Abstract
In the central nervous system, inhibition shapes neuronal excitation. In spinal cord glycinergic inhibition predominates, whereas GABAergic inhibition predominates in the brain. The retina uses GABA and glycine in approximately equal proportions. Glycinergic crossover inhibition, initiated in the On retinal pathway, controls glutamate release from presynaptic OFF cone bipolar cells (CBCs) and directly shapes temporal response properties of OFF retinal ganglion cells (RGCs). In the retina, four glycine receptor (GlyR) α-subunit isoforms are expressed in different sublaminae and their synaptic currents differ in decay kinetics. GlyRα1, expressed in both On and Off sublaminae of the inner plexiform layer, could be the glycinergic isoform that mediates On-to-Off crossover inhibition. However, subunit-selective glycine contributions remain unknown because we lack selective antagonists or cell class-specific subunit knockouts. To examine the role of GlyRα1 in direct inhibition in mature RGCs, we used retrogradely transported adeno-associated virus (AAV) that performed RNAi and eliminated almost all glycinergic spontaneous and visually evoked responses in PV5 (OFFαTransient) RGCs. Comparisons of responses in PV5 RGCs infected with AAV-scrambled-short hairpin RNA (shRNA) or AAV-Glra1-shRNA confirm a role for GlyRα1 in crossover inhibition in cone-driven circuits. Our results also define a role for direct GlyRα1 inhibition in setting the resting membrane potential of PV5 RGCs. The absence of GlyRα1 input unmasked a serial and a direct feedforward GABAAergic modulation in PV5 RGCs, reflecting a complex interaction between glycinergic and GABAAergic inhibition.
Keywords: AAV-RNAi, glycine receptor, inhibitory circuitry, retinal ganglion cell
in the mammalian retina, amacrine cells (ACs) form complex synaptic networks in a series of independent microcircuits (Anderson et al. 2011; Azeredo da Silveira and Roska 2011; Helmstaedter et al. 2013; Marc et al. 2013). To regulate inner retinal excitatory signaling and shape the spiking output of retinal ganglion cells (RGCs), these circuits utilize several mechanisms including feedforward, feedback, crossover, and serial inhibition (reviewed in Zhang and McCall 2012). As one of the main postsynaptic targets of ACs, glycine receptors (GlyRs) are critical components of inhibitory circuits and shape RGC spontaneous and visually evoked responses, e.g., temporal tuning or gain control (Chen et al. 2010, 2011; Manookin et al. 2008; Münch et el. 2009; Murphy and Rieke 2006; Nobles et al. 2012; Pang et al. 2003; Roska et al. 2006; Russell and Werblin 2010; van Wyk et al. 2009).
Four glycinergic α-subunit isoforms have been identified in mouse retina (Dutertre et al. 2012; Wässle et al. 2009), and each GlyR α-subunit exhibits unique current decay kinetics and distinct cell class localization (Heinze et al. 2007; Ivanova et al. 2006; Majumdar et al. 2007, 2009; Weiss et al. 2008). GlyRα1, the isoform with the fastest decay time, is expressed in OFF cone and rod bipolar cells (Eggers and Lukasiewicz 2010; Ivanova et al. 2006) as well as in ON and OFF A-type RGCs (Majumdar et al. 2007). The other GlyR α-subunits are localized on other retinal cell classes (reviewed in Zhang and McCall 2012). The diversity of GlyRs, their different kinetics, and retinal localization suggest that α-subunit-specific circuits regulate visual signaling of either diverse properties within distinct circuits or similar properties across circuits. Supporting the idea of a role in distinct circuits, we showed that GlyRα2 and GlyRα3 selectively mediate inhibition in the retinal On and Off pathways, respectively (Nobles et al. 2012).
On-to-Off pathway glycinergic crossover inhibition onto presynaptic OFF cone bipolar cells (CBCs) (Eggers and Lukasiewicz 2010; Ivanova et al. 2006), as well as onto the RGCs themselves (Majumdar et al. 2007; Manookin et al. 2008; Murphy and Rieke 2006; Pang et al. 2003; van Wyk et al. 2009; Werblin 2010), shapes the visual responses of OFF RGCs. These results and its retinal expression pattern make GlyRα1 the most likely isoform that modulates spontaneous and visually evoked activity of mature OFF α RGCs through crossover inhibition.
The lack of subunit isoform-specific antagonists and cell class-specific/conditional GlyRα1-knockout mice precludes a direct test of this hypothesis. To address this dilemma and define the role of direct GlyRα1 input in shaping mature OFF α RGC responses, we combined several novel approaches. Namely, we 1) infected adult RGCs with retrogradely transported adeno-associated virus (AAV); 2) generated short hairpin RNA (shRNA) against Glra1 to eliminate its expression in RGCs without changing its expression in the upstream circuit; 3) targeted PV5 RGCs (likely OFF αTransient; Münch et al. 2009) in a reporter mouse line (PVCre) with eight identified and fluorescently labeled RGC types (Farrow et al. 2013); and 4) recorded spontaneous and visually evoked responses with two-photon microscopy.
Compared to wild type, RNAi eliminates almost all GlyRα1 expression on the dendrites of PV5 (PV5WT) RGCs, as well as all glycinergic spontaneous and visually evoked postsynaptic currents [spontaneous (sIPSCs) and evoked (eIPSCs) inhibitory postsynaptic currents]. This indicates that the majority of glycinergic input to PV5 RGCs is GlyRα1 subunit specific. Because only the direct GlyRα1 input is eliminated in RGCs and the upstream circuitry is intact, differences in responses between PV5 RGCs with and without GlyRα1 input can be used to define its role in shaping the PV5WT response. We show that the GlyRα1 subunit mediates a previously described On-to-Off crossover synaptic input to OFF αTransient RGCs. We also show that in PV5 RGCs a direct GlyRα1-specific inhibition controls spontaneous activity, facilitates excitatory signaling to luminance decrements (OFF response), and suppresses excitatory signaling to luminance increments (ON response). This improves the signal-to-noise ratio and maintains the correlation between the receptive field (RF) OFF center response and the stratification of the PV5WT RGC dendrites in the Off sublaminae of the inner plexiform layer (IPL). Together these direct inhibitory inputs enhance the fidelity of PV5WT RGCs. We also demonstrate a complex interaction between glycinergic and GABAergic inputs at the level of the PV5WT RGCs. Namely, a serial GABAAergic input modulates GlyRα1 crossover inhibition, and a direct GABAAergic inhibition modulates the PV5WT RGC response at both luminance increment and decrement.
MATERIALS AND METHODS
Animals
PvalbCre homozygous mice (PVCre mice; Hippenmeyer et al. 2005; Jackson Lab stock no. 008069) crossed to Thy1Stp-EYFP homozygous mice (a gift of J. Sanes; Buffelli et al. 2003; Jackson Lab stock no. 005630) were used in all of the experiments. In their retina, yellow fluorescent protein (YFP) is expressed in eight identified RGC classes (Farrow et al. 2013). The PV5 RGC has a large soma and dendritic morphology, similar to OFF αTransient RGCs. Throughout this report, we refer to these OFF αTransient RGCs as PV5 RGCs. Using two-photon microscopy and their fluorescence and morphology, PV5 RGCs were targeted for electrophysiological assessments. Their identity was also verified by immunohistochemistry. All experimental procedures were conducted in accordance with regulations described for the ethical care and treatment of animals in the Society for Neuroscience Policy on the Use of Animals in Neuroscience Research and with the approval of the individual Institutional Animal Care and Use Committees at the University of Louisville and the Friedrich Miescher Institute (FMI).
Viral Vector Construction
The AAV vector plasmid AAV-Ef1a-NLStdTomato-H1 (see Fig. 3A) was constructed by linearizing the viral vector AAV-Ef1a-NLStdTomato at its PmlI site and inserting an H1 promoter. A poly(A) tail was added by seamless cloning using GeneArt 2× enzyme mix (Life Technologies), and the vector was then linearized with SalI/XbaI. The synthesized shRNA cassettes (Sigma-Aldrich) were inserted after the H1 promoter (see Fig. 3A). AAV was chosen because of its high efficiency and low immunogenicity (reviewed in McClements and MacLaren 2013).
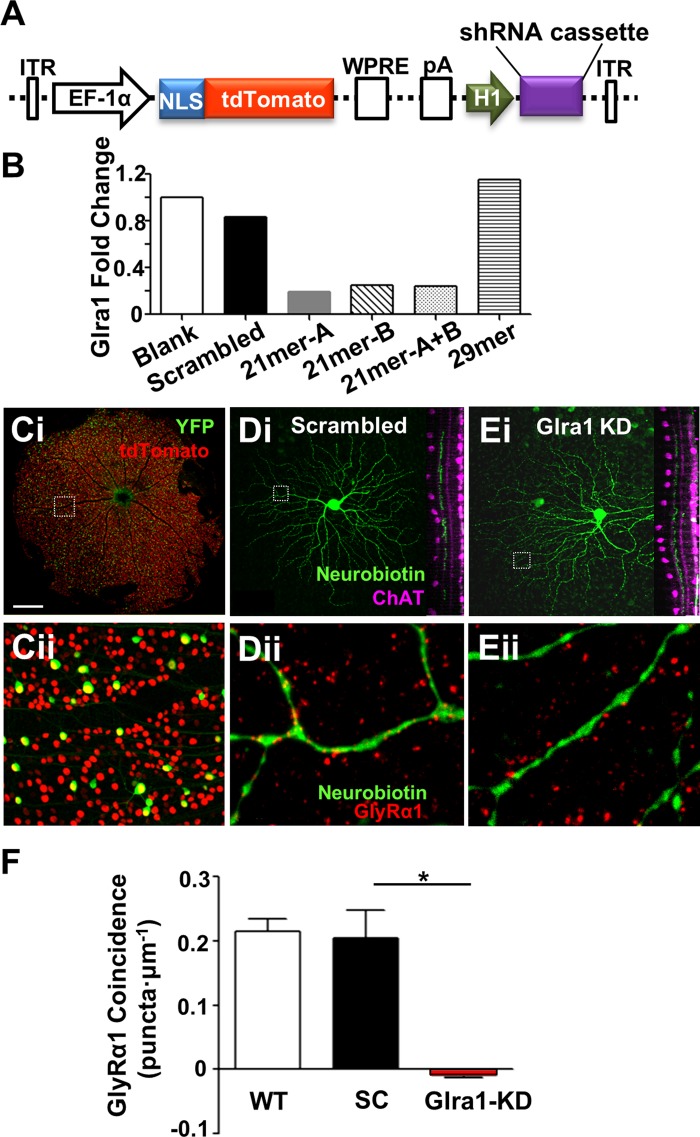
Fig. 3.
