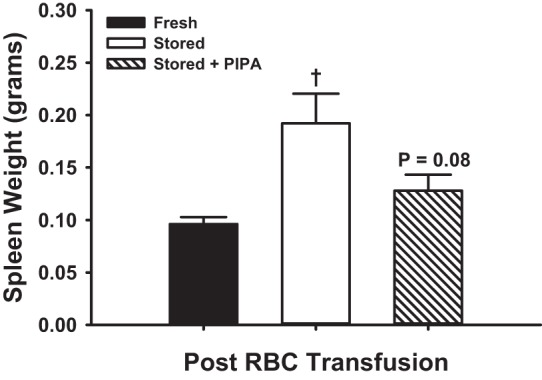
Recipient mouse spleen weights following transfusion of human RBCs. Nude mouse recipient spleen weight was significantly greater following transfusion of human sRBCs than fRBCs. Spleen weight was not significantly greater in PIPA pretreated sRBCs vs. fRBCs, and these treated RBCs were slightly lower than untreated sRBCs. †P < 0.05 vs. fresh.
DISCUSSION
We present the first evidence that sRBCs are functionally defective in their glycolytic capacity to generate intracellular ATP during Hb deoxygenation stimulus and that this is associated with suppressed release of extracellular, anti-adhesive ATP. Moreover, we show that after transfusion, nearly expired banked human RBCs are substantially adherent to the vasculature in vivo, a response that is augmented under low O2 conditions. Conversely, pretreatment of sRBCs with PIPA, a clinically accessible solution containing RBC metabolic precursors, promotes regulated export of ATP in response to Hb deoxygenation, and in turn suppresses RBC adhesion to the microvasculature in vivo under both normoxic and hypoxic conditions. Our findings indicate collectively that restoration of intracellular ATP and the release of ATP from sRBCs may be an effective strategy in efforts aimed to minimize transfusion-related cardiovascular complications while optimizing the benefit of transfusion.
The rationale for the present investigation stems from emerging evidence indicating that RBC transfusions for anemia are not always beneficial and in some patient groups may be associated with exacerbated injury and increased cardiovascular morbidity and overall mortality (5, 18). Specifically, transfusion of human RBCs near the end of shelf-life (vs. <1 to 2 wk storage) has been linked to excess mortality and negative clinical outcomes, including organ failure (11, 12, 17, 19, 20). Banking of RBCs elicits a multitude of deviations from RBC homeostasis, and although some changes are immediate, others evolve progressively over the 5- or 6-wk storage period (2). Two commonly recognized lesions are the discernible drops in intracellular ATP and 2,3-DPG, both resulting from diminished glycolysis, and the consequential elevation in RBC Hb-O2 affinity (2, 33). Therefore, we first investigated whether a functional defect in the sole energy-producing pathway of RBCs - glycolysis - could contribute, recognizing that glycolysis provides ATP and 2,3-DPG, which in turn regulate Hb-O2 binding. To do so, isolated human RBCs were challenged with progressively lower O2 concentration in an effort to elicit a deoxyHb-cdb3 interaction, and thus shift RBC metabolic dominance toward glycolysis (ATP generation) (4, 39). Our results corroborate findings of decreased ATP content in sRBCs under basal conditions and show for the first time a marked defect in the ability of sRBCs to generate intracellular ATP when stimulated. These data collectively demonstrate an impaired glycolytic capacity within banked RBCs (Fig. 1).
Given that all RBC samples were suspended in the same glucose-containing medium, a lack of glucose availability cannot explain our observations. In contrast, supplementation with a mixture rich in pyruvate, inosine, phosphate, and adenine by way of pretreating sRBCs with an additive solution that is “FDA-approved for special circumstances” (i.e., Rejuvesol-equivalent or PIPA) fully overcame the storage-induced defect and allowed deoxygenation-induced increases in intracellular ATP to take place. Consequently, we speculate that long-term banking of RBCs may 1) severely suppress the activity of key glycolytic enzymes, which can then be rescued by high precursor concentration (i.e., concentration feed-forward control), 2) impair band 3-deoxy Hb interactions and subsequently either restrain the release of membrane-bound glycolytic enzymes, thus decreasing available enzyme concentration, or modulate key enzyme regulation (e.g., phosphorylation or allosteric control), or 3) increase ATP-dependent processes to such an extent that ATP consumption rates exceed the ATP accumulation rate. Regardless, our findings clearly demonstrate the inability of sRBCs to generate intracellular ATP to a level similar to that in fRBCs in response to low O2 and that pretreatment with PIPA provides the means necessary for sRBCs to accumulate large quantities of intracellular ATP (Fig. 1).
