Abstract
We tested the hypothesis that the nervous system, and the cortex in particular, is a critical determinant of muscle strength/weakness and that a high level of corticospinal inhibition is an important neurophysiological factor regulating force generation. A group of healthy individuals underwent 4 wk of wrist-hand immobilization to induce weakness. Another group also underwent 4 wk of immobilization, but they also performed mental imagery of strong muscle contractions 5 days/wk. Mental imagery has been shown to activate several cortical areas that are involved with actual motor behaviors, including premotor and M1 regions. A control group, who underwent no interventions, also participated in this study. Before, immediately after, and 1 wk following immobilization, we measured wrist flexor strength, voluntary activation (VA), and the cortical silent period (SP; a measure that reflect corticospinal inhibition quantified via transcranial magnetic stimulation). Immobilization decreased strength 45.1 ± 5.0%, impaired VA 23.2 ± 5.8%, and prolonged the SP 13.5 ± 2.6%. Mental imagery training, however, attenuated the loss of strength and VA by ∼50% (23.8 ± 5.6% and 12.9 ± 3.2% reductions, respectively) and eliminated prolongation of the SP (4.8 ± 2.8% reduction). Significant associations were observed between the changes in muscle strength and VA (r = 0.56) and SP (r = −0.39). These findings suggest neurological mechanisms, most likely at the cortical level, contribute significantly to disuse-induced weakness, and that regular activation of the cortical regions via imagery attenuates weakness and VA by maintaining normal levels of inhibition.
Keywords: muscle, strength, weakness, dynapenia, immobilization, imagery
maximal voluntary force generation, or strength, is controlled by multiple factors. For instance, muscle anatomical and physiological factors are key determinants of strength, or lack thereof (i.e., weakness) (Manini et al. 2012; Mosca et al. 2013). The nervous system has long been suggested to also be a key determinant of strength/weakness (Moritani and deVries 1979). Indeed, dramatic impairments in voluntary (neural) activation (VA) occur following experimentally induced weakness (Clark et al. 2008, 2010; Kawakami et al. 2001). VA reflects the nervous system's ability to fully activate muscle and is assessed by electrically stimulating a peripheral nerve during a maximal voluntary contraction and quantifying the “added force” (Taylor 2009). While findings of impaired VA indicate the nervous system is a key determinant of strength/weakness, it does not provide insight into the neuroanatomical or neurophysiological factors involved in strength/weakness.
Despite the tonic activity of cortico-motoneuronal cells being shown to increase linearly with static force generation in primates more than 3 decades ago (Ashe 1997; Cheney and Fetz 1980), the role of the primary motor cortex (M1) and other high-order cortical regions, and in many instances the entire nervous system, is rarely recognized as being a significant factor in determining muscle strength. Conversely, muscle mass and other muscular mechanisms (e.g., processes involved in excitation-contraction coupling) have received considerably more scientific, as well as popular press, attention (Manini and Clark 2012; Manini et al. 2012; Russ et al. 2012). For instance, M1 has more historically been considered critical for movement coordination and skill acquisition as opposed to maximal force generation of individual muscles (Adkins et al. 2006; Jackson 1873; Remple et al. 2001). More recently, data from humans have begun to accumulate, suggesting that the cortex is a critical determinant of muscle strength. For instance, immobilization-induced weakness results in an increase in intracortical inhibition (Clark et al. 2010), resistance exercise-induced increases in strength results in a decrease in intracortical inhibition (Weier et al. 2012), and mental imagery (MI) of strong muscle contractions increases strength (Ranganathan et al. 2004).
In the present experiment, we sought to test the hypothesis that the cortex is a critical determinant of muscle strength/weakness and VA, and that high levels of intracortical inhibition is an important neurophysiological factor regulating strength/weakness. To test this hypothesis, healthy individuals underwent 4 wk of wrist-hand immobilization to induce weakness. Another group of individuals also underwent 4 wk of immobilization, but they concomitantly performed MI of strong muscle contractions 5 days/wk. We chose to use a cast immobilization paradigm, as it has been shown to dramatically reduce muscle strength and VA and induce a wide range of neuroplastic effects in the central nervous system (Clark et al. 2008, 2010; Crews and Kamen 2006; Kaneko et al. 2003; Lundbye-Jensen and Nielsen 2008; Zanette et al. 1997, 2004). MI has been shown to activate several cortical areas that are involved with actual motor behaviors, including premotor and M1 regions (Hetu et al. 2013). A control group, who underwent no interventions, also participated in this study. Before, immediately after, and 1 wk following immobilization, we measured changes in wrist flexor strength, VA, and the corticospinal silent period [SP; a measure of corticospinal inhibition quantified via transcranial magnetic stimulation (TMS) (Kobayashi and Pascual-Leone 2003; Reis et al. 2008)].
METHODS
Overview of the study design.
