Abstract
Atypical medial olivocochlear (MOC) feedback from brain stem to cochlea has been proposed to play a role in tinnitus, but even well-constructed tests of this idea have yielded inconsistent results. In the present study, it was hypothesized that low sound tolerance (mild to moderate hyperacusis), which can accompany tinnitus or occur on its own, might contribute to the inconsistency. Sound-level tolerance (SLT) was assessed in subjects (all men) with clinically normal or near-normal thresholds to form threshold-, age-, and sex-matched groups: 1) no tinnitus/high SLT, 2) no tinnitus/low SLT, 3) tinnitus/high SLT, and 4) tinnitus/low SLT. MOC function was measured from the ear canal as the change in magnitude of distortion-product otoacoustic emissions (DPOAE) elicited by broadband noise presented to the contralateral ear. The noise reduced DPOAE magnitude in all groups (“contralateral suppression”), but significantly more reduction occurred in groups with tinnitus and/or low SLT, indicating hyperresponsiveness of the MOC system compared with the group with no tinnitus/high SLT. The results suggest hyperresponsiveness of the interneurons of the MOC system residing in the cochlear nucleus and/or MOC neurons themselves. The present data, combined with previous human and animal data, indicate that neural pathways involving every major division of the cochlear nucleus manifest hyperactivity and/or hyperresponsiveness in tinnitus and/or low SLT. The overactivation may develop in each pathway separately. However, a more parsimonious hypothesis is that top-down neuromodulation is the driving force behind ubiquitous overactivation of the auditory brain stem and may correspond to attentional spotlighting on the auditory domain in tinnitus and hyperacusis.
Keywords: DPOAE, efferent feedback, loudness discomfort level, cochlear efferents, posteroventral cochlear nucleus, anteroventral cochlear nucleus, dorsal cochlear nucleus
the medial olivocochlear (MOC) system is one of two efferent systems that exert descending control over the cochlea (e.g., see Guinan 2006 for review). In the MOC system, auditory-nerve fibers project to a subpopulation of multipolar neurons in the posteroventral cochlear nucleus (PVCN), which in turn project to MOC neurons of the superior olivary complex (Brown et al. 2013a; de Venecia et al. 2005; Horváth et al. 2003; Mulders et al. 2007; Thompson and Thompson 1991). MOC neurons then collectively project bilaterally to the outer hair cells of the cochleae, thus controlling cochlear gain and enabling modulation of auditory-nerve activity (Guinan 2006; Guinan and Gifford 1988; Warr 1975). MOC feedback to the cochlea is believed to play roles in deciphering signals such as speech in noisy environments and protecting the cochlea from traumatizing exposures to sound (Liberman et al. 2014; Mishra and Lutman 2014). Anomalous MOC function has been hypothesized, and tested for, in a variety of clinical conditions, including tinnitus, a prevalent and often disruptive condition in which sound is perceived in the absence of actual sound (Shargorodsky et al. 2010; Stouffer and Tyler 1990). Despite a large number of studies, however, it remains unclear whether or not there is a relationship between MOC function and tinnitus (Attias et al. 1996, 2005; Ceranic et al. 1998; Chéry-Croze et al. 1993, 1994; Fávero et al. 2006; Fernandes and Santos 2009; Geven et al. 2011, 2012; Graham and Hazell 1994; Hesse et al. 2005, 2008; Hsu et al. 2013; Lalaki et al. 2011; Lind 1996; Paglialonga et al. 2010, 2011; Riga et al. 2007; Riga et al. in press; Urnau and Tochetto 2012). Results differ even among the more carefully controlled studies of this issue, with some reporting reduced MOC function in tinnitus (Attias et al. 1996; Riga et al. 2007) and others reporting no difference compared with controls (Geven et al. 2011; Lind 1996; Paglialonga et al. 2010, 2011).
In the present study, it was hypothesized that the inconsistent findings of previous MOC/tinnitus studies might be due to a particular uncontrolled variable: the degree to which sound is tolerated or not, based on its level (sound-level tolerance, SLT). Extreme intolerance, or very low SLT, corresponds to clinical hyperacusis, where even moderate-level sounds are considered uncomfortable (Baguley 2003; Tyler et al. 2003). SLT tends to be reduced in people with tinnitus compared with threshold-matched controls without tinnitus, although the ranges of SLT for those with and without tinnitus are overlapping (Gu et al. 2010; Hébert et al. 2013). SLT is a potentially important variable here because the MOC system, as a controller of cochlear gain, is positioned to influence loudness and therefore SLT.
The present study examined MOC function in threshold-, age-, and sex-matched subject groups defined on the basis of tinnitus (having it chronically or not) and SLT (low or high). A widely used test of MOC function was employed that measures the effect of sound to one ear on the otoacoustic emissions (OAE) produced by outer hairs cells of the opposite ear (Hood et al. 1996; Veuillet et al. 1991). When measured in normal human subjects using common protocols, the effect is generally suppressive; that is, the magnitude of the OAE, for instance, distortion-product OAE (DPOAE), is reduced by contralaterally presented sound (commonly broadband noise; Hood et al. 1996; Moulin et al. 1993). In this study, DPOAE suppression was found in all subject groups, but it was greater in the groups with tinnitus and/or low SLT, indicating increased responsiveness of the MOC system.
MATERIALS AND METHODS
Twenty-seven men (30–54 yr, 24 right-handed) were recruited through advertisements and Massachusetts Eye and Ear clinics. Eleven subjects had chronic, subjective tinnitus. All subjects had clinically normal or near-normal audiograms defined as threshold ≤25 dB hearing level (HL) at octave intervals from 250 through 4,000 Hz and ≤35 dB HL at 8,000 Hz. Subjects had no known neurological problems except for subject 129, who had an MRI suggesting a possible telangiectasia in the right pons near the superior olivary complex and trapezoid body. Subject 347 was notable for only intermittent tinnitus (mainly noticeable at night) and a flapping sensation in the right ear in response to certain sounds. No subject had severe depression or anxiety according to standard inventories (Beck et al. 1961, 1988; scores in Table 1). None of the subjects was a professional musician in whom MOC reflex strength might be stronger than in the general population (Perrot and Collet 2014). In a questionnaire asking about musical training, only 4 subjects reported regularly playing musical instruments or singing in the previous 6 mo, and they were distributed across subject groups.
Table 1.
Subject characteristics
| Subject | Age, yr | Sex | Handedness | Depression Score (max = 62) | Anxiety Score (max = 63) | Noise LDL (L, R), dB SPL | SLT Questionnaire Score (max = 1) | DPOAE-Recorded Ear(s) | |
|---|---|---|---|---|---|---|---|---|---|
| No tinnitus | |||||||||
| High SLT | 46 | 47 | M | R | 0 | 0 | >118, >119 | 0.83 | L, R |
| 55 | 47 | M | R | 12 | 4 | 113, 114 | 0.73 | L | |
| 119 | 43 | M | R | 0 | 1 | >118, 119 | 1.0 | R | |
| 131 | 53 | M | R | n/a | 7 | >118, >119 | 1.0 | R | |
| 142 | 48 | M | R | 5 | 3 | 113, 114 | 0.9 | L, R | |
| 146 | 43 | M | R | 6 | 3 | 113, 114 | 0.99 | L, R | |
| 348 | 41 | M | L | 0 | 3 | 113, 117 | 0.97 | R | |
| 409 | 43 | M | R | 11 | 8 | 115, >117 | 0.70 | R | |
| Low SLT | 8 | 47 | M | R | 2 | 0 | 103, 104 | 0.93 | L, R |
| 9 | 53 | M | R | 0 | 0 | 93, 94 | 0.83 | L, R | |
| 125 | 49 | M | R | 0 | 0 | 96, 94 | 0.85 | L | |
| 135 | 35 | M | R | 4 | 7 | >118, >119 | 0.53 | L, R | |
| 137 | 30 | M | R | 8 | 10 | 68, 69 | 0.83 | L | |
| 148 | 36 | M | R | 5 | 0 | 116, 107 | 0.57 | L | |
| 338 | 46 | M | R | n/a | n/a | 95, 87 | 1.0 | L | |
| 435 | 40 | M | R | 0 | 2 | 93, 97 | 1.0 | L | |
| Tinnitus | |||||||||
| High SLT | 72 | 47 | M | L | 5 | 5 | 106, 109 | 0.8 | L, R |
| 85 | 54 | M | L | 0 | 4 | 113, 114 | 0.97 | L, R | |
| 109 | 47 | M | R | 7 | 2 | 116, 114 | 1.0 | L, R | |
| 116 | 45 | M | R | 12 | 16 | >118, >119 | 1.0 | L, R | |
| 160 | 39 | M | R | 11 | 12 | 116, 109 | 1.0 | L, R | |
| Low SLT | 23 | 34 | M | R | 0 | 11 | 83, 89 | 0 | L, R |
| 110 | 41 | M | R | 8 | 7 | 83, 79 | 0.5 | L, R | |
| 128 | 44 | M | R | 20 | 6 | 76, 79 | 0.2 | L, R | |
| 129 | 45 | M | R | 0 | 1 | 73, 77 | 1.0 | L, R | |
| 322 | 42 | M | R | 0 | 1 | >110, 95 | 0.8 | R | |
| 347 | 34 | M | R | 2 | 8 | 75, 77 | 0.87 | L, R | |
M, male; L, left; R, right; LDL, loudness discomfort level; SLT, sound-level tolerance; Quest., questionnaire; DPOAE, distortion-product otoacoustic emission; max, maximum; n/a, not available.