Adeno-associated virus (AAV)-mediated RNAi significantly knocks down GlyRα1 expression on PV5 RGC dendrites. A: schematic diagram of the AAV-RNAi vector backbone, pAAV-Ef1a-NLStdTomato-H1. The short hairpin (sh)RNA cassette (Scrambled or Glra1) was inserted after the H1 promoter. ITR, flanking inverted terminal repeats; EF-1α, elongation factor-1α promoter; NLS, nuclear localization signal for the fluorescent protein tdTomato; WPRE, woodchuck hepatitis posttranscriptional regulatory element; pA, poly(A) tail. B: RT-PCR results show that AAV-Glra1-shRNA 21mer-A produced the largest fold reduction in Glra1 mRNA in transfected HEK293 cells compared with all other constructs. Ci: fluorescence confocal image of the contralateral retina of a PvalbCre × Thy1Stp-EYFP mouse, 4 wk after injection of AAV-scrambled shRNA into the lateral geniculate nucleus (dLGN). Cii: higher-power image of boxed area in Ci, showing yellow fluorescent protein (YFP)-positive PV RGCs (green), AAV-infected RGCs with tdTomato-positive nuclei (red), and double-labeled PV-infected RGCs (green with yellow nuclei). D and E: representative confocal images of neurobiotin-filled PV5 RGCs infected with AAV-Scrambled-shRNA (SC; Di) or AAV-Glra1-shRNA (Glra1-KD; Ei). Dii and Eii: higher-power images of boxed areas in Di and Ei showing the distribution of GlyRα1 expression (red puncta). F: dendrites of PV5 RGCs infected with AAV-Glra1-shRNA (n = 7 cells; N = 14 dendritic fields) have significantly fewer coincident puncta than those infected with AAV-Scrambled-shRNA (n = 4; N = 8), whose expression is similar to PV5WT RGCs (n = 8; N = 16). *P < 0.05. Scale bar (shown in Ci): 600 μm (Ci), 50 μm (Cii), 45 μm (Di and Ei), 3 μm (Dii and Eii).
Two 21-mer and one 29-mer shRNA were designed and synthesized to target different regions of Glra1 mRNA. A scrambled shRNA, designed to no gene, was used as a control. The efficiency of each of three Glra1 and a scrambled shRNA construct were assessed in cultured HEK293T cells after cotransfection with a plasmid expressing GlyRα1 [pCMV6-AC-GFP, carrying mouse Glra1 cDNA open reading frame (OriGene)].
RNA Isolation and cDNA Preparation
Forty-eight hours after transfection of HEK293 cells, the mRNA level of Glra1 was measured. RNA was isolated with TRIzol LS reagent (Invitrogen) according to a standard protocol including DNaseI treatment (Promega) to remove residual genomic DNA. The cDNA was synthesized with 1 μg of RNA and random primers (Promega) according to the SuperScript III Reverse Transcriptase kit (Invitrogen).
RT-PCR
RT-PCR was performed to determine mRNA levels of Glra1 with the StepOne Real-Time PCR System (Applied Biosystems). Each 20-μl reaction mixture included 2 μl of cDNA, 10 μl of SYBR Green mix (Invitrogen), and 1 μl of Glra1 or 18S RNA primer set (10 μM). For each cDNA sample, three PCR replicates were performed using each primer set. The PCR cycling conditions were incubation at 50°C for 2 min, denaturation at 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. With 18S RNA as internal control (Krol et al. 2010), the fold change of Glra1 in each cDNA sample was calculated with the ΔΔCT method. Glra1 RT-PCR primer forward 5′-CCGTCTGGCCTACAATGAAT-3′ and Glra1 RT-PCR primer reverse 5′-CACGTCTGTACATCCATCGG-3′ were used.
AAV Production
Recombinant AAVs (serotype 2/7) were made according to a standard triple-plasmid protocol, by cotransfection of HEK293T cells with the AAV vector plasmid, AAV helper plasmid (harboring Rep/Cap), and Ad-helper plasmid (pGHTI-adeno1). Transfected cells were lysed and treated with Benzonase (Sigma-Aldrich catalog no. E8263). Packaged AAVs were concentrated and purified from total cell lysates by iodixanol gradient centrifugation (Sigma-Aldrich, OptiPrep) and collected in the 40% iodixanol band. Genome copy (GC) number titration was evaluated with RT-PCR (Applied Biosystems, TaqMan reagents). High titers were produced for both scrambled shRNA (1.36 × 1012 GC/ml) and Glra1 shRNA (1.14 × 1012 GC/ml).
Viral Injections in Dorsal Lateral Geniculate Nucleus
Mice were sedated with chlorprothixene (5 mg/kg). Anesthesia was induced and maintained throughout the procedure with isoflurane administered through a mask mounted in front of the nose of the mouse. Body temperature was maintained at 37°C with a feedback-controlled heating pad. The head of the mouse was secured in a stereotaxic frame with ear bars and a bite bar. The skull was exposed with a midline incision and then leveled with reference to sagittal sutures. A craniotomy was performed between the bregma and lambdoid sutures to access the left dorsal lateral geniculate nucleus (dLGN). Tips of borosilicate glass pipettes (inner diameter 50–100 μm) were filled with 2–3 μl of virus and positioned 2.25 μm posterior and 2.25 μm left of bregma and lowered 2.5–2.7 μm from the brain surface into the dLGN. Using pressure, 1–1.5 μl of virus was injected into the dLGN. Four weeks after AAV injection RGC nuclei were positive for AAV-induced tdTomato expression (see Fig. 3C), and expression was stable up to at least 10 wk after injection. Animals 4–10 wk after injection of AAV-Glra1-shRNA and AAV-Scrambled-shRNA were used for all experiments.
Immunohistochemistry
To examine the morphology and expression pattern of GlyRα1 in whole mount retina, the mouse was anesthetized with CO2 and killed by cervical dislocation. With previously published techniques, the eyes were removed and retinas were dissected and fixed in 4% paraformaldehyde in PBS for 30 min (Nobles et al. 2012). Whole mount retinas were reacted with mouse monoclonal anti-GlyRα1 primary (1:500, Synaptic Systems catalog no. 146 111) and donkey anti-mouse IgG Alexa 555 secondary antibody (1:200, Life Technologies catalog no. A31570). The IPL sublamination pattern was defined with goat anti-choline acetyltransferase (ChAT) [1:200, Millipore (CHEMICON/Upstate/Linco) catalog no. AB144] primary and a donkey anti-goat IgG Alexa 405 (1:200, Abcam catalog no. ab175664) secondary antibody. After electrophysiological recordings PVCre RGCs labeled with neurobiotin were reacted with streptavidin-Alexa 633 (1:200, Life Technologies catalog no. S21375) to visualize their somatic and dendritic morphology.
Confocal Image Acquisition and Colocalization Analysis
Retinas were imaged on a Zeiss LSM 700 confocal microscope. Z-stack images of neurobiotin-filled PV5 RGCs were acquired with 0.30-μm z-step using a 40× oil immersion lens, NA 1.2. For each RGC, two nonoverlapping dendritic areas (70 × 70 μm) were randomly selected and imaged with 0.17-μm z-step using a 63× oil-immersion lens, NA 1.3. Prior to colocalization analyses using Imaris (Bitplane), images were deconvolved with Huygens software (Scientific Volume Imaging). For each RGC, we examined all the dendritic processes within the selected areas. To evaluate colocalization a channel was built for each dendritic area (ImarisColoc tool; Fig. 1C) that contained only areas where GlyRα1 puncta were coincident with identified RGC dendrites. Thresholds were set to include most potential coincident pixels and exclude nonspecific colocalization due to background noise. The total number of coincident GlyRα1 puncta (POriginal) was counted (Imaris Spot function), and the total length of dendrite (LDendrite) was measured (Imaris Filament function). To evaluate specific coincidence, we compared this coincidence rate to a coincidence rate (PRandom) we measured when we flipped the GlyRα1-positive puncta channel along its horizontal/vertical axis. The corrected rate was computed as follows: coincidence rate = (POriginal − PRandom)/LDendrite.
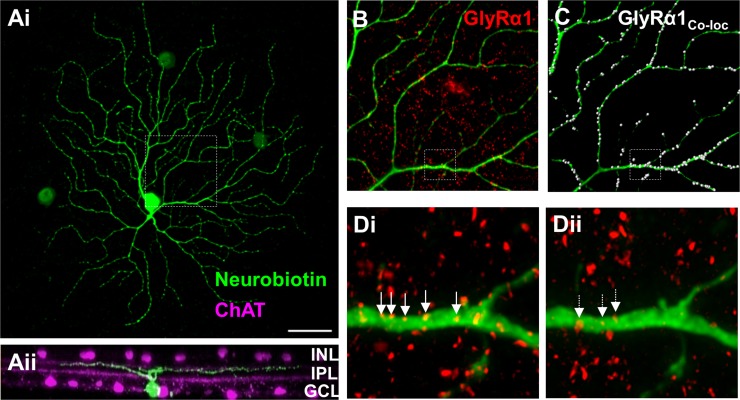
Fig. 1.
The GlyRα1 subunit is expressed on PV5WT retinal ganglion cell (RGC) dendrites. Ai: representative confocal image of a neurobiotin-labeled PV5 RGC (green) in a retinal whole mount shows its characteristic A2 type morphology. ChAT, choline acetyltransferase. Aii: rotated view of the same RGC shows its dendritic lamination pattern in the Off sublaminae of the inner plexiform layer (IPL) relative to the bands formed by the processes of the cholinergic amacrine cells (ACs; magenta). B: representative portion of the dendritic arbor of the same PV5WT RGC (see box; 70 × 70-μm area) and the punctate pattern of GlyRα1 subunit expression (red). C: colocalized GlyRα1 puncta (white dots) on the dendrite of the same RGC. Di: magnified and deconvolved image from B (see box). Arrows indicate a subset of representative colocalized GlyRα1 puncta on the PV5 dendrite. Dii: illustration of random association of GlyRα1 puncta that results from superposition of the channel containing the dendrite with a duplicated and 180°-rotated channel with the GlyRα1 puncta. Arrows indicate what are considered randomly colocalized GlyRα1 puncta. The number of randomly colocalized puncta was used to correct GlyRα1 puncta coincidence rate. INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar (shown in Ai): 40 μm (A), 16 μm (B and C), 2.5 μm (D).