Twenty-nine healthy adults completed 4 wk of wrist-hand immobilization of the nondominant limb, and 15 adults served as a control group. A subset of study participants in the immobilization group (n = 14) were also assigned to perform MI training 5 days/wk. Descriptive statistics are provided in Table 1. The Ohio University Institutional Review Board approved this study, and subjects provided written consent. Potential participants were excluded if they were taking any medications or supplements, had any major medical issues, or had any known neurological or musculoskeletal limitations of the upper limbs. The nondominant arm was assessed for isometric muscle strength, VA, and SP duration during a 15% maximal voluntary isometric contraction (MVC) at baseline, 4 wk later (during which the immobilization groups were immobilized), and 5 wk after baseline (1 wk after cast removal and the restoration of normal activity for participants in the immobilization groups). Subjects abstained from alcohol (24 h) and caffeine (4 h) prior to the sessions. Testing sessions were performed at the same time of day for each subject. Individuals involved in assessments were blinded to experimental group assignment. Subjects were not randomly assigned to treatment group per se, but rather were assigned based on whether they were willing to undergo the immobilization procedures as well as the investigators opinion on whether subjects would comply with the imagery training (e.g., feasibility of their schedule availability for permitting them to report to the facilities 5 days/wk for imagery training).
Table 1.
Descriptive statistics of the study participants
| Group | N (%female) | Age, yr | Height, cm | Weight, kg | BMI, kg/m2 |
|---|---|---|---|---|---|
| Immobilization | 15 (46) | 21.2 ± 3.5 | 170.8 ± 10.9 | 70.1 ± 10.8 | 24.2 ± 4.2 |
| Immobilization + MI | 14 (40) | 20.9 ± 3.6 | 179.4 ± 9.1 | 78.4 ± 16.1 | 24.1 ± 3.0 |
| Control | 15 (47) | 21.5 ± 3.4 | 170.0 ± 10.2 | 67.4 ± 13.7 | 23.3 ± 3.8 |
Values are means ± SD; N, no. of subjects. BMI, body mass index; MI, mental imagery. Note: No significant differences were observed between groups.
Cast immobilization.
Subjects in the immobilization groups were fitted with a rigid wrist-hand cast on the nondominant forearm (model 1101–1103, Orthomerica, Orlando, FL), as previously described (Clark et al. 2008, 2010). In brief, lightweight polyethylene casts were applied, which extend from just below the elbow past the fingers (eliminates wrist flexion/extension movements and finger usage). Casts were removed 3–4 times/wk under supervision to wash the arm and inspect for complications. During the recovery period, subjects in the immobilization groups were instructed to return to their normal daily activities, but not begin rehabilitation or a strengthening protocol.
MI training.
MI training was performed 5 times/wk. For each session, subjects performed 52 imagined maximal contractions of the casted wrist flexor muscles in a quiet room. The duration of each imagined contraction was 5 s, followed by 5 s of rest. Training was performed in four blocks of 13 imagined contractions each with 1 min of rest between the blocks. During the imagery sessions, subjects were instructed to relax their arm muscles, and to maximally activate the brain, but not the muscles. The electromyogram (EMG) was recorded from the flexor carpi radialis (FCR) muscle to ensure that muscle activation did not occur and real-time feedback was provided. Quantitative analyses of these EMG signals were not performed, as we did not visually observe any voluntary interference EMG activity beyond nominal levels that occasionally occurred during the first session. More specifically, an unblinded scientist supervised these sessions. The imagery script was digitized such that this person did not have to actually read the script. Rather, they were charged with monitoring the EMG recordings in real time on a computer monitor and to provide feedback to the subject if any interference EMG was subjectively noted (i.e., activity is observable above baseline noise with the y-axis scale such that very small increases in activity were noticeable).
On a verbal signal to begin, subjects were instructed to “imagine that you are maximally contracting the muscles in your left (or right) forearm and imagine that you are making your wrist flex and push maximally against a hand grip with your hand. We will ask you to do this for 5 s at a time followed by a 5-s rest period for a total time of around 2 min. When we tell you to start, we want you to imagine that you are pushing in against a handgrip as hard as you can and continue to do so until we tell you to stop. After a 5-s rest we will ask you to repeat this. Ready, and begin imagining that you are pushing in as hard as you can with your left wrist, push, push, push… and stop (5 s of silence). Start imagining that you are pushing in again as hard as you can, keep pushing, keep pushing… and stop (5 s of silence).” This verbal cuing and imagery continued for 2 min, at which time the study participant was instructed that they would have a short break (1 min), and then the next blocks would subsequently begin. It should be noted that this mental exercise was not simply a visualization of oneself performing the task; rather, the performers were instructed to adopt a kinesthetic imagery approach, in which they urged the muscles to contract maximally (Ranganathan et al. 2004).
Muscle strength and VA.
To quantify wrist flexion forces, subjects were seated with the elbow at 90°, the hand pronated and the forearm supported and restricted while the head rested on a pad (Fig. 1A) (Biodex System 4, Biodex Medical Systems, Shirley, NY). The wrist joint was aligned to the rotational axis of a torque motor to which a constant-length lever arm was attached. The signal was scaled to maximize its resolution (208.7 mV/Nm; Biodex Researchers Tool Kit Software), smoothed over a 10-point running average, and sampled at 625 Hz (MP150 Biopac Systems). Subjects received visual feedback of all exerted forces on a computer monitor located 1 m directly in front of them.
Fig. 1.