Tinnitus subjects completed questionnaires assessing tinnitus-related distress (Tinnitus Reaction Questionnaire; Wilson et al. 1991) and general tinnitus characteristics such as the location and quality of the tinnitus percept. Tinnitus was bilateral or “in the head” for 8 of the 11 tinnitus subjects. The remaining three subjects reported unilateral tinnitus during testing but had a history of occasional tinnitus in the other ear, as well. The tinnitus of eight subjects could be modulated somatically, that is, altered in loudness or pitch by contractions of head and/or neck muscles (Levine et al. 2003). Additional tinnitus characteristics are given in Table 2.
Table 2.
Tinnitus characteristics
| Subject | Tinnitus Duration, yr | Tinnitus Location | Tinnitus Quality | Tinnitus Pitch (L, R), kHz | Tinnitus Loudness (L, R), dB SL | Minimum Masking Level, dB SL | Tinnitus Reaction Questionnaire Score (max = 104) | Residual Inhibition | Somatic Modulation | |
|---|---|---|---|---|---|---|---|---|---|---|
| High SLT | 72 | 15 | Both ears, worse in R | Ringing, whistling | >8, >8 | 25, 25 | 40 | 45 | No | Yes |
| 85 | “Many” | Both ears, worse in R | Pulsing, hissing, tonal | >12, n/a | 18, 15 | 35 | 18 | Yes | Yes | |
| 109 | “Lifelong,” worse in past 10–15 yr | In head, center | Ringing, tonal | 10, 12 | 30, 30 | 45 | 7 | No | Yes | |
| 116 | 19 | Both ears, equally | Ringing | 12, 12 | 15, 10 | 35 | 61 | No | Yes | |
| 160 | 2 | In head, center | Hissing, electronic | 10, 10 | 25, 15 | 50 | 14 | No | n/a | |
| Low SLT | 23 | 13 | Both ears, worse in L | Tonal | >8, >8 | 35, 40 | 45 | 46 | No | Yes |
| 110 | 3 | Both ears, worse in L | Ringing, buzzing | 1–2, 1–2 | 20, 10 | 25 | 61 | Yes | Yes | |
| 128 | 10 | R ear* | Ringing, hissing | —, 6 | —, 60 | 55 | 78 | No | Yes | |
| 129 | 6 | R ear† | Ringing | —, 2–3 | —, 25 | 30 | 5 | Yes | Yes | |
| 322 | 20 | R ear* | Ringing, tonal | —, 11 | —, 15 | 70 | 13 | No | n/a | |
| 347 | 0.5 | Both ears, worse in R | Intermittent, ringing | n/a, n/a | n/a, n/a | n/a, n/a | 22 | No | n/a |
Subject also has occasional episodes of left ear tinnitus that are distinct from the right ear tinnitus. †Subject's tinnitus was bilateral when first noticed and then became unilateral.
Subjects provided informed written consent prior to participation. The study was approved by the Human Studies Committee at the Massachusetts Eye and Ear Infirmary.
All of the following testing was performed in a double-walled sound-attenuating booth.
Audiograms.
Pure tone thresholds were measured from 0.125 through 8 kHz at half-octave intervals and at 9, 10, 11.2, 12.5, 14, and 16 kHz. Thresholds for 8 kHz and below were measured using TDH-39P headphones and an Interacoustics audiometer (AC33 or AC40). Thresholds above 8 kHz were measured using Sennheiser HDA 200 headphones and either the Interacoustics AC40 audiometer or a custom system calibrated to the same specifications (ANSI S3.6-2004).
Measures of SLT.
Two measures of sound tolerance were used: loudness discomfort level (LDL) and a questionnaire assessment of SLT. LDL was determined for broadband noise and 500-Hz warble tone stimuli as follows (Cox et al. 1997). Each sound was presented monaurally over headphones (TDH-39P) at progressively higher levels beginning at 35 dB SPL. The noise was generated in MATLAB (The MathWorks, Natick, MA) and played from a sound card to the tape input of an Interacoustics audiometer (AC33 or AC40). The 500-Hz warble tone (i.e., frequency-modulated tone) was produced by the audiometer. As sound level was incremented in 5-dB steps, subjects rated the perceived loudness from 1 (very soft) to 7 (uncomfortably loud). Sound level at 7 was the LDL for the stimulated ear. If a subject's rating never reached 7, the highest testable level served as a lower bound on the LDL.
The SLT questionnaire consisted of three items about sound tolerance in everyday life (Gu et al. 2010; Tyler et al. 2003). Subjects rated their agreement with the following statements on a scale from 0 (completely disagree) to 100 (completely agree): 1) Many everyday sounds are unbearably loud to me; 2) Sounds that others believe are moderately loud are too loud for me; and 3) I hear very soft sounds that others with normal hearing do not hear. Ratings were averaged across statements and normalized to yield a score between 0 (low tolerance) and 1 (high tolerance).
Measures of tinnitus.
In tinnitus subjects, the following were measured: tinnitus pitch, tinnitus loudness, the minimum level of binaural broadband noise needed to fully mask the tinnitus percept (minimum masking level), and residual inhibition following 1 min of binaural broadband noise 10 dB re minimum masking level (Table 2). Tinnitus loudness and minimum masking level are expressed relative to the detection threshold of the broadband noise (that is, in dB sensation level; dB SL). Residual inhibition was defined as a reduction in volume or absence of tinnitus for any length of time following cessation of the masking noise.
DPOAE measurement with and without contralateral noise.
DPOAEs were measured by means of the Mimosa HearID system consisting of measurement probe, input-output card, and acquisition software running on a laptop computer (Mimosa Acoustics, Champaign, IL). The probe, consisting of a measurement microphone and two earphones for primary tone delivery, was coupled to the ear canal via a foam ear tip (Etymotic Research ER10C-14A). Quadratic distortion products (2f1 − f2; f2/f1 = 1.2) were measured with and without 60-dB SPL broadband noise presented to the contralateral ear (Fig. 1). Primary levels known to allow for robust contralateral suppression were used (L1 = 55, L2 = 40 dB SPL; Williams and Brown 1997) and were verified by in-ear calibration.
Fig. 1.
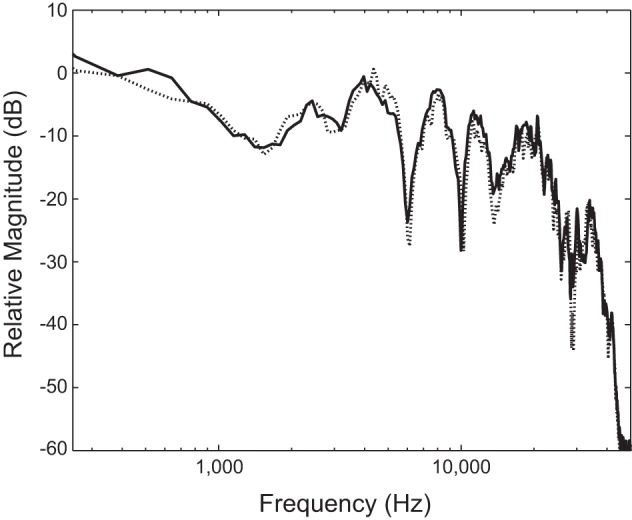
Magnitude spectrum of noise delivered to ears contralateral to distortion-product otoacoustic emissions (DPOAE) measurement. Spectra were measured at the output of the Sennheiser HDA200 headphones positioned on a Larson-Davis AEC101 artificial ear. Dotted and solid lines correspond to noise delivered to right and left ears, respectively.
The contralateral noise stimulus was delivered via Sennheiser HDA 200 headphones. Subjects wore the full HDA 200 headset with an extra earmuff cushion added to the headphone over the DPOAE-recorded ear to prevent contact between headphone and DPOAE measurement probe. Noise was delivered to the opposite headphone. The noise was generated by MATLAB software controlling a laptop sound card. The sound card output was fed to the tape input of the audiometer, which was then used to control the noise level.
DP-grams, consisting of DPOAE magnitude vs. frequency, were measured from 1 to 4 kHz at 28 points/octave in noise/no-noise pairs, meaning that broadband noise was played in the contralateral ear during acquisition of one DP-gram, whereas the contralateral ear was unstimulated during the other DP-gram. During the first half of a recording session, the no-noise DP-gram of each pair was measured first and the noise DP-gram second. In the second half, after the subject was allowed to stretch, the order was reversed. At least 10 no-noise/noise pairs were obtained during a session. Primary levels were calibrated prior to each pair of no-noise/noise DP-grams. In cases with primary level fluctuations >3 dB from baseline at any frequency, the entire DP-gram was discarded. This yielded an average of 13 usable DP-gram pairs per ear (range 3–20).
DPOAE measurements were made in one or both ears in each subject (Table 1, far right). Measurements for a given ear were usually made in a single 3-h session during which subjects reclined, remained awake, and were allowed to read. In 19 instances, measurements for a given ear were distributed over 2 sessions.
Stapedial reflex.