Whole Mount Retinal Preparation for Electrophysiological Recordings
Retinas were prepared and dissected under infrared illumination in Ringer solution (in mM: 110 NaCl, 2.5 KCl, 1 CaCl2, 1.6 MgCl2, 10 d-glucose, and 22 NaHCO3, bubbled with 5% CO2-95% O2, pH 7.4). They were mounted in the center of a piece of filter paper (Millipore) with a 4 × 4-mm square aperture and ganglion cell side up in the microscope recording chamber. During the experiment, the retinas were superfused with oxygenated Ringer solution at 36°C.
Targeting RGCs with Two-Photon Microscopy
In wild-type (WT) retina, PV5 RGCs with YFP-positive soma were targeted based on the soma size and the morphology of their dendrites (Farrow et al. 2013). AAV-infected PV5 RGCs were targeted by their YFP-positive soma with tdTomato-positive nuclei.
Electrophysiology and Pharmacology
Electrophysiological recordings were performed both at the University of Louisville and at the FMI. In both labs, recordings used an Axon Multiclamp 700B amplifier (Molecular Devices) and signals were digitized at 10 kHz (National Instruments) and acquired with software written in LabVIEW (National Instruments). In Louisville, the acquisition rate for spiking activity and for spontaneous and evoked currents in PV5WT RGCs was 10 kHz. Similarly, spiking activity for PV5WT, PV5Glra1-KD, and PV5SC RGCs was recorded at 10 kHz at the FMI. However, the acquisition rate for RGC spontaneous and evoked currents was 1 kHz. RGC spiking activity was recorded in loose patch configuration with electrodes pulled from borosilicate glass electrodes (Sutter Instrument) with 3–5 MΩ resistance and filled with Ringer solution. RGC currents were recorded in whole cell voltage-clamp mode, with 6- to 8-MΩ electrodes filled with (in mM) 112.5 CsCH3SO3, 1 MgSO4, 7.8 × 10−3 CaCl2, 0.5 BAPTA, 10 HEPES, 4 ATP-Na2, 0.5 GTP-Na3, 5 lidocaine N-ethyl bromide (QX314-Br), and 7.5 neurobiotin chloride (pH 7.2 adjusted with CsOH). All voltages were corrected for the measured liquid junction potential of 17 mV. The calculated Cl reversal potential is −73 mV. Excitatory postsynaptic currents (EPSCs) and IPSCs were isolated while holding the RGC membrane potential at −77 mV and 0 mV, respectively. For pharmacological characterization of inhibitory inputs, glycinergic inputs were blocked by bath application of 10 μM strychnine and GABAAergic inputs by application of 20 μM picrotoxin (PTX; Sigma-Aldrich).
Visual Stimulation
Stimuli were generated with a DLP projector controlled by custom software. The light spectrum ranged from ∼400 to 760 nm with a peak at ∼550 nm. The sIPSCs were recorded on a photopic background luminance of 24,000 R*·rod−1·s−1. Stimulus-evoked responses of PV5 RGCs were recorded to a luminance decrement, a dark spot (6,000 R*·rod−1·s−1) on the same background, or to a luminance increment, the offset of the dark spot back to background. The visual stimulation protocol began with 15 s at background luminance, followed by presentation of a stationary dark spot centered on the PV5 RGC soma. The duration of the stimulus was 2 s followed by a 5-s interstimulus interval, and the outer diameter increased in six steps from 125 μm to 1,250 μm. Spiking responses were recorded first in loose-patch mode to five to eight repetitions of this stimulus protocol (25 RGCs) and evoked eEPSCs and eIPSCs were recorded in whole cell voltage-clamp mode to three to five repetitions of the protocol (15 RGCs). Eight RGCs were recorded in loose patch mode followed by whole cell recording.
Electrophysiological Data Analysis
Extracellular recordings.
Spiking activity was analyzed with Spike2 as described previously (Cambridge Electronic Design; Nobles et al. 2012). Spikes were accumulated within a 50-ms bin width and displayed as both raster plots of individual trials and average poststimulus time histograms (PSTHs). Mean spontaneous activity (SA) was measured from the 15-s background illumination at the beginning of the stimulation protocol, and this mean was subtracted from all measures of the stimulus-evoked responses. Several aspects of the spiking response were measured from the stimulus-evoked average PSTH of the PV5 RGCs (see Nobles et al. 2012 and Fig. 6). To a luminance decrement, the transient peak amplitude (0–0.4 s of stimulus onset) and the sustained mean firing rate (0.4–2 s of stimulus onset) were measured. To a luminance increment, the rebound peak firing rate was measured.
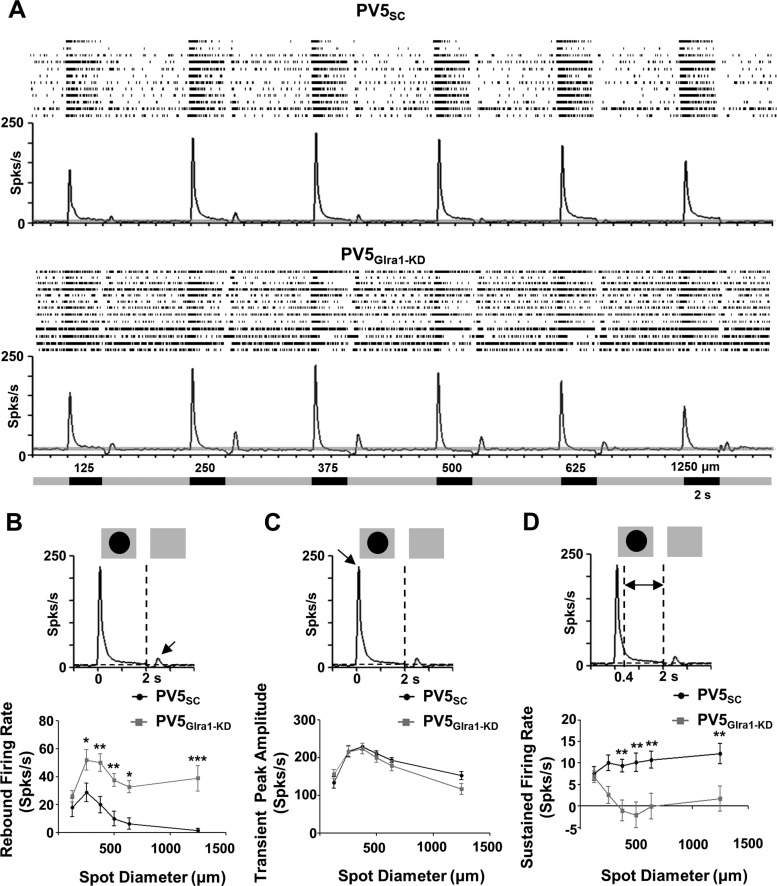
Fig. 6.
GlyRα1 input modulates PV5 RGC spontaneous activity (SA) and excitatory rebound poststimulus suppression to a luminance increment. A: average poststimulus time histograms (PSTHs) and individual raster plots (top) of the spiking responses of PV5SC (n = 12) and PV5Glra1-KD (n = 13) RGCs as a function of spot diameter. Mean SA is indicated by horizontal dashed lines on the PSTHs, and gray shading indicates ±2 SE. Spot diameter and the onset/offset of the stimulus are represented in the diagram below the responses. B: to a luminance increment, the transition between the presence of a dark spot and the background, rebound firing rates are significantly higher and the rebound response is retained across spot diameter in PV5Glra1-KD compared with PV5SC RGCs. C and D: transient peak amplitude (C) does not differ between the 2 groups, while sustained firing rates (D) of PV5Glra1-KD RGCs are significantly lower than those PV5SC RGCs. *P < 0.05, **P < 0.01, ***P < 0.001.
Whole cell patch-clamp recordings.
sIPSCs were analyzed with the Mini Analysis program (Synaptosoft). Stimulus-evoked eEPSCs and eIPSCs of PV5 RGCs were analyzed with Clampfit (Axon Instruments). Only RGCs with series resistance smaller than 25 MΩ were used for analysis, and only sIPSCs that were well-separated events with a single peak and an amplitude that exceeded 15–20 pA (≥2 × root mean square of the noise) were used for analysis. We measured the frequency and interevent interval of these sIPSCs and estimated their decay from the time at which the sIPSC declined to 37% of its peak amplitude (D37; Fig. 2Aiii). We plotted the amplitude and D37 for the population of sIPSCs and found no correlation, confirming that our criteria were unbiased. We compared the decay time for PV5WT sIPSCs acquired with sampling rates of 10 and 1 kHz (n = 5 and 7 cells), found that sampling did not affect the D37 (2.74 ± 0.04 ms vs. 2.6 ± 0.1 ms, P > 0.05), and combined the data (Fig. 2). For comparisons to PV5SC and PV5Glra1-KD RGCs, we only used PV5WT data collected at the same rate (1 kHz). For eEPSCs and eIPSCs, the transient peak (0–0.4 s) and mean sustained current (0.4–2 s) at stimulus onset and the charge transfer at stimulus offset were measured and corrected for average baseline, measured from 2 s before each stimulus onset.
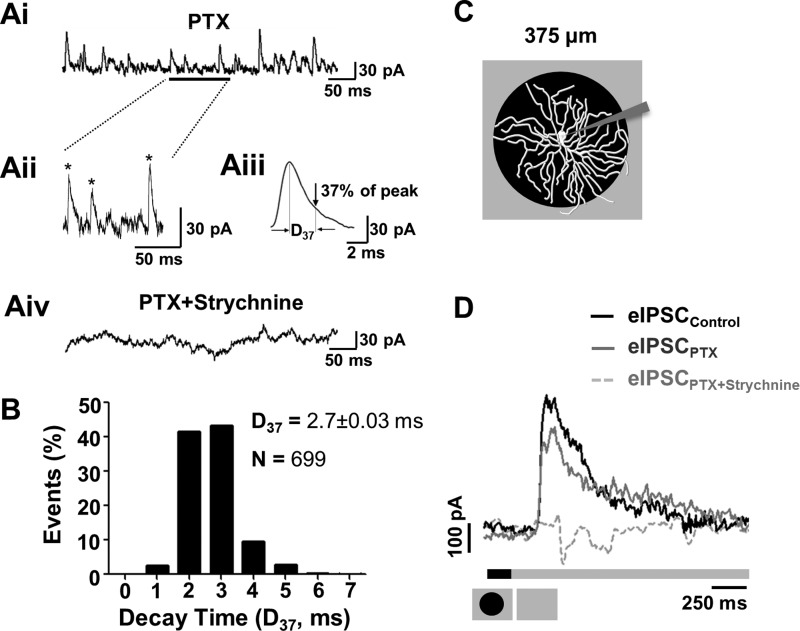
Fig. 2.