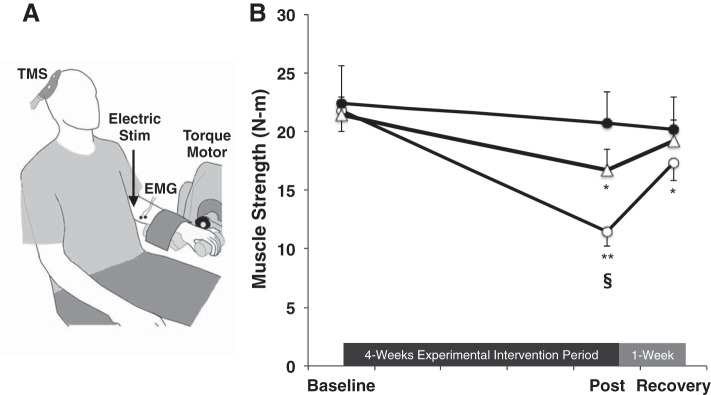
A: setup for assessing wrist flexion strength, voluntary activation (VA), and the cortical silent period (SP). TMS, transcranial magnetic stimulation; EMG, electromyogram. B: immobilization (open circles; n = 15) resulted in a 45% reduction in strength. Mental imagery training (open triangles; n = 14), however, attenuated the loss of muscle strength by ∼50% (strength loss of 24%). No changes were observed in the control group (solid circles; n = 15). Values are means ± SE. Significant differences vs. *baseline, **baseline and recovery, §control group value.
To assess maximal wrist flexion strength, subjects performed a minimum of three MVCs with a 1- to 2-min rest period between each contraction. If subjects continually recorded more force with increasing trials, or if the two highest trials were not within 5% of each other, additional trials were performed until a plateau was reached. Verbal encouragement was provided during testing. The highest value was considered the MVC.
To determine what percentage of the total force-generating capacity of the wrist flexors can be produced voluntarily, a combination of voluntary and electrically stimulated contractions was performed (Fig. 2A). Electrical stimulation (0.2-ms pulse duration) was delivered to the median nerve in the cubital fossa groove via stimulating electrodes (Ag-AgCl, 35 × 45 mm, no. 2015; Nikomed, Doylestown, PA). Stimuli were administered at increasing stimulation intensities until the FCR peak-to-peak (p-p) EMG amplitude reached a plateau (Mmax), and for VA testing the intensity was subsequently increased 20% above that eliciting Mmax (DS7AH; Digitimer, Hertfordshire, UK). To assess VA, a supramaximal 100-Hz electrical doublet was delivered while the subject performed a 4- to 5-s MVC. The increase in force immediately following the stimulation was expressed relative to a potentiated response evoked 1–2 s after the MVC, and VA was calculated as follows.
Fig. 2.
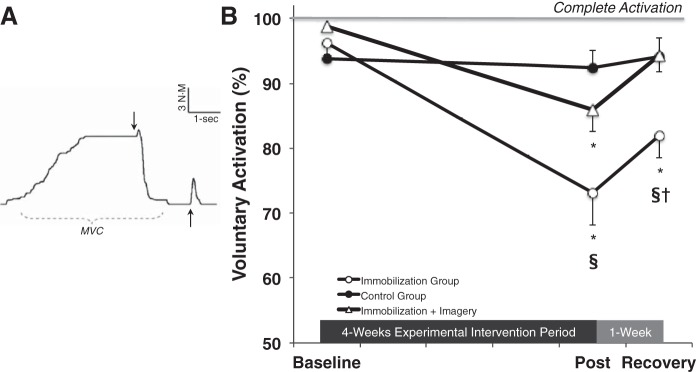
A: example of a force trace assessing VA. Arrows represent the delivery of a 100-Hz electrical doublet to the peripheral nerve while an individual is maximally contracting (first arrow) and ∼2 s after the completion of the contraction (second arrow). MVC, maximum voluntary contraction. B: immobilization (open circles; n = 15) reduced VA ∼25%. Mental imagery training (open triangles; n = 14), however, attenuated the impairment in VA by ∼50%. No changes were observed in the control group (solid circles; n = 15). Values are means ± SE. Significant differences vs. *baseline, §control group value, †imagery group value.
TMS.