The noise level used (60 dB SPL) was less than typically required to activate a stapedial reflex, but each DPOAE-recorded ear was nonetheless tested for stapedius muscle contractions in response to the same contralaterally presented noise used during the noise/no-noise DPOAE measurements. This was done by using the DPOAE recording system and tones as close to one another in frequency as the system allowed (f2/f1 = 1.05). Tone levels were the same as for DPOAE measurement, which is low enough to avoid contributions from stimulus-frequency OAE (L1 = 55 dB SPL and L2 = 40 dB SPL). Tone magnitude in the ear canal was measured for frequencies from 0.5 to 4 kHz at half-octave intervals, alternately with and without noise presented to the contralateral ear. Stapedial reflex strength was quantified at each frequency as the change in primary tone magnitude (ratio of no noise to noise, expressed in dB).
Data analyses.
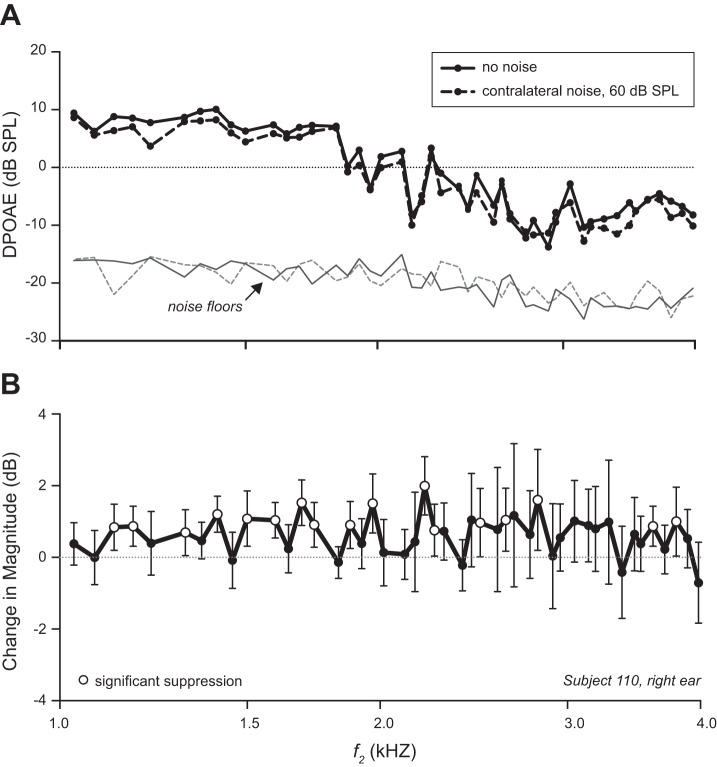
For each DPOAE measurement pair (e.g., Fig. 2A), the change in DPOAE magnitude elicited by contralateral noise was quantified as a magnitude ratio (no noise/noise) and expressed in decibels. The mean change at each frequency was then calculated for each DPOAE-recorded ear. A second analysis identified significant suppression (or facilitation) of DPOAE magnitude at each f2 value in individual ears. Mean magnitude changes were considered significant when their 95% confidence intervals excluded zero. Positive changes are referred to as suppression, negative as facilitation. Figure 2B illustrates with open circles instances of significant suppression in a representative subject. In a third analysis, the difference was taken between the complex DPOAE pressures (real and imaginary parts) recorded with contralateral noise and without. The magnitude of the resulting difference provided a measure of noise-elicited changes in either the magnitude or phase of the DPOAE (or both). This measure showed no significant differences between groups and is not discussed further.
Fig. 2.
Measures of change in DPOAE magnitude elicited by noise to the contralateral ear. A: typical no-noise/noise pair of DP-grams in 1 ear. B: change in magnitude (i.e., magnitude without noise/magnitude with noise expressed in dB) averaged over all 18 of the no-noise/noise pairs for the same ear as in A. Error bars indicate 95% confidence intervals. For f2 values showing significant suppression (confidence intervals exclude 0), the data are plotted with open, rather than filled, circles. There was no significant facilitation in this example.
RESULTS
Figure 3 shows, for both tinnitus and no-tinnitus subjects, the SLT data for each ear in which DPOAE recordings were made. Score on the SLT questionnaire is plotted vs. LDL for noise in Fig. 3A and vs. LDL for the 500-Hz warble tone in Fig. 3B. Noise LDLs, 500-Hz warble tone LDLs, and SLT questionnaire scores each had a range of values that overlapped between tinnitus subjects (asterisks and circles) and no-tinnitus subjects (open and shaded squares). For the primary analysis below, DPOAE-recorded ears were divided into four groups on the basis of tinnitus and the data of Fig. 3A (noise LDL and SLT questionnaire score). Two groups comprised tinnitus subjects with low and high SLT (asterisks and open circles, respectively), whereas the remaining two groups comprised no-tinnitus subjects with low and high SLT (open and shaded squares, respectively). The division between low and high SLT was set so that the four groups included similar numbers of subjects and DPOAE-recorded ears. DPOAE-recorded ears with a noise LDL ≥105 dB SPL and SLT questionnaire score ≥0.7 were designated high SLT, and the rest were designated low SLT. Supplementary analyses, also described below, examined the sensitivity of the results to grouping criteria (e.g., using the LDL data for 500-Hz warble tones).
Fig. 3.
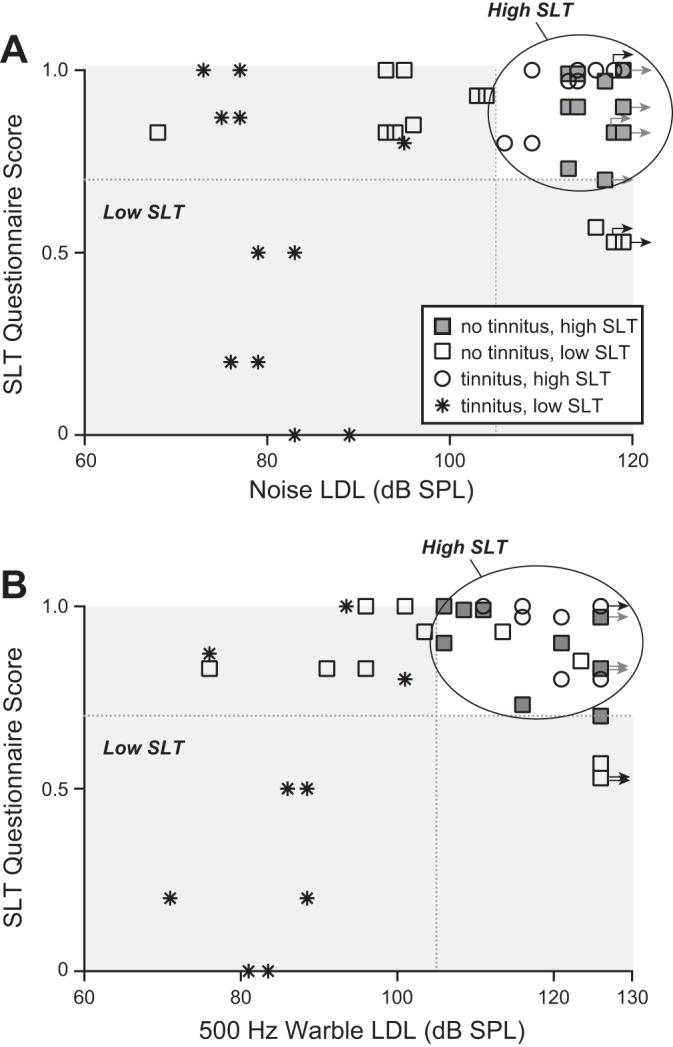
Categorization based on tinnitus and sound-level tolerance (SLT) assessed via questionnaire and loudness discomfort level (LDL). Plotted is score on an SLT questionnaire vs. LDL for noise stimulus (A) and LDL for 500-Hz warble tone (B). Gray shading indicates SLT questionnaire and LDL range corresponding to “low SLT.” Unshaded region corresponds to “high SLT.” In both A and B, each point corresponds to a particular subject and DPOAE-recorded ear. Right-pointing arrows next to some points indicate that LDL exceeded the upper limit of stimulation. Vertical and horizontal dashed lines correspond, respectively, to an LDL of 105 dB SPL and an SLT questionnaire score of 0.7.
