Abstract
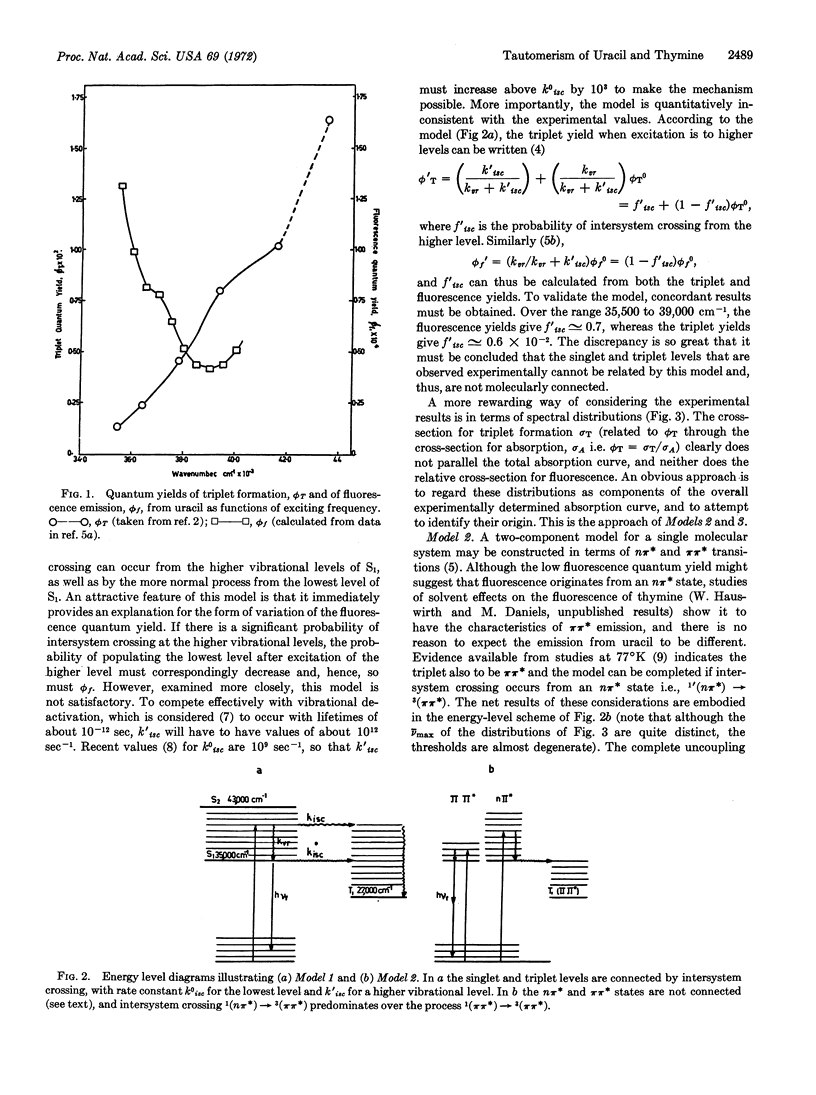
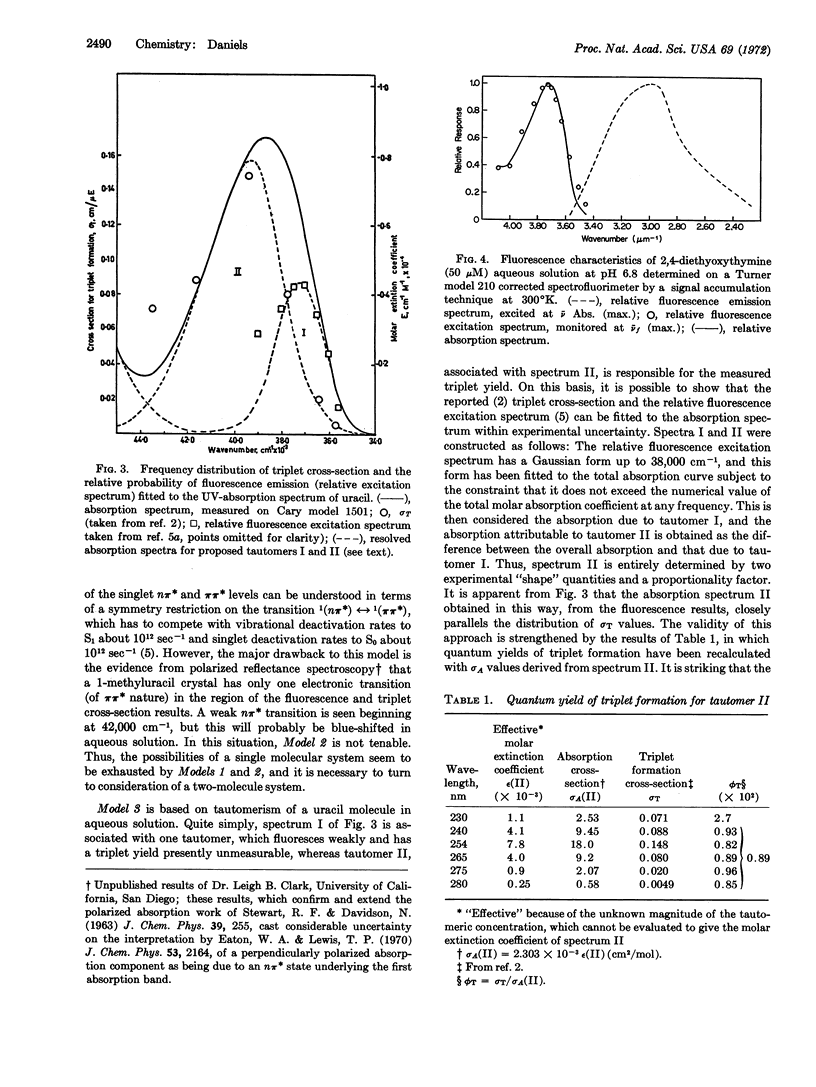
Excitation spectra for triplet state formation and fluorescence emission from uracil and thymine in neutral aqueous solution at room temperature are anomalous when compared to the absorption spectra of uracil and thymine.
The experimental data are critically examined with respect to three molecular models; Model 1, which is characterized by increased intersystem crossing from higher vibrational levels of S1; Model 2, in which fluorescence is attributed to a (π,π*) state, whereas intersystem crossing occurs from an (nπ*) state; and Model 3, in which fluorescence is attributed to a tautomer I, while triplet yields originate from tautomer II. Reasons are presented for discarding Models 1 and 2, and it is demonstrated that the observed excitation spectra complement each other with respect to the absorption spectrum, such that the quantum yields of triplet formation and fluorescence emission become independent of exciting wavelength. It is suggested that the fluorescing tautomer I has the N3-C4 enol structure, while the triplet-forming tautomer II is most likely the predominant diketo form.
Keywords: lactam, lactim, enol, keto, triplet states, fluorescence