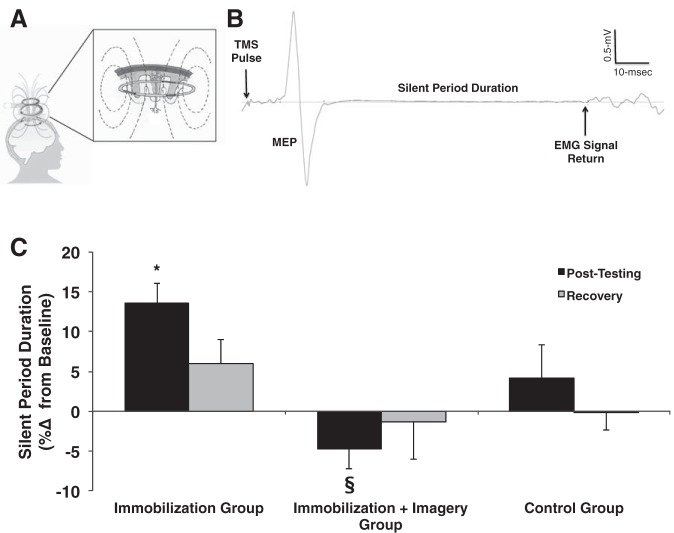
EMG was recorded from the nondominant FCR muscle using bipolar surface electrodes located longitudinally over the muscle on shaved and abraded skin with a reference electrode just distal to the medial epicondyle (Ag/AgCl electrodes with a 25-mm interelectrode distance). The EMG signals were amplified ×1,000, band-pass filtered (10–500 Hz), and sampled at 5,000 Hz (MP150, BioPac Systems, Goleta, CA). Single-pulse, monophasic waveform magnetic stimuli were delivered using a Magstim 2002 (The Magstim, Whitland, UK) magnetic stimulator with a 70-mm figure-of-eight focal coil positioned tangential to the scalp with the handle pointing backwards and laterally at 45° from midline. The stimulation location that elicited the largest p-p amplitude of the FCR motor-evoked potential (MEP) was identified and marked on a lycra cap for coil placement. This procedure was repeated for each testing session. Next, resting motor threshold (MT) was determined while study participants were seated in the dynamometer by delivering single pulses at gradually increasing stimulation intensities, as our laboratory previously described (Clark et al. 2008; Damron et al. 2008). Resting MT was determined and expressed as a percentage of the maximal stimulator output (SO). MT was determined by delivering TMS pulses at a low stimulus intensity and gradually increasing the intensity in 2% increments until MEPs were observed. Resting MT was defined as the stimulation intensity that elicited MEPs with a p-p amplitude of ≥50 μV in at least four of eight trials. During this assessment, the muscle was completely relaxed as monitored by the EMG signal. We should note that our laboratory has previously reported that the resting MT does not change following immobilization (Clark et al. 2008), which is consistent with what we observed in the present study. SP duration was quantified during brief 15% contractions (Fig. 3A). Here, eight single pulses were delivered at 130% of resting MT, and the SP was quantified and averaged. A single, blinded investigator visually defined the return of the interference EMG signal, and the duration between this TMS pulse and this event was quantified to represent the SP. We have previously reported that this quantification method displays high interrater reliability (r = 0.97) (Damron et al. 2008).
Fig. 3.
A: the TMS coil induces a magnetic field and a subsequent Eddy current that stimulates neurons within the motor cortex. B: example of an EMG trace illustrating a motor evoked potential (MEP) and SP. In this study, single TMS pulses were delivered to the primary motor cortex during 15% of maximum contraction to quantify the SP duration as an index of GABAB-mediated inhibition. C: immobilization resulted in a 12% prolongation in the SP (n = 15). Mental imagery training (n = 14), however, eliminated prolongation of the SP. No changes were observed in the control group (n = 15). Values are presented as a %change for clarity, but it should be noted that no baseline differences in groups were observed (baseline measures for control group: 108.5 ± 4.3 ms, immobilization group was 107.5 ± 4.4 ms, and immobilization + imagery group was 110.5 ± 4.9 ms). Values are means ± SE. Significant differences vs. *baseline, §immobilization group value.
Sample size justification.
Our sample size was calculated based on our observed effect size (ES) for imagery training to minimize disuse-induced strength loss (η2 = 0.11) (Clark et al. 2006b). The power calculation was based on the assumption of a mixed-model, within-between interaction ANOVA with α at 0.05 and power at 0.95. Based on this calculation our estimated sample size to detect significant changes in strength from preimmobilization to postimmobilization between the immobilization and the immobilization plus imagery groups was 15 subjects/group (G*Power 3.0.3, Universität Kiel, Kiel, Germany). We chose to set power to 0.95 because the success of this project was vitally dependent upon imagery training maintaining strength.
Statistical analyses.
Mixed-model ANOVAs [group (3 between-subject factors) × time (3 within-subject factors)] followed by Sidak post hoc tests were utilized to determine changes over time between groups. We should also note that we conducted additional contrast analyses with only the immobilization and immobilization + imagery groups included, and these analyses yielded essentially the same findings as those when the control group was included in the model. Correlation coefficients (r) were calculated to examine the relation between 1) the percent change in strength and percent change in VA following immobilization for the immobilization and immobilization + imagery groups, and 2) the percent change in strength and the percent change in SP duration following immobilization for the immobilization and immobilization + imagery groups. A preset α-level of significance equal to 0.05 (two-sided) was required for significance. The SPSS statistical package (version 19.0 for Mac, Chicago, IL) was used for data analysis. Data are presented as means ± SE. Additionally, to further aid in interpretation, we also report the ES (partial η2), which represents the proportion of total variation attributable to a given factor when partialing out other factors from the total nonerror variation.