RBCs can export ATP in a regulated fashion from within the intracellular space to the extracellular milieu. This response is in part dependent on the number of unoccupied Hb O2-binding sites; that is, ATP export follows Hb conformation rather than merely low PO2 per se, consistent with evidence documenting impaired ATP export when cdb3 is inhibited (1, 3, 10, 13). Although the export of extracellular ATP from RBCs is now well described, its relation to intracellular ATP content is not. Here we demonstrate in healthy fRBCs a very close relationship between intracellular ATP and the release of extracellular ATP in response to progressive Hb deoxygenation (r2 = 0.94; Fig. 3, B and C). Our group recently documented a loss of regulated ATP release from sRBCs (43), but as noted above, sRBCs have both depressed intracellular ATP content and a left-shifted OEC (thus less Hb T-state conformation for a given PO2 and, consequently, less “stimulus” for release). In the present study, we exposed RBCs to varying degrees of low O2 so as to produce overlapping fractional O2 saturation curves. We demonstrate that sRBCs have blunted ATP export even when the Hb conformation is taken into account (Fig. 1C), thus substantiating previous reports for impaired ATP export in these RBCs (41, 43).
The hypoxia-induced increases in extracellular ATP we measured are consistent with genuine export of ATP and are unlikely the result of simple RBC lysis. Experimental protocols were rigorously and deliberately designed to curtail hemolysis (e.g., use of albumin in PSS and osmolality-controlled solutions) (15, 43). Moreover, the resulting percent hemolysis values did not differ when gas concentrations were altered, nor was there a significant trend between percent hemolysis and the measured extracellular ATP within or across all RBC samples. Historic reports highlight that hemolysis must be thoroughly controlled to ensure the minimization of RBC rupture, therefore increasing detection capability and an unmasking of authentic ATP export (3). Along these lines, the recent publication by Sikora et al. (36) reemphasizes the need to stringently monitor hemolysis, although surprisingly, their limited study design did not specifically target approaches that safeguarded against hemolysis-induced increases in extracellular ATP, thus limiting generalization and interpretation of the investigation. Nonetheless, our present observations confirm those of numerous other investigators who have, like us, reported inducible and lysis-independent ATP export from intact, healthy human RBCs (3, 7, 13, 15, 24, 38, 41, 43).
When compared with untreated sRBCs, PIPA-pretreated sRBCs had the expected decrease in O2 affinity (Fig. 3A), providing evidence for an increase in glycolytic flux and production of the intracellular allosteric modulators, ATP and 2,3-DPG. This directional shift in Hb allostery and consequent increase in deoxyHb could, in theory, independently augment ATP export for a given PO2 (1, 13). However, increased ATP export from PIPA-treated RBCs was significant only at the two lowest percent O2 exposures (Fig. 2). These data in combination with the poor ability of sRBCs to export ATP at any O2 saturation suggest that simply shifting Hb-O2 affinity in the absence of robust glycolytic flux is not sufficient to promote marked ATP export. In addition, the present data show that ATP export by sRBCs pretreated with PIPA is quantitatively similar to that by fRBCs during moderate or severe hypoxia (Fig. 2B). Of note however, despite this rectified exportation response from PIPA treated sRBCs during hypoxia, the absolute amount of extracellular ATP under basal normoxia or hypoxic stress never reached that seen from fRBCs (Fig. 2, A and C). These observations suggest a secondary defect unrectified by a combination of substantially lowered O2 affinity and boosted intracellular ATP content for a given oxy-Hb fraction (Figs. 1C and 3C). Given the key role for pannexin1 channels in ATP export from RBCs in response to deoxygenation (24, 38), we previously considered whether sRBCs have less protein expression of pannexin1. However, protein abundance for pannexin1 does not decline during RBC banking (unpublished observations; TM and H. Zhu), therefore the secondary underlying mechanisms for impairment are unclear at this time. Nevertheless, PIPA supplementation is an effective means to rectify hypoxia-sensitive ATP export from sRBCs.
sRBCs have been implicated as more adherent than fRBCs (6, 21, 43). Nevertheless, limited data exist describing the potential mechanism for such phenomena. Our group recently demonstrated that treating fRBCs with ATP export inhibitors or scavengers of extracellular ATP significantly augments RBC adhesivity to endothelial cells ex vivo, and conversely, that the addition of extracellular ATP attenuates RBC adhesion (43). These data provide strong evidence that the loss of facile export of anti-adhesive ATP may be a mediator of the proadhesive effects of RBC storage. In the present study, we show that stored human RBCs pretreated with PIPA were less adherent to the microvasculature in vivo compared with untreated sRBCs in both normoxia and hypoxia (Fig. 5). In addition, we observe that treatment of sRBCs can overcome the proadhesive effect of hypoxia (Fig. 5B). Taken together, these findings indicate that PIPA improves intracellular ATP generation and facilitates an increase in extracellular ATP release from RBCs in response to hypoxia (Figs. 1 and 2).