GlyRα1-meditated spontaneous inhibitory postsynaptic currents (sIPSCs) and glycinergic stimulus-evoked IPSCs (eIPSCs) are present in PV5WT RGCs. Ai: glycinergic sIPSCs were recorded from PV5WT RGCs held at 0 mV in the presence of picrotoxin (PTX; 20 μM). Aii: same trace as Ai with an expanded timescale. Asterisks indicate sIPSCs that met our criterion (see text) and were used in our analyses. Aiii: average waveform of PV5 RGC sIPSCs that met our criterion (170 events) and an illustration of their average decay (time at which sIPSC declined to 37% of peak amplitude, D37). Aiv: all sIPSCs were eliminated in presence of PTX (20 μM) and strychnine (10 μM). B: frequency of decay times (D37) in sIPSCs of PV5WT RGCs (n = 12; N = 699 events). C: schematic representation of the visual stimulus, a dark spot (6,000 R*·rod−1·s−1) centered on the PV5WT RGC soma and presented on a background (24,000 R*·rod−1·s−1). A spot outer diameter of 375 μm matches the PV5 RGC dendritic arbor. D: averaged PV5WT RGC eIPSC evoked by the offset of the dark spot in control solution. PTX (20 μM) produces a small reduction in the eIPSC, indicating a small GABAergic input. The eIPSC is completely eliminated when strychnine also is present (PTX 20 μM + strychnine 10 μM), indicating that the majority of synaptic inhibitory input is glycinergic.
Statistical Analysis
Statistical analyses were performed with Prism5 Software (GraphPad). Distributions of each parameter were tested for normality with the D'Agostino and Pearson omnibus test, and appropriate parametric or nonparametric statistical analyses were used. For each morphological and electrophysiological measure, we used a t-test with Welch's correction to compare RGCs infected with AAV-scrambled (SC) shRNA either to WT RGCs or to RGCs infected with AAV-Glra1-shRNA [Glra1 knockdown (KD)]. We used Student's paired t-tests to compare responses of the same RGC in control or in the presence of antagonist. We used a two-way ANOVA with Bonferroni's post hoc tests to compare response components across spot size across RGCs with scrambled or Glra1-KD in the presence of antagonists. In all figures, data are plotted as means ± SE.
RESULTS
PV5WT RGCs Express GlyRα1 and Have GlyRα1-Mediated Synaptic Inputs
We first examined the glycinergic isoform-specific expression and currents in PV5WT RGCs. Previous reports observed expression of GlyRα1 puncta and sIPSCs in A2 OFF RGCs (Sun et al. 2002), which should be the equivalent of the PV5 RGC type, and their absence in the mouse mutant Glra1spd-ot (Majumdar et al. 2007). Using two-photon microscopy, we targeted YFP-positive RGCs with large somas and filled them with neurobiotin. We verified their identity as PV5 RGCs in retinas triple-stained for neurobiotin (Fig. 1), ChAT (Fig. 1Aii), and GlyRα1 (Fig. 1, B–D), using their large soma and dendritic field size as well as the stratification of their dendrites, slightly proximal to the Off ChAT band in the IPL (Fig. 1Aii; see also Farrow et al. 2013; Münch et al. 2009). Every PV5WT RGC (n = 8) had significantly more coincident GlyRα1-positive puncta compared with random coincidence (Fig. 1Di, 0.27 ± 0.03 vs. Fig. 1Dii, 0.05 ± 0.08 puncta/μm of dendrite; P < 0.0001). The corrected GlyRα1 coincidence, 0.21 ± 0.02 puncta/μm, is similar to previous estimates in A2 RGCs (Majumdar et al. 2009). This is consistent with the idea that PV5WT are A2 OFF RGCs.
In PV5WT RGCs in the presence of the GABAA antagonist PTX (20 μM), the majority (97%) of glycinergic sIPSCs (Fig. 2, Ai–Aiii) had decay times (D37) < 5 ms, and the distribution average was 2.7 ± 0.03 ms (Fig. 2B; 699 events, 12 PV5WT RGCs). This is identical to estimates of τ for GlyRα1 sIPSCs in rat AII cells recorded at physiological temperature (Gill et al. 2006), although faster than sIPSCs in WT A2 OFF RGCs recorded at 25°C (Majumdar et al. 2007). Our mean D37 also is much faster than estimates for GlyRα2, -α3, and -α4 subunits (Wässle et al. 2009). In the presence of both PTX and strychnine, all sIPSCs were eliminated (Fig. 2Aiv) and the RGCs exhibited oscillatory current activity. Our immunohistochemical and electrophysiological results indicate that that GlyRα1 inputs are the predominant glycinergic synaptic inputs of PV5 RGCs.
Stimulus-Evoked Response Profiles of PV5WT RGCs
Even though we used higher-contrast stimuli, the RF center/surround organization and stimulus-evoked spiking response properties of PV5WT RGCs are consistent with previous descriptions (Farrow et al. 2013, cf. Fig. 2; Münch et al. 2009, cf. Fig. 3). Namely, to a luminance decrement, PV5WT RGCs respond with an initial transient excitatory response followed by a sustained response slightly above spontaneous activity. To a luminance increment, there is a suppression in firing rate followed by a rebound excitation when spot diameters are small. The eEPSCs and eIPSCs that underlie the spiking response of PV5WT RGCs also are consistent with previous descriptions. A luminance decrement (onset of a 375-μm dark spot, which matched the RF center diameter; Fig. 2C) evokes only an eEPSC, whereas a luminance increment (offset of a dark spot) evokes only an eIPSC (Fig. 2D). The magnitude of the eIPSC was unchanged when PTX eliminated GABAAergic inhibition (Fig. 2D; n = 3; 205.84 ± 66.01 vs. 173.34 ± 58.77 nA·ms). In contrast, the eIPSC was completely eliminated when strychnine (10 μM) also was included. Our morphological and electrophysiological results suggest that the previously described glycinergic input to OFF αTransient RGCs is GlyRα1 (Münch et al. 2009; Murphy and Rieke 2006; van Wyk et al. 2009) and that this isoform subunit input is a critical element of a crossover circuit and is maintained at high luminance and contrast levels.
AAV-Mediated RNAi Eliminates GlyRα1 Expression in PV5Glra1-KD RGCs
In examining the role of glycinergic input in the responses of RGCs, previous studies have used the competitive glycine antagonist strychnine, which eliminates glycinergic signaling throughout the retinal circuit. We tested whether an RNAi approach could be used to selectively eliminate direct GlyRα1 isoform-specific inhibition in RGCs while leaving the remaining upstream glycinergic inhibition intact. To this end, we injected AAVs expressing a Glra1-shRNA cassette into the dLGN to selectively target the AAV to RGCs. The viral vector plasmid (pAAV-Ef1a-NLStdTomato-H1; Fig. 3A) also expressed the tdTomato fluorescence protein with a nuclear localization signal, which we used to determine the timing and extent of RGC viral infection across the retina. We used RT-PCR and the cDNA isolated from HEK293 cells cotransfected with a plasmid expressing GlyRα1 along with either a AAV scrambled shRNA construct or one of three AAV Glra1-shRNA (a 29-mer and two 21-mer) constructs and evaluated the change in Glra1 transcription compared with a blank control vector (tdTomato only). One AAV construct, 21-mer-A Glra1-shRNA, showed the most (81%) downregulation of Glra1 mRNA levels (Fig. 3B), whereas the scrambled shRNA had no effect on Glra1 mRNA levels. All subsequent experiments in vivo used 21-mer-A Glra1-shRNA AAV. The sequences of the scrambled and Glra1 shRNAs are scrambled shRNA: 5′-GTCGAAACCCGCAATAATAAT and Glra1 shRNA: 5′-GCACTACAACACAGGTAAATT-3′.
Four weeks after LGN injection, we observed tdTomato nuclear expression throughout but confined to the RGC layer in the contralateral eye (Fig. 3Ci). Almost all YFP-expressing PVcre RGCs (green with yellow nuclei) were infected (Fig. 3Cii). The remaining experiments targeted double-labeled PV5 RGCs infected with AAVs generating scrambled (PV5SC) or Glra1-shRNA (PV5Glra1-KD).
To evaluate the effect of knockdown of Glra1 expression, we quantified coincident GlyRα1 puncta on the dendrites of PV5SC or PV5Glra1-KD (Fig. 3, D and E). When corrected for random coincidence, there were no coincident GlyRα1-immunoreactive puncta on the dendrites of PV5Glra1-KD (n = 7) RGCs (−0.009 ± 0.003 puncta/μm; P = 0.02). The density of GlyRα1-immunoreactive puncta on the dendrites of PV5SC (n = 4) was the same as PV5WT (n = 8) RGCs (Fig. 3F; 0.20 ± 0.04 and 0.21 ± 0.02 puncta/μm). We conclude that AAV-RNAi of Glra1 significantly downregulates GlyRα1 protein expression in PV5Glra1-KD RGCs and that the scrambled construct has no off-target effects. For the remainder of the experiments, we used PV5SC RGCs as controls.