RESULTS
There were no group differences at baseline for strength (P = 0.94, ES < 0.01; immobilization group: 21.8 ± 1.8 N·m, immobilization + imagery group: 21.4 ± 1.6 N·m, control group: 22.4 ± 3.2 N·m), VA (P = 0.16, ES = 0.08; immobilization group: 96.2 ± 1.8%, immobilization + imagery group: 98.7 ± 0.6%, control group: 93.8 ± 2.2%), MT [P = 0.09, ES = 0.11; immobilization group: 51.9 ± 2.3% of SO, immobilization + imagery group: 44.4 ± 2.2% SO, control group: 47.1 ± 2.1% SO], or SP duration (P = 0.89, ES < 0.01; immobilization group: 107.5 ± 4.4 ms, immobilization + imagery group: 110.5 ± 5.0 ms, control group: 108.4 ± 4.3 ms). We observed group × time interactions for the dependent variables of strength (P < 0.001, ES = 0.31), VA (P = 0.004, ES = 0.16), and SP duration (P = 0.015, ES = 0.13). Follow-up analyses indicated that immobilization significantly decreased strength by 45.1 ± 5.0% (Fig. 1), impaired VA capacity by 23.2 ± 5.8% (Fig. 2), and prolonged the SP by 13.5 ± 2.6% (Fig. 3). MI training, however, attenuated the loss of strength and VA by ∼50% (Figs. 1 and 2; 23.8 ± 5.6% and 12.9 ± 3.2% reductions, respectively), and also eliminated prolongation of the SP (Fig. 3; 4.8 ± 2.8% reduction). No changes over time were observed in the control group for any of the outcomes (Figs. 1–3). We did not observe a group × time interaction for MT (P = 0.11, ES = 0.08; immobilization group: 51.9 ± 2.3%, 53.1 ± 2.5%, and 51.6 ± 2.5% of SO at baseline, post, and recovery; immobilization + imagery group: 44.4 ± 2.2%, 45.5 ± 2.7%, and 41.9 ± 2.5% SO; control group: 47.1 ± 2.1%, 44.6 ± 1.7%, and 45.2 ± 2.1% SO).
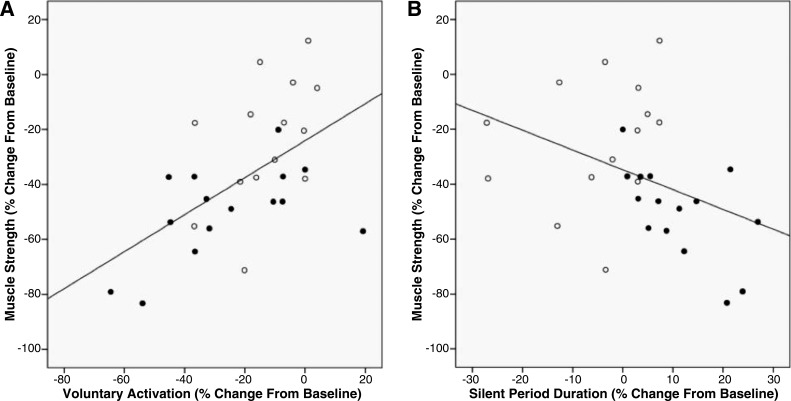
We observed a positive association between the percent change in strength and the percent change in VA following immobilization (Fig. 4A; r = 0.56, P < 0.01). We observed a negative association between the percent change in strength and the percent change in SP duration following immobilization (Fig. 4B; r = −0.39, P = 0.03).
Fig. 4.
A: there was a positive association between the percent change in muscle strength and the percent change in VA following 4 wk of cast immobilization (solid symbols: immobilization group; open symbols: immobilization + imagery group). This finding indicates that individuals who experienced the largest immobilization-induced loss of muscle strength also experienced the largest immobilization-induced impairments in voluntary (neural) activation (r = 0.56, P < 0.01). B: there was a negative association between the percent change in muscle strength and the percent change in the cortical SP duration following 4 wk of cast immobilization (solid symbols: immobilization group; open symbols: immobilization + imagery group). This finding indicates that individuals who experienced the largest immobilization-induced loss of muscle strength also experienced the largest immobilization-induced prolongation in the cortical SP (r = −0.39, P = 0.03).
DISCUSSION
In this study, we utilized MI as a manipulation to minimize the immobilization-induced loss of strength and VA to better elucidate the role of the cortex in regulating muscle strength/weakness by examining the association/dissociation between the respective without concomitantly affecting muscle properties. The novel, and most notable, findings of this study are that 1) imagery attenuated the loss of strength and VA by 50%, while also concomitantly eliminating the prolongation of the SP; and 2) we observed significant associations between the percent changes in a) muscle strength and VA, and b) muscle strength and SP duration. Below we discuss the interpretation, significance, and impact of these findings.