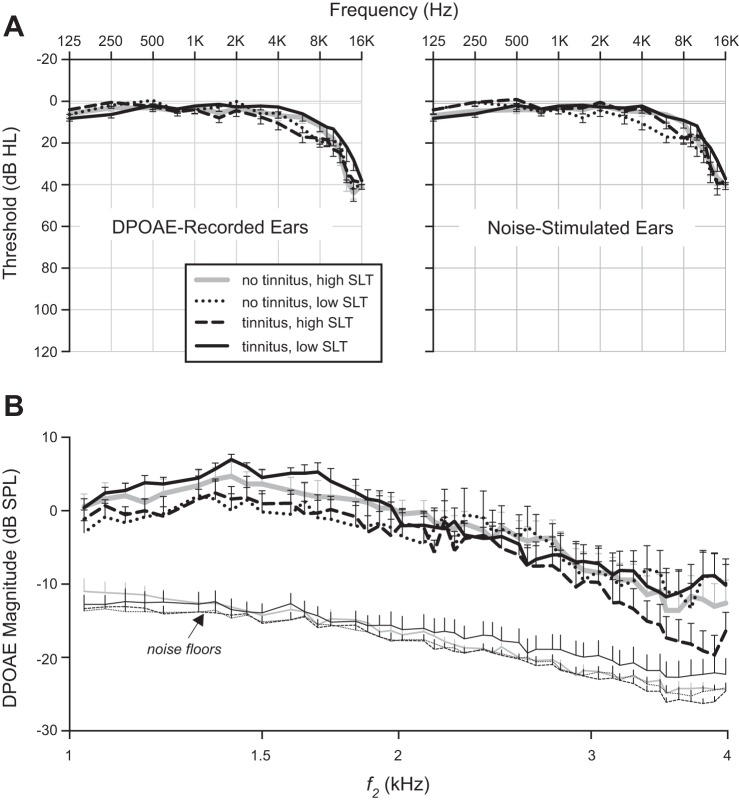
The groups formed for the primary analysis were closely matched: mean threshold for the DPOAE-recorded ears, and for the noise-stimulated ears, of each group differed by 7 dB or less at any given frequency below 4 kHz and by 16 dB or less at 4 kHz and above (Fig. 4A). Mean age differed between any pair of groups by 0–6 yr (Table 3). Sex was automatically matched between groups because all subjects were male. Mean baseline DPOAE magnitude (no contralateral noise) was well above the mean noise floor in all groups for all but the highest f2 values (Fig. 4B).
Fig. 4.
Audiograms and DP-grams for ears contributing to Fig. 5. A: mean audiograms for DPOAE-recorded and noise-stimulated ears of each group. B: mean baseline (no noise) DP-grams for each group. Error bars indicate SE.
Table 3.
Group characteristics
| Age, yr | Depression Score | Anxiety Score | Tinnitus Reaction Questionnaire | |
|---|---|---|---|---|
| No tinnitus, high SLT | 46 ± 1* | 5 ± 1† | 3 ± 1* | |
| No tinnitus, low SLT | 43 ± 2* | 3 ± 1† | 3 ± 1† | |
| Tinnitus, high SLT | 46 ± 2† | 7 ± 1† | 8 ± 2† | 29 ± 7† |
| Tinnitus, low SLT | 40 ± 1* | 7 ± 2* | 6 ± 1* | 40 ± 8* |
Values are means ± SE across ears (*11 ears; †10 ears).
Change in DPOAE magnitude with contralateral noise: primary analysis.
Figure 5A shows the average change in DPOAE magnitude produced by contralateral noise for each subject group defined in Fig. 3A. This change (no-noise/noise magnitude ratio in dB) was generally positive for all groups, indicating a reduction in DPOAE magnitude by the contralateral noise (suppression). However, the two groups with tinnitus (black solid and dashed lines), as well as the group with no tinnitus but low SLT (dotted line) tended to show greater suppression than the no-tinnitus/high-SLT group (gray solid line) over much of the f2 range (1–3 kHz). This tendency for greater suppression (that is, greater magnitude change) can also be seen in Fig. 5B, where the data for individual DPOAE-recorded ears were first averaged over half-octave f2 bands and then across ears. The difference in mean magnitude change between the two no-tinnitus groups suggested that the data for the two groups, which differed in SLT, came from different underlying populations. This was confirmed by a Kolmogorov-Smirnov test, which demonstrated significantly greater magnitude change in the no-tinnitus/low-SLT group in two f2 bands (1–1.4 kHz, P = 0.02; 2–2.8 kHz, P = 0.02). At the same time, the no-tinnitus/low-SLT group showed no significant differences in any f2 band compared with each of the two groups with tinnitus (1–1.4 kHz, P = 0.08, tinnitus/low SLT; all other comparisons, P ≥ 0.34). Thus the no-tinnitus/low-SLT group bore greater similarity to the tinnitus groups than to the other no-tinnitus group and has been combined with the tinnitus groups in subsequent analyses. The no-tinnitus/low-SLT group, combined with the two tinnitus groups, showed significantly greater magnitude change (greater suppression) compared with the no-tinnitus/high-SLT control group in two f2 bands [1–1.4 kHz, P = 0.003; 2–2.8 kHz, P = 0.001; Mann-Whitney, uncorrected for multiple (that is, 4) comparisons].
Fig. 5.
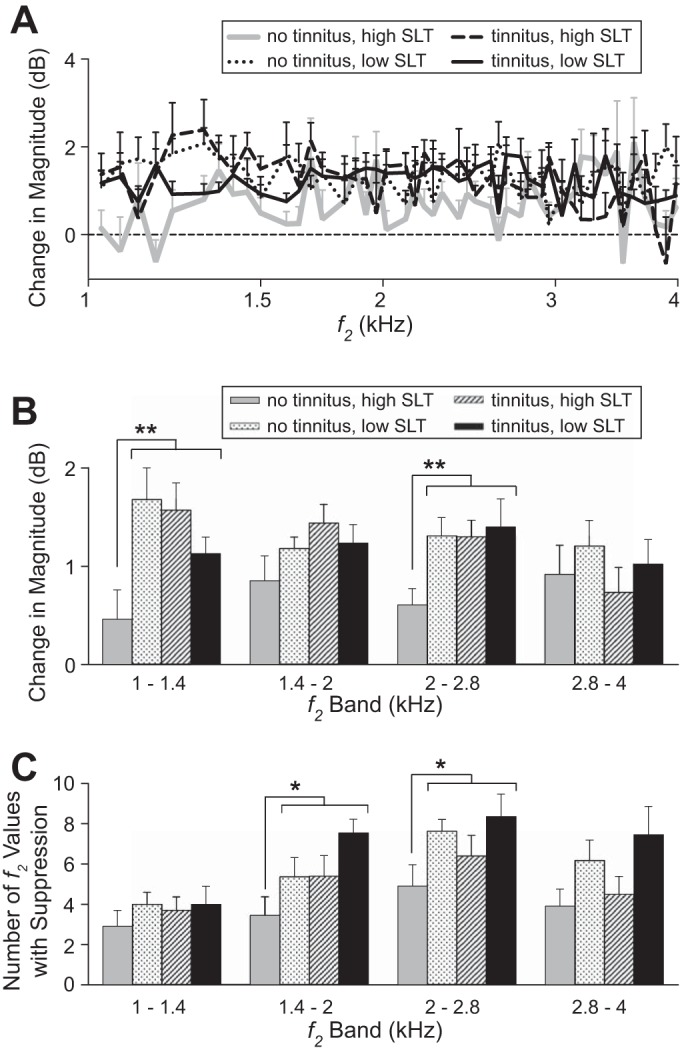
Effect of contralateral noise on DPOAE magnitude in each of the subject groups defined in Fig. 3A. A: change in magnitude elicited by contralateral noise averaged across DPOAE-recorded ears. B: same as A except that the magnitude change for each ear was first averaged over half-octave bands in f2 and then averaged across ears. C: number of f2 values showing significant suppression in half-octave bands averaged across ears. Error bars indicate SE. Asterisks indicate significance: *P < 0.05; **P < 0.01 (not corrected for multiple comparisons). The number of subjects and DPOAE-recorded ears per group are as follows: 8 subjects, 11 ears (no tinnitus, high SLT); 8 subjects, 11 ears (no tinnitus, low SLT); 5 subjects, 10 ears (tinnitus, high SLT); and 6 subjects, 11 ears (tinnitus, low SLT).
An alternative quantification of the changes in DPOAE magnitude produced by contralateral noise is shown in Fig. 5C. The alternative analysis separately analyzed each DPOAE-recorded ear to identify each f2 value at which significant suppression (or facilitation) occurred along the DP-gram (e.g., Fig. 2B). Thus the analysis took into account the fact that the effect of contralateral noise varies across frequency (i.e., along the DP-gram) in a manner related to the specific DPOAE fine structure of individual ears (Abdala et al. 2009). Figure 5C plots, for each subject group, the average number of f2 values showing significant suppression in half-octave f2 bands from 1 to 4 kHz. Collectively, the three groups with tinnitus and/or low SLT showed more instances of significant suppression than the no-tinnitus, high-SLT group [P = 0.01 (f2 = 1.4–2 kHz); P = 0.04 (f2 = 2–2.8)]. Significant facilitation of DPOAE magnitude also occurred, but far less often than significant suppression, averaging between 0 and 0.73 depending on octave band and subject group. One interpretation is that the same underlying mechanism accounts for both suppression and facilitation (reduced magnitude of 1 of 2 underlying DPOAE sources; e.g., see Abdala et al. 2009), in which case instances of facilitation should be added to those of suppression. When this was done, the main result was unchanged and indicated that contralateral noise had a greater effect on DPOAE magnitude in the groups with tinnitus and/or low SLT than in the group with no tinnitus and high SLT.