AAV-Mediated RNAi Eliminates GlyRα1 Synaptic Inputs in PV5Glra1-KD RGCs
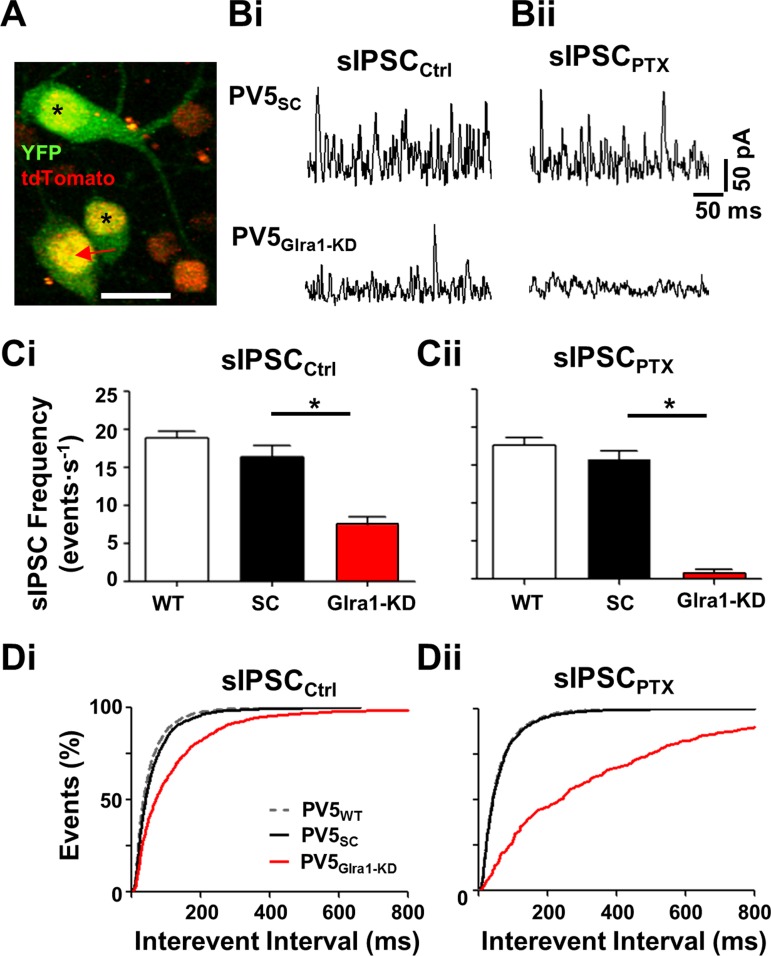
To evaluate the effect of knockdown on direct GlyRα1 synaptic inputs to PV5 RGCs, we used two-photon-guided whole cell patch clamp to target (Fig. 4A), characterize, and compare the frequency and kinetics of PV5Glra1-KD and PV5SC sIPSCs (Fig. 4, B–D). In control solution, the frequency of sIPSCs in PV5SC (Fig. 4Bi) and PV5WT RGCs was the same (Fig. 4Ci; n = 5 and 7; 16.44 ± 1.44 and 18.87 ± 0.95 events/s), indicating that the scrambled vector does not produce off-target knockdown. Under the same conditions, the frequency of sIPSCs in PV5Glra1-KD was significantly lower than PV5SC RGCs (Fig. 4, Bi and Ci; n = 7; 7.56 ± 0.96 events/s; P = 0.003) and the mean interevent interval was significantly longer (Fig. 4Di; n = 1,026 vs. 2,069; 125.1 ± 5.6 ms vs. 62.3 ± 1.5 ms; P = 0.0003). Elimination of GABAAergic inputs with PTX did not alter sIPSC frequency in PV5SC RGCs (Fig. 4, Bii and Cii; 15.70 ± 1.24 events/s) or the mean D37 (n = 116; 2.6 ± 0.1 ms) compared with PV5WT RGCs. The peak amplitude of the isolated glycinergic sIPSCs was the same in PV5SC and PV5WT RGCs (n = 116 and 389; 60.5 ± 2.9 and 62.2 ± 1.4 pA, respectively). Unexpectedly, elimination of GABAAergic inputs significantly decreased the already low sIPSC frequency in PV5Glra1-KD RGCs (0.86 ± 0.42 events/s; P = 0.0002) and lengthened the mean interevent interval (Fig. 4Dii; n = 244; 354.3 ± 25.5 ms; P <0.0001). The remaining sIPSCs (n = 45) had a mean D37 of 3.18 ± 0.18 ms, with 86% of events < 5 ms, which overlaps the distribution of sIPSCs in PV5WT and PV5SC RGCs. The remaining sIPSCs were glycinergic, as they were eliminated by strychnine (see Fig. 2Aiii), and their peak amplitude was significantly smaller than PV5SC RGCs (28.9 ± 1.8 pA; P < 0.0001). Our results show that retrograde AAV-RNAi significantly reduces GlyRα1 expression and the majority (∼95%) of glycinergic synaptic inputs. Without this glycinergic input, a PTX-sensitive GABAAergic synaptic input is seen that is not evident in PV5WT or PV5SC RGCs. We speculate that synaptic inhibition to PV5WT RGCs is modulated by two GABAAergic inputs. One inhibits a glycinergic AC and modulates spontaneous glycine release onto PV5 RGCs (see Fig. 8A, blue pathway). The other (see Fig. 8B, blue) modulates direct spontaneous GABA release onto PV5 RGCs. We speculate that in PV5WT and PV5SC RGCs PTX blocks the first, serial GABAAergic input and increases spontaneous glycine release. This offsets a decrease in sIPSCs due to the PTX block of the direct spontaneous GABA release. The serial GABAAergic pathway is eliminated in PV5Glra1-KD RGCs, and the direct GABAAergic synaptic input can be observed as a consequence of its sensitivity/elimination by PTX.
Fig. 4.
Knockdown of Glra1 eliminates GlyRα1 sIPSCs in PV5 RGCs. A: representative 2-photon Z-stack image of a PV5Glra1-KD RGC infected by AAV-Glra1-shRNA (arrow) that expresses YFP (green) in its cytoplasm and nucleus and tdTomato (yellow) in its nucleus. Double-labeled cells (asterisks) represent other PVcre RGC types. Single-labeled RGCs represent infected non-PVcre RGC types (red). Scale bar, 40 μm. Bi: representative sIPSCs in PV5SC and PV5Glra1-KD RGCs. Bii: representative sIPSCs of the same RGCs in the presence of PTX (20 μM). Ci: in control solution, the average sIPSC frequency in PV5Glra1-KD RGCs (n = 7) is significantly lower compared with PV5WT (n = 7) and PV5SC (n = 5) RGCs, which are similar. Cii: in the presence of PTX, the average sIPSC frequency in PV5Glra1-KD RGCs (n = 7) is also significantly lower compared with PV5WT (n = 7) and PV5SC (n = 5) RGCs, which are similar. The sIPSC frequency in PV5Glra1-KD RGCs is significantly reduced in control vs. PTX. *P < 0.05. D: interevent intervals in PV5Glra1-KD RGC sIPSCs are longer than PV5SC RGCs in control (Di) and in the presence of PTX (Dii). Interevent intervals in PV5Glra1-KD RGCs are significantly longer in control vs. PTX.
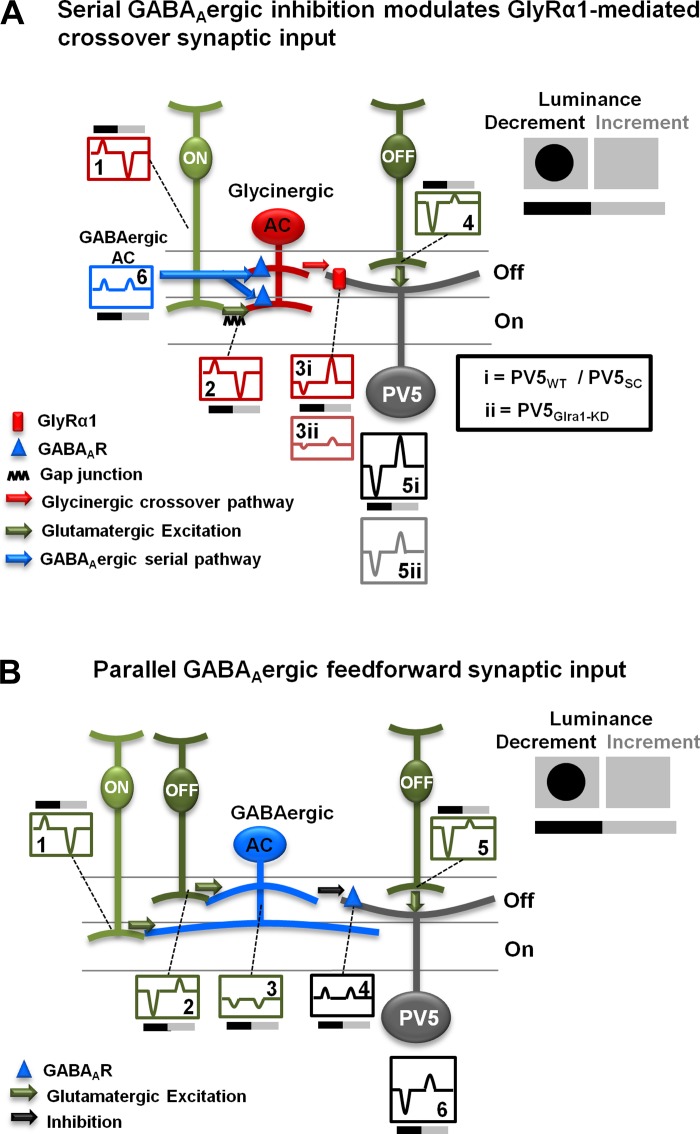
Fig. 8.
Light-evoked responses of PV5WT RGCs are modulated by both GlyRα1 and GABAAergic inputs: schematic diagrams of the circuits controlling light-evoked responses of PV5WT RGCs. Top right: diagram illustrating the stimulus, a dark spot presented on a light adapting background and centered on the RF center. Bars below indicate the timing of spot onset (luminance decrement) and offset (luminance increment). Note: Current onset and offset are accurately depicted, but all currents are shown as transient. A: a GlyRα1-mediated crossover inhibitory circuit modulates the responses of PV5 RGCs at luminance increment and decrement. PV5WT, PV5SC, and PV5Glra1-KD ON cone bipolar cells (CBCs; box 1) drive bistratified glycinergic ACs (box 2). In PV5WT and PV5SC this glycinergic input produces inhibition via their GlyRα1 postsynaptic receptors (rectangle on dendrite). A synaptic rectification occurs between the ON CBC and the bistratified glycinergic AC, resulting in a small outward current at luminance decrement and a large inward current at luminance increment (box 2). Through this receptor the glycinergic ACs evoke currents of inverted polarity in the PV5 RGC (box 3i). The net result (box 5i) is disinhibition at luminance decrement and inhibition at luminance increment. Knockdown of Glra1 eliminates this crossover inhibition and reduces excitation at luminance decrement and inhibition at luminance increment (boxes 3ii and 5ii). The glycinergic AC is regulated by serial GABAAergic input (triangle) at luminance decrement and increment (box 6). B: a feedforward GABAAergic inhibition arises in both the On and the Off pathway (boxes 1 and 2) and suppresses PV5WT, PV5SC, and PV5Glra1-KD RGC responses at luminance increment and decrement, respectively (boxes 4 and 6). It is conducted by multistratified GABAergic ACs (box 3).