The finding that imagery, a neurological-based intervention strategy, attenuated immobilization-induced weakness, coupled with the observation that 32% (obtained by calculating R2 from the correlation coefficient) of the between-subject variability in the loss of strength was explained by the loss of ability to voluntarily activate the musculature indicates that neurological factors are critical contributors to weakness (at least in the context of a disuse model). “Neural factors” have long-been considered to be key contributors to muscle performance (Moritani and deVries 1979), as incomplete motor unit recruitment and/or the inability to mount high motor unit discharge rates are both factors that can result in weakness (Kamen 2005). As such, this finding is not surprising per se. In fact, our laboratory's own previous studies (Clark et al. 2006a, 2006b), and those of others (Kawakami et al. 2001), indicate a moderate-to-strong association between the loss of VA capacity and loss of strength following prolonged disuse. Similarly, the finding that imagery attenuated the loss of strength is also expected as several studies have shown that its imagery training (in the absence of disuse) increases muscle strength (Fontani et al. 2007; Ranganathan et al. 2004; Yue and Cole 1992; Zijdewind et al. 2003); however, to our knowledge, this finding is novel as it is the first report of imagery training significantly attenuating the loss of strength following prolonged disuse. To our knowledge there have been two other studies examining the potential for imagery to attenuate losses of motor function following prolonged disuse (Clark et al. 2006b; Crews and Kamen 2006). Neither of these observed significant effects. Specifically, Crews and Kamen (2006) reported that imagery training (performed 4 times) did not ameliorate the effects of 7 days of cast immobilization on a motor control task changes in a motor control task following 7 days of cast immobilization. Similarly, our own group did not observe a significant effect on mitigating the loss of strength following prolonged lower limb unweighting (Clark et al. 2006b). It should be noted, however, that the former study employed a short (7-day) immobilization period with only 4 days of imagery training occurring during this time period. While our laboratory's prior study used a similar duration for the disuse period (4 wk) and a similar frequency of imagery training (4 days/wk), there were only six subjects in the imagery group, and, based on the observed ESs, it is likely that it was underpowered. Thus our findings that imagery attenuated the loss of muscle strength provides proof-of-concept for it as a therapeutic intervention for muscle weakness; however, double-blind placebo-controlled studies should be conducted to more fully explore this potential.
Another novel aspect of this work is that our findings, collectively, provide support for our global hypothesis that the cortex is a critical determinant of muscle strength and VA and that high level of intracortical inhibition is an important neurophysiological factor regulating force generation of muscle. The cortex, and the motor cortex in particular, has historically been considered critical for movement coordination/control and skill acquisition as opposed to maximal force generation of individual muscles (Adkins et al. 2006; Jackson 1873; Remple et al. 2001), but growing evidence is now suggesting that it is a critical determent of muscle strength/weakness. We believe our finding of 1) MI [an interventional strategy that activates the motor cortical areas (Hetu et al. 2013)] attenuating the loss of muscle strength while concomitantly eliminating the prolongation of the SP (a neurophysiological outcome of corticospinal inhibition), coupled with the finding of 2) an association between the changes in strength and the SP, provide strong support for the notion that the cortex is a critical determinant of muscle strength/weakness. If our interpretation is correct, these findings suggest that increases in intracortical inhibition are mechanistically associated with muscle weakness.
Our above-mentioned interpretation is based on the predication of imagery and actual movements sharing, at least in part, common cortical substrates, which has been shown in neuroimaging studies (see Hetu et al. 2013 for a meta-analytical review). For example, a host of brain functional imaging studies indicate that imagery activates several cortical areas, including the M1, supplementary and premotor areas, and cingulated gyrus (Malouin et al. 2003; Porro et al. 1996; Rao et al. 1993; Roth et al. 1996; Stephan et al. 1995), all of which are known to contain corticospinal neurons in monkeys (Dum and Strick 1996). Additionally, TMS studies have shown that MI acutely increases the excitability of the specific representation in the contralateral M1 (Bakker et al. 2008; Facchini et al. 2002). Furthermore, imagery training has been shown to increase muscle strength in a variety of muscles (Herbert et al. 1998; Ranganathan et al. 2004; Yue and Cole 1992; Zijdewind et al. 2003), with the strength gain being accompanied by significant increases in the EEG-derived cortical potential, suggesting imagery training enhances cortical output and increases VA (Ranganathan et al. 2004). Most recently, Yao and colleagues (2013) also demonstrated that kinesthetic imagery (as used herein) increased muscle strength and the movement-related cortical potential on scalp locations over M1 and the supplementary motor cortices, with the authors suggesting that imagery changes the activity level of cortical motor control networks that translates into greater descending command to the target muscle and increase its strength. So, based on these findings, we postulate that in the present study imagery training had a similar effect on the motor cortical areas, providing support for our interpretation. However, it should be noted that there are reports that imagery training slightly increases the H-reflex excitability (Cowley et al. 2008) and has a selective facilitatory effect on the stretch reflex pathways (Aoyama and Kaneko 2011), and, as such, we cannot fully exclude that peripheral nervous system factors could have contributed to the differential results we observed in the imagery group. Similarly, we cannot fully exclude that the SP is mechanistically indicative of cortical level changes. The duration of the SP is dependent on the intensity of stimulation (Cantello et al. 1992; Inghilleri et al. 1993), and the first 50-ms are widely assumed to be spinally mediated, through mechanisms such as after-hyperpolarization of the motoneurons and recurrent inhibition, with the latter part due to supraspinal inhibition (Cantello et al. 1992; Fuhr et al. 1991; Inghilleri et al. 1993; Wilson et al. 1993; Ziemann et al. 1993). This SP is generally believed to be caused by activation of long-lasting GABAB-mediated inhibition (Kobayashi and Pascual-Leone 2003; McDonnell et al. 2006; Reis et al. 2008). With this stated, it should be noted that recent data suggest that the SP can be influenced by spinal-mediated factors such muscle lengthening (Butler et al. 2012) and that the underlying mechanisms may also be linked to shifts in cortical glutamate + glutamine concentrations (Tremblay et al. 2013). Thus our interpretation of our current findings must be considered within the context of the somewhat limited understanding of the SP. However, our mechanistic interpretation of increases in intracortical inhibition being linked to weakness is indeed consistent with findings from other studies (Clark et al. 2010; Weier et al. 2012), although it should be noted that it is inconsistent with others (Plow et al. 2013).