Although the subject groups were close in age, there were still some intergroup differences in mean age and age range. The data were therefore reexamined in two ways taking age into account. The second of these analyses also automatically accounted for the slight differences between groups in high-frequency threshold (≥8 kHz, Fig. 4), because high-frequency threshold was correlated with age. In the first analysis, change in DPOAE magnitude over a narrowed age range was examined (38 ≤ age < 48 yr). As for the full age range, change in DPOAE magnitude over the narrowed range was greater in the tinnitus and/or low-SLT groups than in the no-tinnitus/high-SLT group in f2 bands from 1 to 2.8 kHz. In a second analysis, a correction for age was made to the data covering the full age range of subjects. Based on plots of magnitude change vs. age, correction via linear regression was determined to be appropriate (Fig. 6). Figure 7 shows the age-corrected results. Magnitude change remained greater for the tinnitus and/or low-SLT groups across f2 bands from 1–2.8 kHz, as did the number of f2 values showing significant suppression (Fig. 7, A and B). There was almost no difference in age-corrected hearing threshold between the no-tinnitus/high-SLT group and the combined tinnitus and/or low-SLT group (Fig. 7C). Therefore, neither age nor hearing threshold accounts for the greater magnitude change present, on net, in subjects with tinnitus and/or low SLT.
Fig. 6.
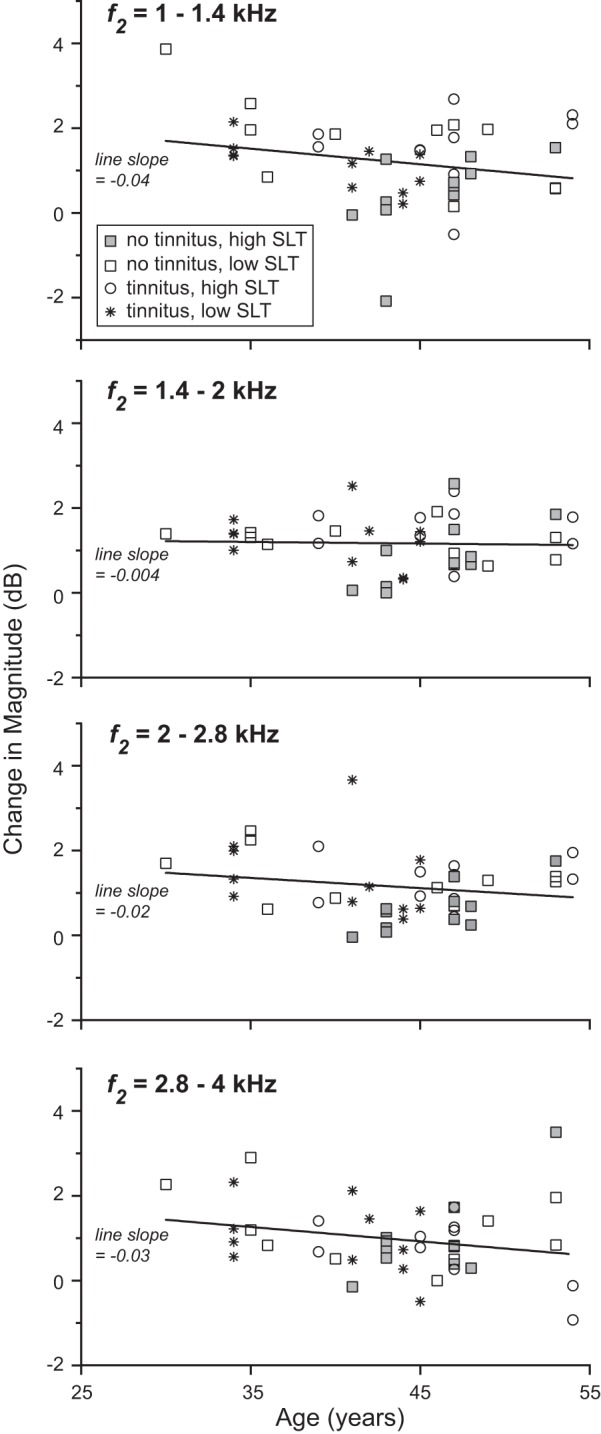
Change in magnitude vs. age in four f2 bands. Each point corresponds to a DPOAE-recorded ear. A linear, least mean-square error fit to the data is shown for each band.
Fig. 7.
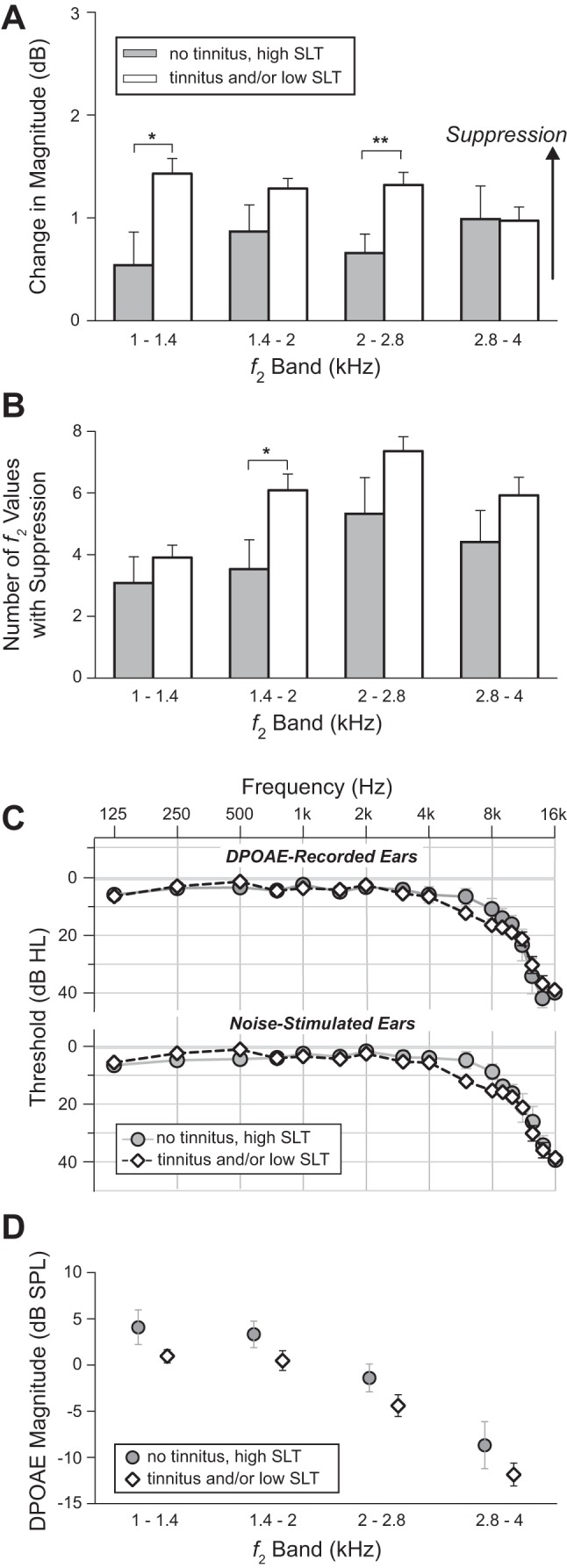
After age correction. A: the change in DPOAE magnitude remains greater in the combined tinnitus and/or low-SLT group compared with the no-tinnitus/high-SLT group. B: the number of f2 values showing suppression also remains greater in the tinnitus and/or low-SLT group. C: mean audiograms for the 2 groups are even more closely matched. D: baseline (no noise) DPOAE magnitude shows no significant difference between groups. *P < 0.05; **P < 0.01 (not corrected for multiple comparisons). Exact P values are as follows: A, 1–1.4 kHz, P = 0.01, uncorrected; A, 2–2.8 kHz, P = 0.004; B, 1.4–2 kHz, P = 0.02.
Age-corrected baseline (no noise) DPOAE magnitude was also compared between the no-tinnitus/high-SLT group and the combined tinnitus and/or low-SLT group (Fig. 7D). The two groups did not differ significantly in any f2 band (P ≥ 0.08, all f2 bands; Mann-Whitney). However, because 1) there was a nonsignificant but nevertheless systematic tendency, across f2 bands, toward greater DPOAE magnitude in the no-tinnitus/high-SLT group, and 2) there was a weak linear relationship between change in magnitude and baseline DPOAE magnitude (decreasing magnitude change with increasing DPOAE magnitude), age-corrected magnitude change was further corrected for DPOAE magnitude via linear regression, as was the number of f2 values showing suppression. The main result again remained: change in magnitude was greater in the combined tinnitus and/or low-SLT group (magnitude change, 2–2.8 kHz: P = 0.02; number of f2 values, 1.4–2 kHz: P = 0.02).
Negligible effect of the stapedial reflex.
Stapedial reflex strength, measured as a change in ear canal tone level elicited by 60-dB contralateral noise, averaged <0.2 dB in any given subject group and f2 band and averaged <0.1 dB in the frequency ranges showing the greatest difference in DPOAE magnitude change across subject groups (f2 = 1–1.4, 2–2.8 kHz). Tone frequencies tested were at approximately half-octave intervals over the frequency range of DPOAE measurement (from 1 through 4 kHz) and also at lower frequencies (515 and 750 Hz), where one would expect the greatest effects of stapedius muscle contraction. These measurements indicate that noise-induced stapedius muscle contractions made little or no contribution to the noise-induced changes in DPOAE magnitude reported in this study.
Test of sensitivity to SLT criterion.