AAV-Mediated RNAi Eliminates Stimulus-Evoked GlyRα1 Currents in PV5Glra1-KD RGCs
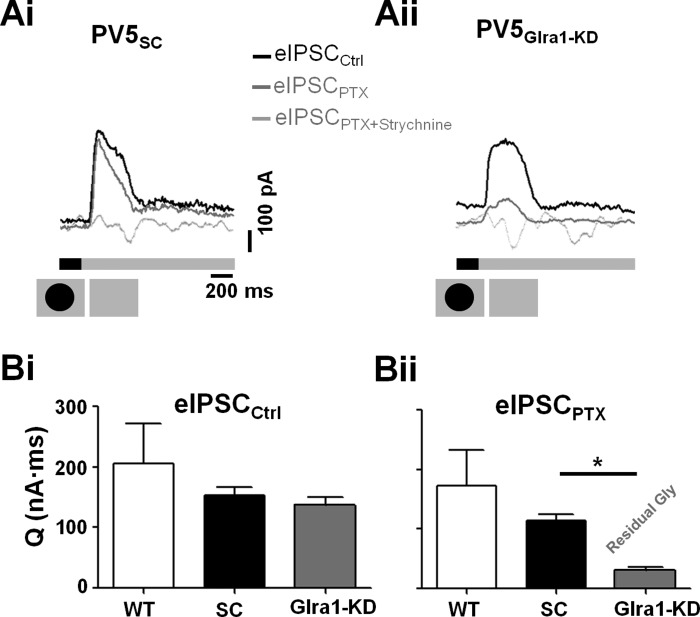
A luminance increment depolarizes ON CBCs, which initiates excitatory signaling in the On pathway. This stimulus culminates as a glycinergic inhibitory input onto PV5WT OFF RGCs (dark spot offset; see Fig. 2D). The eIPSC total charge transfer evoked by this stimulus was similar across PV5WT, PV5SC, and PV5Glra1-KD RGCs (Fig. 5, A and Bi; n = 3, 5, and 7; 205.83 ± 66.01, 153.33 ± 13.54, and 136.62 ± 13.34 nA·ms, respectively). However, an initial transient peak, found in the eIPSCs of both PVWT and PV5SC RGCs, was absent in PV5Glra1-KD eIPSCs (Fig. 5, Ai vs. Aii, respectively). This flattened their eIPSCs and significantly lengthened estimates of time to peak (157.6 ± 30.4 vs. 236.4 ± 5.3 ms; P = 0.02). In the same cells, the presence of PTX did not alter the eIPSC (eIPSCPTX) of PV5SC RGCs (Fig. 5, Ai and Bii; 114.43 ± 10.43 nA·ms). In contrast, eIPSCPTX in PV5Glra1-KD RGCs was significantly reduced (Fig. 5, Aii and Bii; 30.49 ± 6.31 nA·ms; P = 0.001). The eIPSCPTX in PV5Glra1-KD RGCs were glycinergic as they were eliminated by strychnine (Fig. 5Aii) and likely result from the few GlyRα1 receptors that are expressed in PV5Glra1-KD RGCs. Furthermore, their charge transfer was significantly smaller (∼27%) than the glycinergic eIPSC in PV5SC RGCs (Fig. 5, A and Bii; P < 0.0001). All of these changes in the eIPSC are similar to those observed in sIPSCs, suggesting that the same two, GABAAergic, inputs modulate both synaptic and stimulus-evoked responses (see Fig. 8).
Fig. 5.
Knockdown of Glra1 eliminates GlyRα1 input to PV5 RGCs at luminance increments. A: representative eIPSCs evoked by the offset of a dark spot (schematic, bottom) from PV5SC (Ai; n = 5) and PV5Glra1-KD (Aii; n = 7) RGCs in control solution (black traces), in the presence of PTX (dark gray traces), and in the presence of both PTX and strychnine (light gray traces). B: in control (Bi) the charge transfer (Q) does not differ across PV5WT, PV5SC, or PV5Glra1-KD groups, whereas the presence of PTX (Bii) significantly reduces the charge transfer in PV5Glra1-KD compared with PV5SC RGCs. *P < 0.05.
Our comparisons of sIPSCs and eIPSCs across PV5WT, PV5SC, and PV5Glra1-KD RGCs show that 1) the majority of glycinergic input to PV5WT RGCs is mediated by the GlyRα1 isoform, 2) this RNAi approach effectively eliminates the majority of the synaptic and evoked glycinergic currents, 3) viral infection has no nonspecific effect on spontaneous or evoked currents and reveal 4) the presence of two GABAAergic mechanisms that modulate synaptic inhibition in PV5 RGCs.
PV5SC RGC Stimulus-Evoked Responses
Similar to WT, PV5SC RGCs respond to a luminance decrement (onset of a dark spot) with a transient excitatory peak (≤400 ms of stimulus onset) followed by maintained firing whose rate was above SA and matched to stimulus duration (2,000 ms; Fig. 6, A, C, and D). A luminance increment (offset of a dark spot) evokes a short-latency suppression of spiking that is followed by a small rebound excitatory response (∼500 ms from stimulus offset; Fig. 6, A and B). The amplitude of the rebound response increases and then declines with increasing spot diameter and is not significantly above SA once the spot diameter exceeds the cell's RF center (>500 μm). Given the difference in the stimulation conditions, our responses are comparable to previously published PV5WT responses (Farrow et al. 2013) and do not vary with time after AAV infection.
GlyRα1 Inhibitory Input Modulates PV5WT RGC Spontaneous Spiking Activity and Postsuppression Rebound Responses to Luminance Increments
To define the roles of direct GlyRα1-mediated inhibition in PV5WT RGC visual function, we compared and defined the differences in the spontaneous and stimulus-evoked spiking responses of PV5SC (n = 12) and PV5Glra1-KD (n = 13) RGCs. Consistent with a role for a tonic GlyRα1 inhibitory synaptic input to PV5WT RGCs, the absence of GlyRα1 current significantly increased the spontaneous spiking rate in PV5Glra1-KD compared with PV5SC RGCs (Fig. 6A; 13.95 ± 2.60 and 4.11 ± 1.02 spikes/s; P = 0.003). This mismatch in SA precluded quantitative comparisons of spiking suppression to a luminance increment (Fig. 6A). To correct for the difference in all other evoked spiking responses, we subtracted the mean SA. The absence of GlyRα1 input did not alter RF center diameter in PV5Glra1-KD compared with PV5SC RGCs (336.54 ± 27.64 vs. 343.75 ± 27.20 μm), and therefore we directly compared responses as a function of stimulus diameter.
To parse the role of GlyRα1 in feedforward and crossover inhibitory mechanisms, we examined responses to luminance increments and decrements, respectively. In PV5SC RGCs, a luminance increase induced a suppression in the spiking response that was followed by a postsuppression rebound response, as long as the spot diameter was less than or equal to the RGC RF center diameter. In PV5Glra1-KD the rebound response did not decline significantly with increasing spot diameter, although it was significantly larger than PV5SC RGCs at all but the smallest spot diameter (Fig. 6, A and B; 2-way ANOVA, P < 0.0001). The rebound response had a slow onset and had no directly associated eEPSC. Although a small inward current was recorded with similar timing (Fig. 7A), it did not differ across PV5WT, PV5SC, and PV5Glra1-KD RGCs. This result, along with the observation that an intrinsic rebound response can be induced in WT OFF RGCs by injecting negative current (Margolis and Detwiler 2007), suggests that an absence of tonic GlyRα1 input causes PV5Glra1-KD RGC resting membrane potential to be more depolarized. At the end of spike suppression, which results from a release from inhibition, spiking threshold is reached sooner in the more depolarized PV5Glra1-KD RGCs and results in an enhanced rebound response.
Fig. 7.
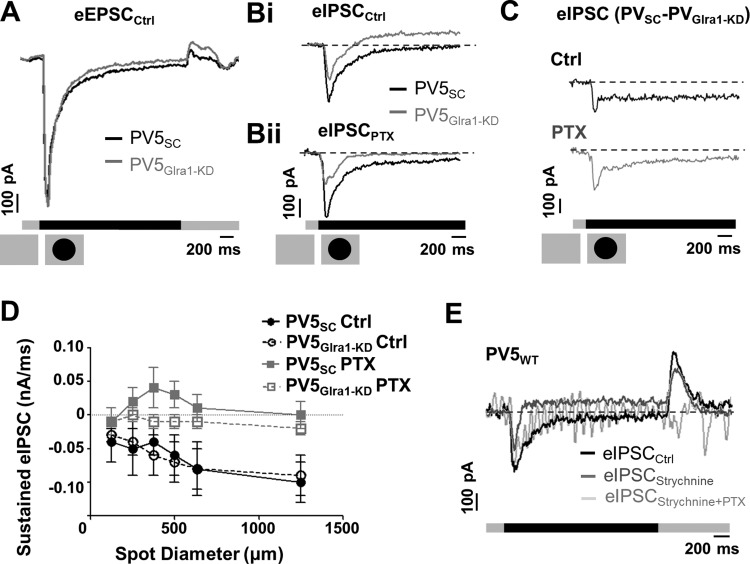
Stimulus-evoked responses to luminance decrement are modulated by glycinergic and GABAAergic inputs. A: to a luminance decrement (375-μm-diameter spot), the average evoked excitatory postsynaptic currents (eEPSCs) of PV5SC (black, n = 4) and PV5Glra1-KD (gray, n = 5) RGCs are similar. B: to the same stimulus, the average eIPSCs of PV5Glra1-KD (n = 7) are significantly elevated compared with PV5SC RGCs (n = 5) in control solution (black) and in the presence of PTX (gray). C: subtraction of the average eIPSC of PV5Glra1-KD from that of PV5SC under control conditions and in the presence of PTX shows a prominent decrease in eIPSC at luminance decrement, suggesting a disinhibition. This decrease also is seen in the presence of PTX (gray). D: mean sustained eIPSC of PV5SC (black circles) and PV5Glra1-KD RGCs (gray squares) in control solution (filled symbols) and in the presence of PTX (open symbols) as a function of spot diameter. The significantly elevated eIPSC level in PV5Glra1-KD RGCs in control and in the presence of PTX indicates the elimination of glycinergic disinhibition. The absence of glycinergic disinhibition unmasks a GABAAergic input in PV5Glra1-KD RGCs, which is blocked by PTX. E: eIPSC from a PV5WT RGC in the presence of strychnine (gray) exhibits a response profile similar to PV5Glra1-KD RGCs in control solution that differs from control (black). Application of both strychnine and PTX eliminated all eIPSCs (light gray).
GlyRα1 Disinhibitory and GABA Inhibitory Inputs Use Push-Pull Mechanism to Modulate PV5WT RGC Responses to Luminance Decrements
A luminance decrease depolarizes OFF CBCs and initiates excitatory signaling in the Off pathway that culminates with an excitatory response in PV5WT RGCs. The transient excitatory peak amplitudes of PV5SC and PV5Glra1-KD RGCs were similar (Fig. 6C), but the sustained firing rate was significantly lower in PV5Glra1-KD RGCs across spot diameter (Fig. 6D; P < 0.0001). This result appears inconsistent with the observation that no inhibitory current is evoked in PV5WT RGCs to this stimulus (Fig. 7E; see also Münch et al. 2009). To address this discrepancy, we characterized and compared the stimulus-evoked currents of PV5SC and PV5Glra1-KD eIPSCs to a luminance decrement.