We should note that there are certainly muscular factors that likely contributed to the observed muscle weakness as well. Due to pragmatic reasons (e.g., associated costs), we did not obtain measures pertaining to muscle size; however, it is very likely that muscle atrophy as well as other muscular adaptations contributed to the losses in muscle strength. In fact, based on the observation that MI attenuated ∼50% of the loss of strength and VA, it seems that one can conclude that around one-half of the induced weakness was due to muscular adaptations and that the other one-half was due to reductions in neural drive.
There are several limitations of this study that should be acknowledged. First, our subjects were asked to produce a contraction intensity at their relative strength level at all time points of testing (as opposed to the same absolute force level), and it is possible that selected outcomes in particular (e.g., SP duration) could have been influenced by the absolute amount of force produced. Second, we did not actually record the amount of muscle activity/usage during the immobilization protocol, and as such it is not possible to know whether subtle differences in activity across groups could explain the results. We should note that our casting protocol involves the splint platform extending well beyond the fingers, which minimizes the potential for study participants to engage the wrist flexion musculature, but, nonetheless, we can exclude this as a source of variance in the present study. Third, we were not able to quantify how successful study participants were in actually performing the MI, and, as such, it is possible, if not probable, that there was a reasonable amount of heterogeneity in the ability of study participants to actually perform MI that we were not able to control for.
In conclusion, the cortex as a determinant of strength/weakness has received limited attention. We used immobilization to induce weakness and impairments in VA, and used MI to activate the cortex during immobilization. We measured the SP duration by stimulating the brain during a contraction to provide an index of GABAB-mediated inhibition. Our findings most likely suggest that neurological mechanisms arising at the cortical level are a substantial contributor to disuse-induced muscle weakness, and that regular activation of the motor cortical regions via MI attenuates disuse-induced losses in strength and VA by maintaining normal levels of inhibition.
GRANTS
Research reported in this publication was supported by grants from the National Institute on Aging (R01-AG-044424 to B. C. Clark), the National Center for Complimentary and Alternative Medicine (R01-AT-006978 to B. C. Clark and J. S. Thomas), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R15-AR-055802 to B. C. Clark) of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.C.C. conception and design of research; B.C.C. and N.K.M. performed experiments; B.C.C. and M.N. analyzed data; B.C.C., M.N., T.D.L., and J.S.T. interpreted results of experiments; B.C.C. prepared figures; B.C.C. drafted manuscript; B.C.C., N.K.M., M.N., T.D.L., and J.S.T. edited and revised manuscript; B.C.C., N.K.M., M.N., T.D.L., and J.S.T. approved final version of manuscript.
REFERENCES
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol 101: 1776–1782, 2006. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Kaneko F. The effect of motor imagery on gain modulation of the spinal reflex. Brain Res 1372: 41–48, 2011. [DOI] [PubMed] [Google Scholar]
- Ashe J. Force and the motor cortex. Behav Brain Res 87: 255–269, 1997. [DOI] [PubMed] [Google Scholar]
- Bakker M, Overeem S, Snijders AH, Borm G, van Elswijk G, Toni I, Bloem BR. Motor imagery of foot dorsiflexion and gait: effects on corticospinal excitability. Clin Neurophysiol 119: 2519–2527, 2008. [DOI] [PubMed] [Google Scholar]
- Butler JE, Petersen NC, Herbert RD, Gandevia SC, Taylor JL. Origin of the low-level EMG during the silent period following transcranial magnetic stimulation. Clin Neurophysiol 123: 1409–1414, 2012. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology 42: 1951–1959, 1992. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 44: 773–791, 1980. [DOI] [PubMed] [Google Scholar]
- Clark BC, Fernhall B, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting. I. Skeletal muscle contractile properties and applied ischemia efficacy. J Appl Physiol 101: 256–263, 2006a. [DOI] [PubMed] [Google Scholar]
- Clark BC, Issac LC, Lane JL, Damron LA, Hoffman RL. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol 105: 868–878, 2008. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM, Bolanowski SJ, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting. II. Neurological properties and motor imagery efficacy. J Appl Physiol 101: 264–272, 2006b. [DOI] [PubMed] [Google Scholar]
- Clark BC, Taylor JL, Hoffman RL, Dearth DJ, Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve 42: 363–372, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley PM, Clark BC, Ploutz-Snyder LL. Kinesthetic motor imagery and spinal excitability: the effect of contraction intensity and spatial localization. Clin Neurophysiol 119: 1849–1856, 2008. [DOI] [PubMed] [Google Scholar]
- Crews RT, Kamen G. Motor-evoked potentials following imagery and limb disuse. Int J Neurosci 116: 639–651, 2006. [DOI] [PubMed] [Google Scholar]
- Damron LA, Dearth DJ, Hoffman RL, Clark BC. Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. J Neurosci Methods 173: 121–128, 2008. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci 16: 6513–6525, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study. Acta Neurol Scand 105: 146–151, 2002. [DOI] [PubMed] [Google Scholar]