One last pair of analyses was conducted to test the robustness of the main results. These analyses tested for sensitivity to the criterion for grouping ears into low vs. high SLT. A first analysis still used LDL and SLT questionnaire score to divide low from high SLT, but used LDL for a 500-Hz warble tone instead of noise (Fig. 3B). Two ears were reallocated between low and high SLT. Magnitude change remained greater in the group with tinnitus and/or low SLT (1–1.4 kHz, P = 0.05; 1.4–2, P = 0.03; 2–2.8, P = 0.001). A second analysis assigned ears strictly according to noise LDL (without regard to SLT questionnaire score; high SLT:LDL ≥ 105), which moved three ears from low to high SLT. In both analyses, the main results persisted. Magnitude change again remained greater in the group with tinnitus and/or low SLT (1–1.4 kHz, P = 0.04; 2–2.8, P = 0.02).
Comparison with depression, anxiety, and tinnitus variables.
Mean depression and mean anxiety differed across subject groups, but the trend across groups did not parallel that of magnitude change (Table 3).
For the tinnitus subjects, each of the following variables from Table 2 was cross-correlated with magnitude change to test for relationships: minimum masking level, tinnitus loudness, and score on Tinnitus Reaction Questionnaire. There were no significant correlations for any f2 band (minimum masking level: |r| ≤ 0.36, P ≥ 0.13; tinnitus loudness: |r| ≤ 0.44, P ≥ 0.07; Tinnitus Reaction Questionnaire score: |r| ≤ 0.16, P ≥ 0.50; Spearman correlation, P values uncorrected for multiple comparisons).
For most tinnitus subjects (8 of 10), tinnitus pitch exceeded the frequency range over which suppression was measured (Table 2; tinnitus pitch ≥6 kHz). Therefore, increased suppression was not limited to tinnitus frequency(s). In one ear of one of the two subjects with lower tinnitus pitch (subject 110), suppression in the 2- to 2.8-kHz band was greater than in any other subject. Otherwise, however, the other ear of this subject and that of the other subject with low tinnitus pitch (subject 129) showed suppression that was well within the range of the other tinnitus subjects. Thus there was no obvious relationship between tinnitus pitch and the degree to which contralateral suppression was increased in tinnitus subjects.
To test for a relationship between magnitude change and lateralization of the tinnitus percept, an index of magnitude change asymmetry was calculated for all subjects in which both the right and left ears served as a DPOAE-recorded ear, including two subjects with unilateral tinnitus (subjects 128 and 129). The index was calculated as the absolute value of the difference in magnitude change between the right and left ears, divided by the average magnitude change for the two ears. Using the age-corrected magnitude change data, asymmetry indexes were calculated for each subject and half-octave band from 1 to 4 kHz. All but one of the asymmetry indexes for the unilateral tinnitus subjects fell within the range for no-tinnitus and bilateral tinnitus subjects. The only exception was for subject 129 in the 2.8- to 4-kHz band, where the index (3.4) slightly exceeded the maximum for the no-tinnitus/bilateral tinnitus subjects (3.2). Thus the data showed no clear relationship between magnitude change in the right vs. left ear and tinnitus laterality. The lack of asymmetry in magnitude change may reflect the fact that the unilateral tinnitus subjects had low SLT that was equally reflected in the two ears (note similarly low LDLs for the left and right ears of subjects 128 and 129 in Table 1). In other words, the tinnitus percept was asymmetric, but (low) SLT was symmetric.
DISCUSSION
The results demonstrated that, on net, noise delivered to one ear had a greater effect on DPOAE magnitude in the other ear in subjects with tinnitus and/or low SLT. The nature of the effect was suppressive; that is, DPOAE magnitude was reduced with delivery of contralateral noise (contralateral suppression). Importantly, the experimental design included crucial controls that have not generally been incorporated into tests of contralateral suppression in tinnitus. For instance, possible effects of middle ear muscles were tested for and found to be negligible. Additionally, hearing threshold at both clinical and supraclinical (>8 kHz) frequencies was controlled in both the DPOAE-recorded and noise-stimulated ears, as was baseline DPOAE magnitude. Mean threshold, DPOAE magnitude, and also age were highly similar across groups to begin with. (Sex was identical.) Nevertheless, the data were corrected via linear regression for remaining differences to test whether the differences, although small, might still account for the greater contralateral suppression in subject groups with tinnitus and/or low SLT. They did not. The main result, greater contralateral suppression in subjects with tinnitus and in subjects without tinnitus but with low SLT, remained.
Importance of considering SLT and closely matching subject groups.
The present data demonstrate the importance of factoring SLT into the design of any study examining contralateral suppression in tinnitus. If SLT had been ignored, and the two tinnitus groups (high and low SLT) had been compared with the two no-tinnitus groups, little or no difference in contralateral suppression would have been found. An important point is that lowered tolerance of sound was not usually reported spontaneously by the low-SLT subjects of the present study (see also Gu et al. 2010, 2012). Lower tolerance only became apparent through questionnaire responses and LDL measurement. In other words, it is easy to see how low- and high-SLT subjects could have been mixed together in the no-tinnitus groups of previous reports without the investigators realizing it, resulting in little or no difference between tinnitus and no-tinnitus groups. To our knowledge, no previous examination of contralateral suppression and tinnitus has taken SLT into account while also incorporating crucial controls needed to prevent the enhancements in contralateral suppression from being obscured (see following paragraph).
One might expect that at least some previous studies comparing tinnitus and no-tinnitus subjects would have, by chance, included a majority of high-SLT subjects in the no-tinnitus group and thus observed elevated contralateral suppression in the tinnitus group. However, this has not been the case, most likely because of a second issue: inadequate control of hearing threshold, particularly in the noise-stimulated ear. With some exceptions (Attias et al. 1996; Geven et al. 2011; Lind 1996; Paglialonga et al. 2010, 2011; Riga et al. 2007), the previous work leaves room for a systematic offset in hearing threshold between tinnitus subjects and no-tinnitus controls because subjects were screened for clinically normal thresholds without ensuring that mean threshold was matched between tinnitus and no-tinnitus control groups. Screening without matching does not prevent substantial (e.g., 10 dB) threshold differences between groups extending over the entire audiometric frequency range. Given the general tendency for tinnitus and poor hearing to go hand in hand, mean threshold, left unconstrained, is likely to be poorer for any given group of tinnitus subjects with clinically normal thresholds than for a given group of no-tinnitus subjects meeting the same minimum threshold criteria. Systematically poorer thresholds in the noise-stimulated ears of tinnitus subjects would result in lower effective noise stimulation levels, lessening any noise-induced suppression (because suppression decreases with decreasing noise level; Veuillet et al. 1991) and thus counteracting any enhancement of suppression that would have been apparent otherwise. Note that similar logic suggests that some counteraction of enhanced suppression also could have occurred in studies that matched mean threshold for noise-stimulated ears up to 8 kHz, but not beyond, if the noise spectrum as well as significant hearing in the noise-stimulated ears extended to those higher frequencies.
Possible underestimation of MOC hyperresponsiveness in the present study.
Even in the present study, where mean threshold was closely matched across subject groups, effective levels of noise stimulation may have been less in the groups with tinnitus because of suprathreshold diminishment of auditory-nerve responsiveness. This suggestion follows from previous data demonstrating lower ABR wave I amplitude (indicating less sound-evoked auditory-nerve activity) in tinnitus subjects compared with threshold-matched no-tinnitus subjects (Gu et al. 2012; Schaette and McAlpine 2011). If auditory-nerve activity were similarly diminished in the noise-stimulated ears of the present study, the reported levels of contralateral suppression in the tinnitus groups would underrepresent the degree of MOC hyperresponsiveness actually present. In other words, hyperresponsiveness of the MOC system could actually be greater in tinnitus subjects than that reported here.
Possible neural bases for hyperresponsiveness of the MOC system.
The greater DPOAE suppression found in subject groups with tinnitus and/or low SLT (relative to no tinnitus, high SLT) indicates a net hyperresponsiveness of the portion of the MOC system activated by noise stimulation in the present experiments. The exact basis for the measured hyperresponsiveness is unclear but may involve the following: 1) increased responsiveness of MOC interneurons, that is, planar multipolar cells (T stellate cells) of the PVCN, which receive auditory-nerve input from the noise-stimulated ear and provide excitatory input to MOC neurons, which are located in the superior olivary complex (Darrow et al. 2012); 2) increased responsiveness of MOC neurons themselves, as might be mediated by the large, presumably excitatory endings onto these cells that may represent descending inputs from auditory cortex (Brown et al. 2013), 3) increased efficacy of any or all of the synapses in the chain of MOC feedback to the DPOAE-recorded cochlea, including between MOC terminals and outer hair cells.
Some of these bases for hyperresponsiveness might also underlie a secondary observation of the present study, namely, the slight negative correlation between DPOAE magnitude and noise-induced DPOAE suppression. Specifically, if mechanisms leading to increased responsiveness of the MOC system also lead to increased spontaneous activity within the system (hyperactivity), a predicted consequence would be tonic DPOAE suppression. In other words, greater noise-induced DPOAE suppression would co-occur with lower DPOAE magnitude, which is what the present data showed (compare Fig. 7, A and D). Relevant to the proposal of a spontaneously hyperactive MOC system are animal data indicating the development of elevated spontaneous activity in ventral cochlear nucleus unit types following acoustic trauma, a known inducer of tinnitus (Vogler et al. 2011). The elevated activity that develops in onset choppers and transient choppers has particular relevance because there is evidence that both of these unit types (or subgroups thereof) are part of the MOC system, either projecting to MOC neurons (transient choppers, which correspond to T stellate cells) or receiving MOC input (both onset and transient choppers; Darrow et al. 2012; Mulders et al. 2007; Oertel et al. 2011). These animal data demonstrate that hyperactivity can indeed develop within certain elements of the MOC system.