The transient component of the responses of PV5WT, PV5SC, and PV5Glra1-KD RGCs held at 0 mV appears as an inward current (Fig. 2C and Fig. 7B; see also Münch et al. 2009). This reflects incomplete voltage clamp (e.g., space clamp) due to the large dendritic fields and gap junctional coupling in PV5 RGCs. Since the AAV-RNAi does not affect presynaptic inputs and the eEPSCs are similar between PV5SC and PV5Glra1-KD RGCs (Fig. 7A), we conclude that space clamp is similar between the two groups and the absence of GlyRα1 input does not affect the gap junctional coupling or the dendritic field electrotonic properties of PV5 RGCs. Therefore, differences between PV5SC and PV5Glra1-KD eIPSCs reflect the role of GlyRα1 input in their stimulus-evoked responses.
A luminance decrement evoked a prominent sustained eIPSC in PV5Glra1-KD RGCs that was absent in PV5SC RGCs (Fig. 7Bi). Consistent with this, the mean sustained eIPSC was significantly higher in PV5Glra1-KD compared with PV5SC (Fig. 7, C and D; n = 7 vs. 5) both in control (P < 0.0001) and in the presence of PTX (P < 0.0001) and this difference was significant across spot diameters (Fig. 7D). These results define a role for GlyRα1 subunit isoform input in a previously described crossover glycinergic mechanism that facilitates excitation in OFF RGCs to a luminance decrement (Manookin et al. 2008; Murphy and Rieke 2006). Moreover, the glycinergic modulation is spatially invariant and is not altered by recruitment of lateral inhibitory mechanisms with increasing spot diameter. Because the sustained eIPSC eliminated by PTX in PV5Glra1-KD RGC was not evident in PV5SC RGCs (Fig. 7, Bii and D; P = 0.01), we eliminated glycinergic input in PV5WT RGCs with 10 μM strychnine and determined that a GABAAergic eIPSC was present to a luminance decrement that could be reduced by PTX (Fig. 7E).
Our data show that to a luminance decrement the responses of PV5WT RGCs are modulated by GlyRα1 using a mechanism that has been previously described as an On-to-Off crossover glycinergic disinhibition that enhances sustained spiking activity (Manookin et al. 2008; Murphy and Rieke 2006; Pang et al. 2003; van Wyk et al. 2009). In addition, our data define a GABAAergic input that is driven through the Off pathway and reduces spiking activity in PV5 RGCs. This suggests a combination of GABAergic and glycinergic inputs uses a “push-pull” mechanism to control PV5WT RGC responses to a luminance decrement (Fig. 8).
DISCUSSION
As an image processor, the retina dynamically encodes visual inputs using distinct RGC types where different inhibitory mechanisms modulate the excitatory signal and shape visual function. Much of our view of this signaling in the retina has relied on pharmacological manipulations, which alter not only the direct effects of the agonist/antagonist but also effects on these inputs in the upstream circuit. We show that viral RNAi can be used to selectively eliminate both expression and a subunit-specific current in an OFF RGC subtype, the PV5 RGC, while maintaining their upstream circuit intact. In addition, we show that this approach can be used to evaluate the role of a specific glycinergic subunit in visual function in identified RGCs. Our results show that GlyRα1 input to PV5 RGCs is the predominant crossover glycinergic input described previously in OFF αTransient RGCs (Manookin et al. 2008; Murphy and Rieke 2006; Pang et al. 2003; van Wyk et al. 2009). In addition, the data show that both glycinergic and GABAergic inputs shape PV5 RGC spontaneous and visually evoked responses and modulates their interaction differentially at luminance increment and decrement.
AAV-Mediated Infection of RGCs with shRNA Reduces GlyRα1 Expression and Currents
AAV-RNAi has been adopted in a variety of biological systems to induce and/or eliminate gene expression (reviewed in Borel et al. 2014). In the retina, AAVs have been used primarily in gene therapy approaches to treat retinal disease (reviewed in McClements and MacLaren 2013; Sahel and Roska 2013) and more recently in combination with pseudotyped rabies virus to trace presynaptic partners (Cruz-Martin et al. 2014) or target and characterize the visual response properties of identified RGC types (Farrow et al. 2013; Yonehara et al. 2013).
We show that a novel use of retrogradely transported AAV-RNAi eliminates expression of a subunit-specific receptor in an identified RGC type. We use the approach to define the receptor's role in shaping the visually evoked response properties of PV5 RGCs and the underlying excitatory and inhibitory currents. The advantages of this AAV-RNAi approach include 1) its use in mature tissue to circumvent developmental complications, 2) its relatively rapid expression (≤4 wk), 3) its long-term stability of expression (up to 10 wk in our study), and 4) its selectivity for its target. Retrogradely transported AAV-RNAi is a particular benefit, when a circuit, like the retina, expresses a receptor isoform at multiple locations (e.g., glycinergic and GABAAergic inputs) and when subunit-specific agonist/antagonists do not exist. Our results show that AAV-RNAi can be used to selectively eliminate the majority of GlyRα1-mediated inputs to an identified RGC, the PV5. Finally, our results show that the approach dissects complex interactions between GABAergic and glycinergic inputs. Given that retrograde transport is similar throughout the central nervous system, this approach should be broadly applicable to both inhibitory and excitatory receptor-mediated inputs.
Tonic GlyRα1 Input Modulates Spontaneous Activity and Rebound Excitation of PV5WT RGCs
We used differences between PV5Glra1-KD and PV5SC RGC responses to define GlyRα1-mediated inputs to PV5WT RGCs that are initiated in both the On and Off pathways. In general, the glycinergic mechanisms that modulate PV5 RGC spontaneous and stimulus-evoked responses act to enhance the fidelity of their spiking responses and the signal that they convey to the rest of the brain.
Under a steady background, GlyRα1 mediates a tonic input to PV5 RGCs that reduces their spontaneous spiking activity. This is likely the same as the glycinergic current that has been previously observed to lower spontaneous activity and improve the signal-to-noise ratio in OFF RGCs (Margolis and Detwiler 2007; Pang et al. 2003; Zaghloul et al. 2003). We conjecture that this control occurs via a GlyRα1 input that helps to set the resting membrane potential of the PV5 RGCs. In the absence of GlyRα1-mediated currents, we found GABAAergic synaptic inputs whose role is unknown.
A luminance increment depolarizes ON CBCs and culminates in a transient suppression of spiking activity followed by rebound excitation in PV5WT RGCs. The absence of GlyRα1 input enhances this rebound excitation. We observed a small inward current that is temporally related to this response, which could be generated by the excitatory input from BCs (Kastner and Baccus 2013; Nikolaev et al. 2013) or could be related to the intrinsic rebound response that is evoked when negative current is applied and all synaptic input is blocked to OFF RGCs (Margolis and Detwiler 2007). Alternatively, if GlyRα1 input contributes to the PV5 RGC resting membrane potential, it should make the PV5 RGC more hyperpolarized and lower the probability that the release from inhibition will initiate an excitatory response to a luminance increase. Regardless of its exact role, the presence of GlyRα1 input maintains the correlation between PV5 RGC dendritic lamination in the Off IPL sublaminae and their RF OFF center response.
Both Feedforward and Crossover Inhibitory Circuits Regulate PV5WT RGCs Light Responses at Luminance Decrement
A luminance decrement depolarizes OFF CBCs (Fig. 8A, box 4, inward current), whereas ON CBCs hyperpolarize (Fig. 8A, box 1, outward current). This culminates in a large transient spiking response in PV5WT RGCs, which is followed by a much lower sustained spiking response matched in duration to the stimulus. The absence of GlyRα1 input does not alter the transient peak or their eEPSC but results in a significantly lower sustained spiking rate compared with PV5SC and PV5WT RGCs. This suggests that the GlyRα1 inhibitory input is likely to be a previously described glycinergic On-to-Off crossover tonic input (Demb and Singer 2012; Ke et al. 2014; Münch et al. 2009; Murphy and Rieke 2008), facilitating excitation in PV5 RGCs (glycinergic disinhibition; Fig. 8A, boxes 3i and 5i). If this is the case, this means that the AII, defined as the presynaptic AC in the crossover circuit, operates at the high background and contrast levels used here. Alternatively, one of the other multistratifed ACs (Buldyrev et al. 2012; Menger et al. 1998) could be the presynaptic AC. We found that this stimulus evokes a direct GABAAergic input (Fig. 8B, box 4), which should suppress spiking activity and indicates that a push-pull mechanism mediated by GABAAergic and GlyRα1 inputs sets the spiking output of PV5WT RGCs to a luminance decrement.
Glycine and GABA Inputs Suppress PV5 RGCs Light Responses at Luminance Increment
The sustained suppression to a luminance increment (discussed above) is followed by a rebound excitatory response. Although a fast, transient GlyRα1 input is eliminated in PV5Glra1-KD RGCs, the suppressive response remains. This suggests that spiking suppression is produced through an On-to-Off crossover pathway consisting of a transient GlyRα1 and a prolonged GABAAergic input. Unlike the push-pull combination of feedforward GABAAergic and crossover glycinergic inputs at luminance decrement, the GABAAergic and glycinergic crossover inputs at luminance increment work together.
A Serial GABA-Glycine Circuit Masks the Direct GABAAergic Input in PV5WT RGCs
In the retina, serial connections among ACs (Anderson et al. 2011; Chen et al. 2011) are thought to fine-tune downstream activity. Serial GABA-to-glycine or glycine-to-GABA inhibition has been observed in both RGCs and BCs (Eggers et al. 2007; Eggers and Lukasiewicz 2010; Zhang et al. 1997) and modulates RGC RF center activity (Russell and Werblin 2010; Venkataramani and Taylor 2010). We interpret our results to indicate that PV5 (OFF αTransient) RGCs receive a serial GABA-glycine inhibition (Fig. 8A, box 6) and a direct GABAergic inhibition (Fig. 8B, box 4), both mediated by GABAAR. In the presence of the GABAAR antagonist PTX, both serial and direct GABAergic inhibition are blocked, producing offsetting conductance changes. This makes it virtually impossible to separate the two mechanisms. The selective elimination of the direct glycinergic input in PV5Glra1-KD RGCs also eliminates the GABA-glycine serial modulation and unmasks the direct GABAAergic input. Providing support for this hypothesis, we also could observe a GABAAergic component in PV5WT RGCs when glycinergic input was eliminated in the presence of strychnine (Fig. 7E). While this scenario represents a complex interaction, there is other support for our hypothesis. For example, the likely AC that provides glycinergic crossover inhibition onto the PV5 RGC is the AII, which receives GABAergic input, a required element in our proposed circuit (Bloomfield and Xin 2000; Zhou and Dacheux 2004). Furthermore, a sustained GABAAergic current has been observed in mouse ON α and OFF αSustained RGCs (Di Marco et al. 2013; Murphy and Rieke 2006), and a similar, albeit unidentified inhibitory input was observed in guinea pig OFF αTransient RGCs (Manookin et al. 2008). Finally, we found no change in GABAergic signaling in PV5Glra1-KD compared with PV5SC when we examined the transcription of GABA-related genes using RNA microarray and deep sequencing strategies (negative data not shown).