- Fontani G, Migliorini S, Benocci R, Facchini A, Casini M, Corradeschi F. Effect of mental imagery on the development of skilled motor actions. Percept Mot Skills 105: 803–826, 2007. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 81: 257–262, 1991. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Dean C, Gandevia SC. Effects of real and imagined training on voluntary muscle activation during maximal isometric contractions. Acta Physiol Scand 163: 361–368, 1998. [DOI] [PubMed] [Google Scholar]
- Hetu S, Gregoire M, Saimpont A, Coll MP, Eugene F, Michon PE, Jackson PL. The neural network of motor imagery: an ALE meta-analysis. Neurosci Biobehav Rev 37: 930–949, 2013. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466: 521–534, 1993. [PMC free article] [PubMed] [Google Scholar]
- Jackson JH. On the localisation of movements in the brain. Lancet 101: 232–235, 1873. [Google Scholar]
- Kamen G. Aging, resistance training, and motor unit discharge behavior. Can J Appl Physiol 30: 341–351, 2005. [DOI] [PubMed] [Google Scholar]
- Kaneko F, Murakami T, Onari K, Kurumadani H, Kawaguchi K. Decreased cortical excitability during motor imagery after disuse of an upper limb in humans. Clin Neurophysiol 114: 2397–2403, 2003. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol 84: 7–12, 2001. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2: 145–156, 2003. [DOI] [PubMed] [Google Scholar]
- Lundbye-Jensen J, Nielsen JB. Central nervous adaptations following 1 wk of wrist and hand immobilization. J Appl Physiol 105: 139–151, 2008. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp 19: 47–62, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 67: 28–40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Russ DW, Clark BC. The complex relationship between muscle mass and muscle strength. In: Sarcopenia, edited by Cruz-Jentoft AJ, Morley JE. Chichester, UK: Wiley, 2012, p. 74–103. [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173: 86–93, 2006. [DOI] [PubMed] [Google Scholar]
- Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58: 115–130, 1979. [PubMed] [Google Scholar]
- Mosca B, Delbono O, Laura Messi M, Bergamelli L, Wang ZM, Vukcevic M, Lopez R, Treves S, Nishi M, Takeshima H, Paolini C, Martini M, Rispoli G, Protasi F, Zorzato F. Enhanced dihydropyridine receptor calcium channel activity restores muscle strength in JP45/CASQ1 double knockout mice. Nat Commun 4: 1541, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Cunningham D, Bonnett C, Gohar D, Bayram M, Wyant A, Varnerin N, Mamone B, Siemionow V, Hou J, Machado A, Yue GH. Neurophysiological correlates of aging-related muscle weakness. J Neurophysiol 110: 2563–2573, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci 16: 7688–7698, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan VK, Siemionow V, Liu JZ, Sahgal V, Yue GH. From mental power to muscle power–gaining strength by using the mind. Neuropsychologia 42: 944–956, 2004. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, et al. , et al. Functional magnetic resonance imaging of complex human movements. Neurology 43: 2311–2318, 1993. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586: 325–351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remple MS, Bruneau RM, VandenBerg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res 123: 133–141, 2001. [DOI] [PubMed] [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, Morand S, Gemignani A, Decorps M, Jeannerod M. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport 7: 1280–1284, 1996. [DOI] [PubMed] [Google Scholar]
- Russ DW, Gregg-Cornell K, Conaway MJ, Clark BC. Evolving concepts on the age-related changes in “muscle quality”. J Cachexia Sarcopenia Muscle 3: 95–109, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73: 373–386, 1995. [DOI] [PubMed] [Google Scholar]
- Taylor JL. Point: The interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107: 354–355, 2009. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Beaule V, Proulx S, de Beaumont L, Marjanska M, Doyon J, Pascual-Leone A, Lassonde M, Theoret H. Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. J Neurophysiol 109: 1343–1349, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier AT, Pearce AJ, Kidgell DJ. Strength training reduces intracortical inhibition. Acta Physiol (Oxf) 206: 109–119, 2012. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Lockwood RJ, Thickbroom GW, Mastaglia FL. The muscle silent period following transcranial magnetic cortical stimulation. J Neurol Sci 114: 216–222, 1993. [DOI] [PubMed] [Google Scholar]
- Yao WX, Ranganathan VK, Allexandre D, Siemionow V, Yue GH. Kinesthetic imagery training of forceful muscle contractions increases brain signal and muscle strength. Front Hum Neurosci 7: 561, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue G, Cole K. Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67: 1114–1123, 1992. [DOI] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol 115: 1264–1275, 2004. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalogr Clin Neurophysiol 105: 269–279, 1997. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett 156: 167–171, 1993. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Toering ST, Bessem B, Van Der Laan O, Diercks RL. Effects of imagery motor training on torque production of ankle plantar flexor muscles. Muscle Nerve 28: 168–173, 2003. [DOI] [PubMed] [Google Scholar]