MOC hyperresponsiveness and tinnitus/low SLT: cause-and-effect relationship?
Neither hyperactivity nor hyperresponsiveness of the MOC system provides an obvious way to account for the tinnitus percept or for low SLT as defined in the present study. In contrast to afferent spontaneous hyperactivity, for instance, efferent hyperactivity is not in a position to be carried to more rostral centers for subsequent interpretation as sound in the absence of sound (tinnitus). Also, the frequency range of any hyperactivity, as manifest in reduced DPOAEs, extends well below the tinnitus pitch. Lastly, hyperactivity (or hyperresponsiveness), at first glance, seems to be opposite what would be needed to account for the lowered LDLs of most subjects in the present study's low-SLT group, since it implies greater MOC-mediated reductions in cochlear gain and auditory-nerve activity than would occur normally. Reduced auditory-nerve activity in response to sound in turn implies reduced loudness, not the enhanced loudness that characterizes low SLT.
However, there are many unknowns, so the possibility of a direct role for the MOC system in tinnitus and/or low SLT cannot be dismissed. One major question is how the central auditory system is informed of, and takes into account, MOC-mediated cochlear gain changes. It is known that MOC axons, in addition to projecting to the cochlea, also project to the cochlear nucleus (especially the edges, where they likely terminate on dendrites of T stellate cells), thus providing the central auditory system with a copy of the control signals sent to the outer hair cells (Brown 1993, 2011). A direct indicator of what the outer hair cells actually do (i.e., reduce cochlear gain) may be conveyed centrally by type II auditory-nerve fibers acting in a manner akin to proprioceptive neurons in muscular motor control (Jagger and Housley 2003). Regardless of which neural elements report the action of the outer hair cells to the central auditory system, if the neural elements are damaged or destroyed, there is an opportunity for a mismatch between the actual gain of the cochlea and what the central auditory system thinks the gain is. In the event of a mismatch, there is the potential for sound-evoked (or spontaneous) auditory-nerve input to the brain to be misinterpreted such that the level of sound (and ultimately its loudness) is incorrectly perceived (for example, leading to low SLT) or the level of spontaneous activity is misconstrued to be great enough to come from sound (leading to tinnitus).
Instead of MOC hyperactivity/hyperresponsiveness leading to tinnitus/low SLT, the opposite is equally possible, that tinnitus/low SLT leads to changes in the MOC system. In humans, auditory discrimination, signal detection in noise, and stimulus-counting tasks, for instance, have been reported to result in increased activation of the MOC system (e.g., Mishra and Lutman 2014; Smith et al. 2012). The increase may be mediated by known descending projections: directly from inferior colliculus or auditory cortex to MOC neurons, or indirectly from auditory cortex to cochlear nucleus (Brown et al. 2013b; Mellott et al. 2011; Mulders et al. 2000a, 2000b; Schofield et al. 2011). These same pathways and mechanisms may be responsible for increasing MOC activity in tinnitus and low SLT. Specifically, tinnitus and/or low sound tolerance may heighten arousal or draw attention to the auditory domain, either overtly or covertly, triggering top-down-mediated activity increases in the MOC system.
Ubiquitous brain stem hyperresponsiveness/hyperactivity associated with tinnitus and low sound tolerance: a result of top-down neuromodulation?
The present results add to previous data indicating brain stem hyperresponsiveness and/or hyperactivity associated with tinnitus and/or lowered sound tolerance. Elevated responses to sound in the inferior colliculi of people with low SLT have been demonstrated with functional magnetic resonance imaging (Melcher et al. 2009; Gu et al. 2010). Wave V of the auditory brain stem response, which is generated by pathways originating in the anteroventral cochlear nucleus (AVCN), has been found to be elevated in people with tinnitus compared to those without (Gu et al. 2012). Acoustic startle, also likely mediated through AVCN in humans, is elevated in people with tinnitus (Fournier and Hébert 2013; Knudson and Melcher 2014). There have been numerous reports of elevated spontaneous and sound-driven activity in the dorsal cochlear nucleus (DCN) in animal models of tinnitus (for review, see Kaltenbach and Godfrey 2008). And finally, in the present study, in people with tinnitus and/or low sound tolerance, we found elevated responsiveness of the MOC system, which involves neuronal types in yet another division of the cochlear nucleus, the PVCN. In other words, there is evidence for hyperactivity and/or hyperresponsiveness associated with tinnitus and low sound tolerance in structures ranging from the cochlea (efferent feedback; present study) to inferior colliculus (Bauer et al. 2008; Gu et al. 2010; Vogler et al. 2014) and in neural pathways distributed across every major division of the cochlear nucleus, the source of all ascending signals in the central auditory pathway. We propose that hyperactivity/hyperresponsiveness is ubiquitous in the auditory brain stem of people with tinnitus and/or low sound tolerance.
Prior to the present study, when hyperactivity/hyperresponsiveness had only been demonstrated within the highly plastic, cerebellum-like circuitry of the DCN (Tzounopoulos 2008) and more recently in evoked responses mediated by a subset of neurons of the AVCN (Gu et al. 2012), it seemed plausible that excesses of activity or responsiveness, triggered by cochlear damage, might develop independently in each of the involved neural populations, presumably via different mechanisms given their very different circuitries, and this may be true. However, in our view, the plausibility of this scenario, in which hyperactivity/hyperresponsiveness develops separately in different neuronal pathways, is diminished by the present data, which add another (PVCN mediated) neural system with distinct innervation and neurochemistry to the list of those manifesting overactivation in tinnitus and low sound tolerance. In light of the existing data, it is worth considering a more parsimonious hypothesis, that overactivation of the auditory brain stem arises from forebrain-mediated neuromodulation broadly distributed throughout the brain stem, and for which there is evidence (Schofield et al. 2011; see also Geven et al. 2014; Roberts et al. 2013). We propose that the patterns of activity in ascending auditory brain stem pathways (e.g., spontaneous activity distribution across characteristic frequency) determine what tinnitus will sound like once heard, but for the tinnitus to be heard, those ascending activity patterns must have an attentional spotlight shone on them via top-down neuromodulation.
GRANTS
Support for this work was provided by the Tinnitus Research Consortium and National Institute of Deafness and Other Communications Disorders Grant P30 DC005209.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.K., C.A.S., and J.R.M. conception and design of research; I.M.K. performed experiments; I.M.K. and J.R.M. analyzed data; I.M.K., C.A.S., and J.R.M. interpreted results of experiments; I.M.K. prepared figures; I.M.K., C.A.S., and J.R.M. edited and revised manuscript; I.M.K., C.A.S., and J.R.M. approved final version of manuscript; J.R.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank M. Christian Brown for many helpful discussions and comments on the manuscript, John Guinan, Jr. for suggesting the method used to measure the stapedius muscle reflex and comments on the manuscript, Barbara Norris and Jianwen Wendy Gu for assistance with data taking, and Barbara Norris for assistance with the figures.