GlyRα1-Mediated Crossover Inhibition Directly onto PV5 RGC Improves Fidelity of RGC Responses
In the retina, glycinergic inhibition has been primarily associated with an On-to-Off crossover pathway, which appears to enhance excitatory/inhibitory responses of OFF RGCs to luminance decrement/increment (Buldyrev et al. 2012; Buldyrev and Taylor 2013; Demb and Singer 2012; Liang and Freed 2010; Manookin et al. 2008; Murphy and Rieke 2006; Nobles et al. 2012; Roska et al. 2006), regulate contrast gain control (Beaudoin et al. 2008), contribute to the linearity of the retinal output (Molnar et al. 2009), and shape RGCs responses to specific visual stimuli (Cafaro and Rieke 2013; Münch et al. 2009).
Within the IPL On-to-Off crossover inhibition has been observed both at the dendrites of OFF RGCs, contributing to an inhibitory conductance, and at the terminals of OFF CBCs, contributing to an excitatory conductance in the OFF RGCs (Manookin et al. 2008; Molnar and Werblin 2007; Murphy and Rieke 2006; Münch et al. 2009; Roska et al. 2006). Although these studies inferred a role for glycinergic inhibition at both levels of crossover inhibition, the lack of a subunit-specific glycine antagonist made it difficult to directly isolate synapse-specific glycinergic regulation and separate it from GABAergic regulation at the same synaptic level. As a consequence, the relative contribution of modulation at each input level of OFF RGC visual activity remains unclear.
For example, direct inhibition is the major drive to OFF αTransient RGC under rod-dominant conditions or when both rods and cones are active (Manookin et al. 2008; Murphy and Rieke 2006). Other studies under similar light levels suggest that crossover modulation at the CBC level and its control of excitation in OFF RGCs are critical (Pang et al. 2003; van Wyk et al. 2009). Moreover, the contribution of excitation vs. inhibition appears to change with increasing contrast (Manookin et al. 2008). By eliminating GlyRα1 expression in PV5 RGCs, we show that glycinergic crossover inhibition at the RGC level is critical for regulating cone-driven responses within the RF center. Specifically, GlyRα1 inputs lower the spontaneous spiking activity to improve signal-to-noise ratio in PV5 RGCs. GlyRα1 inputs reduce/eliminate rebound excitation at luminance increment as well as enhancing the excitation response at luminance decrement via disinhibition. Together these mechanisms enhance the fidelity of the OFF αTransient RGC RF center response relative to its dendritic stratification and maintain its ability to primarily encode luminance decrements.
GRANTS
The study was supported by Friedrich Miescher Institute funds, an EMBO short-term fellowship (ASTF 359-2012, C. Zhang), National Eye Institute Grant EY-140701 (M. A. McCall), and an unrestricted Research to Prevent Blindness (RPB) grant to University of Louisville Department of Ophthalmology and Visual Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.Z., S.B.R., B.R., and M.A.M. conception and design of research; C.Z. and S.B.R. performed experiments; C.Z. and M.A.M. analyzed data; C.Z. and M.A.M. interpreted results of experiments; C.Z. prepared figures; C.Z. drafted manuscript; C.Z., S.B.R., B.R., and M.A.M. edited and revised manuscript; C.Z., S.B.R., B.R., and M.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Jacek Krol, Yong Li, Ron Gregg, and Thomas Ray for helping with shRNA design. We thank Zoltan Raics for creating the custom-built psychophysics software program. We thank Drs. Tamas Szikra, Karl Farrow, and Bart Borghuis for their comments on the manuscript.
REFERENCES
- Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, Koshevoy P, Grimm B, Tasdizen T, Whitaker R, Marc RE. Exploring the retinal connectome. Mol Vis 17: 355–379, 2011. [PMC free article] [PubMed] [Google Scholar]
- Azeredo da Silveira R, Roska B. Cell types, circuits, computation. Curr Opin Neurobiol 21: 664–671, 2011. [DOI] [PubMed] [Google Scholar]
- Beaudoin DL, Manookin MB, Demb JB. Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell. J Physiol 586: 5487–5502, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J Physiol 523: 771–783, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel F, Kay MA, Mueller C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Mol Ther 22: 692–701, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature 424: 430–434, 2003. [DOI] [PubMed] [Google Scholar]
- Buldyrev I, Puthussery T, Taylor WR. Synaptic pathways that shape the excitatory drive in an OFF retinal ganglion cell. J Neurophysiol 107: 1795–1807, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buldyrev I, Taylor WR. Inhibitory mechanisms that generate centre and surround properties in ON and OFF brisk-sustained ganglion cells in the rabbit retina. J Physiol 591: 303–325, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Rieke F. Regulation of spatial selectivity by crossover inhibition. J Neurosci 33: 6310–6320, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hsueh HA, Greenberg K, Werblin FS. Three forms of spatial temporal feedforward inhibition are common to different ganglion cell types in rabbit retina. J Neurophysiol 103: 2618–2632, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hsueh HA, Werblin FS. Amacrine-to-amacrine cell inhibition: spatiotemporal properties of GABA and glycine pathways. Vis Neurosci 28: 193–204, 2011. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507: 358–361, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Singer JH. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis Neurosci 29: 51–60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco S, Protti DA, Solomon SG. Excitatory and inhibitory contributions to receptive fields of alpha-like retinal ganglion cells in mouse. J Neurophysiol 110: 1426–1440, 2013. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Becker CM, Betz H. Inhibitory glycine receptors: an update. J Biol Chem 287: 40216–40223, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, Yonehara K, Roska B. Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron 78: 325–338, 2013. [DOI] [PubMed] [Google Scholar]
- Gill SB, Veruki ML, Hartveit E. Functional properties of spontaneous IPSCs and glycine receptors in rod amacrine (AII) cells in the rat retina. J Physiol 575: 739–759, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze L, Harvey RJ, Haverkamp S, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha4 subunit. J Comp Neurol 500: 693–707, 2007. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500: 168–174, 2013. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3: e159, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller U, Wassle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci 23: 350–364, 2006. [DOI] [PubMed] [Google Scholar]
- Kastner DB, Baccus SA. Spatial segregation of adaptation and predictive sensitization in retinal ganglion cells. Neuron 79: 541–554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke JB, Wang YV, Borghuis BG, Cembrowski MS, Riecke H, Kath WL, Demb JB, Singer JH. Adaptation to background light enables contrast coding at rod bipolar cell synapses. Neuron 81: 388–401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, Duebel J, Bicker S, Fehling HJ, Schubeler D, Oertner TG, Schratt G, Bibel M, Roska B, Filipowicz W. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141: 618–631, 2010. [DOI] [PubMed] [Google Scholar]
- Liang Z, Freed MA. The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci 30: 5533–5543, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Heinze L, Haverkamp S, Ivanova E, Wassle H. Glycine receptors of A-type ganglion cells of the mouse retina. Vis Neurosci 24: 471–487, 2007. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Weiss J, Wassle H. Glycinergic input of widefield, displaced amacrine cells of the mouse retina. J Physiol 587: 3831–3849, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci 28: 4136–4150, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Anderson JR, Sigulinsky C, Lauritzen S. Retinal connectomics: towards complete, accurate networks. Prog Retin Eye Res 37: 141–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci 27: 5994–6005, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements ME, MacLaren RE. Gene therapy for retinal disease. Transl Res 161: 241–254, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol 401: 34–46, 1998. [DOI] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J Comput Neurosci 27: 569–590, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol 98: 3423–3435, 2007. [DOI] [PubMed] [Google Scholar]
- Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci 12: 1308–1316, 2009. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron 52: 511–524, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci 11: 318–326, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, Leung KM, Odermatt B, Lagnado L. Synaptic mechanisms of adaptation and sensitization in the retina. Nat Neurosci 16: 934–941, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles RD, Zhang C, Muller U, Betz H, McCall MA. Selective glycine receptor alpha2 subunit control of crossover inhibition between the on and off retinal pathways. J Neurosci 32: 3321–3332, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci 23: 6063–6073, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol 95: 3810–3822, 2006. [DOI] [PubMed] [Google Scholar]
- Russell TL, Werblin FS. Retinal synaptic pathways underlying the response of the rabbit local edge detector. J Neurophysiol 103: 2757–2769, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel JA, Roska B. Gene therapy for blindness. Annu Rev Neurosci 36: 467–488, 2013. [DOI] [PubMed] [Google Scholar]
- Schaefer N, Vogel N, Villmann C. Glycine receptor mutants of the mouse: what are possible routes of inhibitory compensation? Front Mol Neurosci 5: 98, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol 451: 115–126, 2002. [DOI] [PubMed] [Google Scholar]
- van Wyk M, Wässle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci 26: 297–308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani S, Taylor WR. Orientation selectivity in rabbit retinal ganglion cells is mediated by presynaptic inhibition. J Neurosci 30: 15664–15676, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the Mammalian retina. Front Mol Neurosci 2: 6, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, O'Sullivan GA, Heinze L, Chen HX, Betz H, Wässle H. Glycinergic input of small-field amacrine cells in the retinas of wildtype and glycine receptor deficient mice. Mol Cell Neurosci 37: 40–55, 2008. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci 27: 1–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Farrow K, Ghanem A, Hillier D, Balint K, Teixeira M, Jüttner J, Noda M, Neve RL, Conzelmann KK, Roska B. The first stage of cardinal direction selectivity is localized to the dendrites of retinal ganglion cells. Neuron 79: 1078–1085, 2013. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci 23: 2645–2654, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McCall MA. Receptor targets of amacrine cells. Vis Neurosci 29: 11–29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci 14: 553–563, 1997. [DOI] [PubMed] [Google Scholar]
- Zhou C, Dacheux RF. All amacrine cells in the rabbit retina possess AMPA-, NMDA-, GABA-, and glycine-activated currents. Vis Neurosci 21: 181–188, 2004. [DOI] [PubMed] [Google Scholar]