We and others (21) observe attenuated adhesion of PIPA-treated sRBCs during normoxia, but the present study did not detect an elevation in basal ATP release from treated sRBCs. This could result from 1) a limited capacity to measure all ATP export, 2) the fact that RBCs are to some extent deoxygenating in vivo (in the microvascular network) even during normoxic gas exposure (Fig. 4), or 3) alternatively, that intracellular ATP concentrations are the key variable dictating vascular RBC adhesion rather than just release per se. In support of the latter proposition, the surface appearance of the adhesion molecule phosphatidylserine (PS) is negatively regulated by an ATP-dependent flippase enzyme and increases during RBC banking, thus raising the possibility that PIPA reduced PS exposure in our studies. Other studies, however, suggest that although PIPA treatment augments intracellular ATP, this only partially modulates flippase and PS exposure (40). We speculate that each of these factors might contribute. Regardless, we clearly observe that stored human RBCs pretreated with PIPA are not greatly adherent to the microvasculature in vivo.
In summary, we show for the first time that human RBCs stored for extended periods (to ∼39 days) have disrupted glycolytic production of intracellular ATP coinciding with suppressed export of antiadhesive ATP, an impairment especially apparent during hypoxia. Despite this, presupplementing sRBCs with RBC metabolic precursors rescues deoxygenation-induced elevations in intracellular ATP and rectifies stimulated ATP export. Finally, we observe that microvascular adhesion of banked human RBCs is abundant in an in vivo setting and, moreover, significantly augmented during hypoxemia. Importantly, our novel observations demonstrate that pretreatment of RBCs with the substrate needed for augmented ATP synthesis (namely, ample glycolytic intermediate/purine/phosphate precursors, here as PIPA) before transfusion significantly suppresses RBC-vascular adhesion regardless of the oxygenation status of the recipient. Modulation of either the intracellular ATP content of RBCs or extracellular ATP export, or the combination, may prove beneficial in efforts intended to improve the balance between risk and benefit in anemic patients transfused with RBCs.
GRANTS
This work was funded by Veterans Affairs Grant BX-000281 (to T. J. McMahon) and National Heart, Lung, and Blood Institute Grants R01 HL107608 and R21 HL119343 (to T. J. McMahon) and T32 HL007057 (to B. S. Kirby).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.S.K., G.H., and T.J.M. conception and design of research; B.S.K. and G.H. performed experiments; B.S.K. and H.C.H. analyzed data; B.S.K., G.H., H.C.H., and T.J.M. interpreted results of experiments; B.S.K. prepared figures; B.S.K. drafted manuscript; B.S.K., G.H., H.C.H., and T.J.M. edited and revised manuscript; B.S.K., G.H., H.C.H., and T.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Duke Cancer Institute and Dr. Gregory Palmer for support and guidance via the shared resource program of Optical Molecular Imaging and Analysis in the mouse transfusion studies. We thank Hongmei Zhu for technical assistance and histology work and Drs. Daniel Riccio, Eduardo Lazarowski, and Vikram Premkumar for insightful comments.