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniels M., Hauswirth W. Fluorescence of the purine and pyrimidine bases of the nucleic acids in neutral aqueous solution at 300 degrees K. Science. 1971 Feb 19;171(3972):675–677. doi: 10.1126/science.171.3972.675. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Shulman R. G. Excited electronic states of DNA. Science. 1968 Sep 27;161(3848):1311–1319. doi: 10.1126/science.161.3848.1311. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Johns H. E. Ultraviolet photochemistry of thymine in aqueous solution. Photochem Photobiol. 1970 Jun;11(6):429–444. doi: 10.1111/j.1751-1097.1970.tb06015.x. [DOI] [PubMed] [Google Scholar]
- Hauswirth W., Daniels M. Fluorescence of thymine in aqueous solution at 300 degrees K. Photochem Photobiol. 1971 Feb;13(2):157–163. doi: 10.1111/j.1751-1097.1971.tb06101.x. [DOI] [PubMed] [Google Scholar]
- Longworth J. W., Rahn R. O., Shulman R. G. Luminescence of pyrimidines, purines, nucleosides, and nucleotides at 77 degree K. The effect of ionization and tautomerization. J Chem Phys. 1966 Oct 15;45(8):2930–2939. doi: 10.1063/1.1728048. [DOI] [PubMed] [Google Scholar]
- SHUGAR D., FOX J. J. Spectrophotometric studies of nucleic acid derivatives and related compounds as a function of pH. I. Pyrimidines. Biochim Biophys Acta. 1952;9(2):199–218. doi: 10.1016/0006-3002(52)90147-9. [DOI] [PubMed] [Google Scholar]
- Vigny P. Fluorescence des bases puriques et pyrimidiques à température ambiante. C R Acad Sci Hebd Seances Acad Sci D. 1971 Apr 26;272(17):2247–2249. [PubMed] [Google Scholar]