REFERENCES
- Abdala C, Mishra SK, Williams TL. Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex. J Acoust Soc Am 125: 1584–1594, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias J, Bresloff I, Furman V. The influence of the efferent auditory system on otoacoustic emissions in noise induced tinnitus: clinical relevance. Acta Otolaryngol (Stockh) 116: 534–539, 1996. [DOI] [PubMed] [Google Scholar]
- Attias J, Zwecker-Lazar I, Nageris B, Keren O, Groswasser Z. Dysfunction of the auditory efferent system in patients with traumatic brain injuries with tinnitus and hyperacusis. J Basic Clin Physiol Pharmacol 16: 117–126, 2005. [DOI] [PubMed] [Google Scholar]
- Baguley DM. Hyperacusis. J R Soc Med 96: 582–585, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res 86: 2564–2578, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571, 1961. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56: 893–897, 1988. [DOI] [PubMed] [Google Scholar]
- Brown MC. Fiber pathways and branching patterns of biocytin-labeled olivocochlear neurons in the mouse brainstem. J Comp Neurol 337: 600–613, 1993. [DOI] [PubMed] [Google Scholar]
- Brown MC. Anatomy of olivocochlear neurons. In: Auditory and Vestibular Efferents. Springer Handbook of Auditory Research 38, edited by Ryugo DK, Fay RR, Popper A. New York: Springer, 2011, p. 17–37. [Google Scholar]
- Brown MC, Mukerji S, Drottar M, Winsor AM, Lee DJ. Identification of inputs to olivocochlear neurons using transneuronal labeling with pseudorabies virus (PRV). J Assoc Res Otolaryngol 14: 703–717, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Lee DJ, Benson TE. Ultrastructure of spines and associated terminals on brainstem neurons controlling auditory input. Brain Res 1516: 1–10, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceranic BJ, Prasher DK, Raglan E, Luxon L. Tinnitus after head injury: evidence from otoacoustic emissions. J Neurol Neurosurg Psychiatry 65: 523–529, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéry-Croze S, Collet L, Morgon A. Medial olivo-cochlear system and tinnitus. Acta Otolaryngol (Stockh) 113: 285–290, 1993. [DOI] [PubMed] [Google Scholar]
- Chéry-Croze S, Truy E, Morgon A. Contralateral suppression of transiently evoked otoacoustic emissions and tinnitus. Br J Audiol 28: 255–266, 1994. [DOI] [PubMed] [Google Scholar]
- Cox RM, Alexander GC, Taylor IM, Gray GA. The contour test of loudness perception. Ear Hear 18: 388–400, 1997. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Benson TE, Brown MC. Planar multipolar cells in the cochlear nucleus project to medial olivocochlear neurons in mouse. J Comp Neurol 520: 1365–1375, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Venecia RK, Liberman MC, Guinan JJ, Jr, Brown MC. Medial olivocochlear reflex interneurons are located in the posteroventral cochlear nucleus: a kainic acid lesion study in guinea pigs. J Comp Neurol 487: 345–360, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favéro ML, Sanchez TG, Bento RF, Nascimento AF. Contralateral suppression of otoacoustic emission in patients with tinnitus. Braz J Otorhinolaryngol 72: 223–226, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes L da Cruz, Santos TM. Tinnitus and normal hearing: a study on the transient otoacoustic emissions suppression. Braz J Otorhinolaryngol 75: 414–419, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P, Hébert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill the gap. Hear Res 295: 16–23, 2013. [DOI] [PubMed] [Google Scholar]
- Geven L, de Kleine E, Free RH, van Dijk P. Contralateral suppression of otoacoustic emissions in tinnitus patients. Otol Neurotol 32: 315–321, 2011. [DOI] [PubMed] [Google Scholar]
- Geven L, Wit HP, de Kleine E, van Dijk P. Wavelet analysis demonstrates no abnormality in contralateral suppression of otoacoustic emissions in tinnitus patients. Hear Res 286: 30–40, 2012. [DOI] [PubMed] [Google Scholar]
- Geven L, Köppl C, de Kleine E, van Dijk P. Plasticity in tinnitus patients: a role for the efferent auditory system? Otol Neurotol 35: 796–802, 2014. [DOI] [PubMed] [Google Scholar]
- Graham RL, Hazell JW. Contralateral suppression of transient evoked otoacoustic emissions: intra-individual variability in tinnitus and normal subjects. Br J Audiol 28: 235–245, 1994. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104: 3361–3370, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, Melcher JR. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol 13: 819–833, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions. Hear Res 33: 97–113, 1988. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27: 589–607, 2006. [DOI] [PubMed] [Google Scholar]
- Hébert S, Fournier P, Norena A. The auditory sensitivity is increased in tinnitus ears. J Neurosci 33: 2356–2364, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse G, Andres R, Schaaf H, Laubert A. DPOAE und laterale Inhibition bei chronischem Tinnitus. HNO 56: 694–700, 2008. [DOI] [PubMed] [Google Scholar]
- Hesse G, Schaaf H, Laubert A. Specific findings in distortion product otoacoustic emissions and growth functions with chronic tinnitus. Int Tinnitus J 11: 6–13, 2005. [PubMed] [Google Scholar]
- Hood LJ, Berlin CI, Hurley A, Cecola RP, Bell B. Contralateral suppression of transient-evoked otoacoustic emissions in humans: intensity effects. Hear Res 101: 113–118, 1996. [DOI] [PubMed] [Google Scholar]
- Horváth M, Ribári O, Répássy G, Tóth IE, Boldogkõi Z, Palkovits M. Intracochlear injection of pseudorabies virus labels descending auditory and monoaminerg projections to olivocochlear cells in guinea pig. Eur J Neurosci 18: 1439–1447, 2003. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Wang PC, Yang TH, Lin TF, Hsu SH, Hsu CJ. Auditory efferent dysfunction in normal-hearing chronic idiopathic tinnitus. B-ENT 9: 101–109, 2013. [PubMed] [Google Scholar]
- Jagger DJ, Housley GD. Membrane properties of type II spiral ganglion neurons identified in a neonatal rat cochlear slice. J Physiol 552: 525–533, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA. Dorsal cochlear nucleus hyperactivity and tinnitus: are they related? Am J Audiol 17: S148–S161, 2008. [DOI] [PubMed] [Google Scholar]
- Knudson IM, Melcher JR. Acoustic startle response in humans with tinnitus and hyperacusis. Assoc Res Otolaryngol Abs 37: 533, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalaki P, Hatzopoulos S, Lorito G, Kochanek K, Sliwa L, Skarzynski H. A connection between the efferent auditory system and noise-induced tinnitus generation. Reduced contralateral suppression of TEOAEs in patients with noise-induced tinnitus. Med Sci Monit 17: MT56–MT62, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res 153: 643–648, 2003. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF. Efferent feedback slows cochlear aging. J Neurosci 34: 4599–4607, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind O. Transient-evoked otoacoustic emissions and contralateral suppression in patients with unilateral tinnitus. Scand Audiol 25: 167–172, 1996. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res 257: 63–74, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott JG, Motts SD, Schofield BR. Multiple origins of cholinergic innervation of the cochlear nucleus. Neuroscience 180: 138–147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Lutman ME. Top-down influences of the medial olivocochlear efferent system in speech reception in noise. PLoS One 9: e85756, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin A, Collet L, Duclaux R. Contralateral auditory stimulation alters acoustic distortion products in humans. Hear Res 65: 193–210, 1993. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Evidence for direct cortical innervation of medial olivocochlear neurones in rats. Hear Res 144: 65–72, 2000a. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Effects on cochlear responses of activation of descending pathways from the inferior colliculus. Hear Res 149: 11–23, 2000b. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Harvey AR, Robertson D. Electrically evoked responses in onset chopper neurons in guinea pig cochlear nucleus. J Neurophysiol 97: 3288–3297, 2007. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wright S, Cao XJ, Ferragamo M, Ramazan B. The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus. Hear Res 276: 61–69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglialonga A, Del Bo L, Ravazzani P, Tognola G. Quantitative analysis of cochlear active mechanisms in tinnitus subjects with normal hearing sensitivity: multiparametric recording of evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx 37: 291–298, 2010. [DOI] [PubMed] [Google Scholar]
- Paglialonga A, Fiocchi S, Del Bo L, Ravazzani P, Tognola G. Quantitative analysis of cochlear active mechanisms in tinnitus subjects with normal hearing sensitivity: time-frequency analysis of transient evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx 38: 33–40, 2011. [DOI] [PubMed] [Google Scholar]
- Perrot X, Collet L. Function and plasticity of the medial olivocochlear system in musicians: a review. Hear Res 308: 27–40, 2014. [DOI] [PubMed] [Google Scholar]
- Riga M, Papadas T, Werner JA, Dalchow CV. A clinical study of the efferent auditory system in patients with normal hearing who have acute tinnitus. Otol Neurotol 28: 185–190, 2007. [DOI] [PubMed] [Google Scholar]
- Riga M, Katotomichelakis M, Danielides V. The potential role of the medial olivocochlear bundle in the generation of tinnitus: controversies and weaknesses in the existing clinical studies. Otol Neurotol. In press. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Husain FT, Eggermont JJ. Role of attention in the generation and modulation of tinnitus. Neurosci Biobehav Rev 37: 1754–1773, 2013. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31: 13452–13457, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Motts SD, Mellott JG. Cholinergic cells of the pontomesencephalic tegmentum: connections with auditory structures from cochlear nucleus to cortex. Hear Res 279: 85–95, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med 123: 711–718, 2010. [DOI] [PubMed] [Google Scholar]
- Smith DW, Aouad RY, Keil A. Cognitive task demands modulate the sensitivity of the human coclea. Front Psychol 3: 30, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord 55: 439–453, 1990. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Posteroventral cochlear nucleus projections to olivocochlear neurons. J Comp Neurol 303: 267–285, 1991. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Bergan C, Preece J, Nagase S. Audiologische Messmethoden de Hyperakusis. In: Hyperakusis 6 (Nelting Med). Stuttgart: Thieme, 2003, p. 39–46. [Google Scholar]
- Tzounopoulos T. Mechanisms of synaptic plasticity in the dorsal cochlear nucleus: plasticity-induced changes that could underlie tinnitus. Am J Audiol 17: S170–S175, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnau D, Tochetto TM. Occurrence and suppression effect of otoacoustic emissions in normal hearing adults with tinnitus and hyperacusis. Braz J Otorhinolaryngol 78: 87–94, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol 65: 724–735, 1991. [DOI] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, Mulders WH. Hyperactivity following unilateral hearing loss in characterized cells in the inferior colliculus. Neuroscience 265: 28–36, 2014. [DOI] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, Mulders WH. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci 31: 6639–6645, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr WB. Olivocochlear and vestibular efferent neurons of the feline brain stem: their location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry. J Comp Neurol 161: 159–182, 1975. [DOI] [PubMed] [Google Scholar]
- Williams DM, Brown AM. The effect of contralateral broad-band noise on acoustic distortion products from the human ear. Hear Res 104: 127–146, 1997. [DOI] [PubMed] [Google Scholar]
- Wilson PH, Henry J, Bowen M, Haralambous G. Tinnitus reaction questionnaire: psychometric properties of a measure of distress associated with tinnitus. J Speech Hear Res 34: 197–201, 1991. [PubMed] [Google Scholar]