REFERENCES
- 1.Akatsu T, Tsukada K, Hishiki T, Suga-Numa K, Tanabe M, Shimazu M, Kitagawa Y, Yachie-Kinoshita A, Suematsu M. T-state stabilization of hemoglobin by nitric oxide to form alpha-nitrosyl heme causes constitutive release of ATP from human erythrocytes. Adv Exp Med Biol 662: 109–114, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA 104: 17063–17068, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovascular Research 26: 40–47, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci USA 102: 2402–2407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J, Investigators F. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 365: 2453–2462, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin-Yee IH, Gray-Statchuk L, Milkovich S, Ellis CG. Transfusion of stored red blood cells adhere in the rat microvasculature. Transfusion 49: 2304–2310, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 24: 107–116, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friesenecker B, Tsai AG, Dunser MW, Mayr AJ, Martini J, Knotzer H, Hasibeder W, Intaglietta M. Oxygen distribution in microcirculation after arginine vasopressin-induced arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol 287: H1792–H1800, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Giardina B, Messana I, Scatena R, Castagnola M. The multiple functions of hemoglobin. Crit Rev Biochem Mol Biol 30: 165–196, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 118: 6675–6682, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 115: 4284–4292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kerger H, Torres Filho IP, Rivas M, Winslow RM, Intaglietta M. Systemic and subcutaneous microvascular oxygen tension in conscious Syrian golden hamsters. Am J Physiol Heart Circ Physiol 268: H802–H810, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet 370: 415–426, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Koch CG, Khandwala F, Li L, Estafanous FG, Loop FD, Blackstone EH. Persistent effect of red cell transfusion on health-related quality of life after cardiac surgery. Ann Thorac Surg 82: 13–20, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 34: 1608–1616, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Koch CG, Li L, Duncan AI, Mihaljevic T, Loop FD, Starr NJ, Blackstone EH. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg 81: 1650–1657, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 358: 1229–1239, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Koshkaryev A, Zelig O, Manny N, Yedgar S, Barshtein G. Rejuvenation treatment of stored red blood cells reverses storage-induced adhesion to vascular endothelial cells. Transfusion 49: 2136–2143, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion 47: 1212–1220, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci USA 106: 18515–18520, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messana I, Orlando M, Cassiano L, Pennacchietti L, Zuppi C, Castagnola M, Giardina B. Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett 390: 25–28, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion 51: 1574–1579, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Oski FA, Travis SF, Miller LD, Delivoria-Papadopoulos M, Cannon E. The in vitro restoration of red cell 2,3-diphosphoglycerate levels in banked blood. Blood 37: 52–58, 1971. [PubMed] [Google Scholar]
- 28.Palmer GM, Fontanella AN, Shan S, Hanna G, Zhang G, Fraser CL, Dewhirst MW. In vivo optical molecular imaging and analysis in mice using dorsal window chamber models applied to hypoxia, vasculature and fluorescent reporters. Nat Protocols 6: 1355–1366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer GM, Fontanella AN, Shan S, Hanna G, Zhang G, Fraser CL, Dewhirst MW. In vivo optical molecular imaging and analysis in mice using dorsal window chamber models applied to hypoxia, vasculature and fluorescent reporters. Nat Protoc 6: 1355–1366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA 104: 17058–17062, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds JD, Bennett KM, Cina AJ, Diesen DL, Henderson MB, Matto F, Plante A, Williamson RA, Zandinejad K, Demchenko IT, Hess DT, Piantadosi CA, Stamler JS. S-nitrosylation therapy to improve oxygen delivery of banked blood. Proc Natl Acad Sci USA 110: 11529–11534, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riccio DA, McMahon TJ. Reversing a red blood cell storage (RBCS) lesion: S-nitrosothiol replenishment in aged RBCS. Nitric Oxide 27: S30, 2012. [Google Scholar]
- 33.Salzer U, Zhu R, Luten M, Isobe H, Pastushenko V, Perkmann T, Hinterdorfer P, Bosman GJ. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion 48: 451–462, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Nielsen K. Energy metabolism, body size, and problems of scaling. Fed Proc 29: 1524–1532, 1970. [PubMed] [Google Scholar]
- 35.Siems WG, Sommerburg O, Grune T. Erythrocyte free radical and energy metabolism. Clin Nephrol 53: S9–S17, 2000. [PubMed] [Google Scholar]
- 36.Sikora J, Orlov SN, Furuya K, Grygorczyk R. Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 124: 2150–2157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprague RS, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML. A selective phosphodiesterase 3 inhibitor rescues low PO2-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control. Am J Physiol Heart Circ Physiol 301: H2466–H2472, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299: H1146–H1152, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefanovic M, Puchulu-Campanella E, Kodippili G, Low PS. Oxygen regulates the band 3-ankyrin bridge in the human erythrocyte membrane. Biochem J 449: 143–150, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhoeven AJ, Hilarius PM, Dekkers DW, Lagerberg JW, de Korte D. Prolonged storage of red blood cells affects aminophospholipid translocase activity. Vox sanguinis 91: 244–251, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Giebink A, Spence DM. Microfluidic evaluation of red cells collected and stored in modified processing solutions used in blood banking. Integr Biol (Camb) 6: 65–75, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Zennadi R, Moeller BJ, Whalen EJ, Batchvarova M, Xu K, Shan S, Delahunty M, Dewhirst MW, Telen MJ. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood 110: 2708–2717, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med 39: 2478–2486, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]