Abstract
Nicotinamide phosphoribosyltransferase (NAMPT) has been implicated in neuroprotection against ischemic brain injury, but the mechanism underlying its protective effect remains largely unknown. To further examine the protective effect of NAMPT against ischemic stroke and its potential mechanism of action, we generated a novel neuron-specific NAMPT transgenic mouse line. Transgenic mice and wild-type littermates were subjected to transient occlusion of the middle cerebral artery (MCAO) for 60 minutes. Neuron-specific NAMPT overexpression significantly reduced infarct volume by 65% (P=0.018) and improved long-term neurologic outcomes (P≤0.05) compared with littermates. Interestingly, neuronal overexpression of NAMPT increased the area of myelinated fibers in the striatum and corpus callosum, indicating that NAMPT protects against white matter injury. The mechanism of protection appeared to be through extracellular release of NAMPT. First, NAMPT was secreted into the extracellular medium by primary cortical neurons exposed to ischemia-like oxygen–glucose deprivation (OGD) in vitro. Second, conditioned medium from NAMPT-overexpressing neurons exposed to OGD protected cultured oligodendrocytes from OGD. Third, the protective effects of conditioned medium were abolished by antibody-mediated NAMPT depletion, strongly suggesting that the protective effect is mediated by the extracellular NAMPT released into in the medium. These data suggest a novel neuroprotective role for secreted NAMPT in the protection of white matter after ischemic injury.
Keywords: NAMPT, NAMPT secretion, neuroprotection, oligodendrocytes, transient MCAO, white matter injury
Introduction
Cerebral ischemic injury leads to complex tissue pathology involving both gray matter and white matter (WM). White matter in the telencephalon encompasses the corpus callosum, internal capsule, anterior commissure, and fiber bundles in the striatum, and is mainly involved in sensorimotor signal transduction to and from the cerebral cortex (CTX). Blood flow within WM is relatively less than in gray matter and there is little collateral circulation, especially in deep WM. Oligodendrocytes (OLs) and OL precursor cells play an essential role in myelin formation in WM and are highly sensitive to ischemia.1 Furthermore, WM becomes even more susceptible to ischemia with age.2 Therefore, WM is highly vulnerable to ischemia and often more severely injured than gray matter.3 Indeed, WM is affected in most cases of human stroke, accounting for half of the lesion volume.4 Histologic changes of WM injury (WMI) range from demyelination, death of OLs, and axonal damage and loss, to mild reactive gliosis. Owing to the critical role of WM in neurotransmission, WMI may lead to sensorimotor disruption, neurobehavioral syndromes, and cognitive impairments.5, 6, 7 Despite the essential roles for WM in neural function and its susceptibility to ischemia, WMI has largely been overlooked in animal studies as well as in clinical treatments.8 Thus, complete neuroprotection cannot be truly attained without WM protection, and complete analyses of WMI in models of cerebral ischemia and potential WM protectants are essential.
Nicotinamide phosphoribosyltransferase (NAMPT) converts nicotinamide to nicotinamide mononucleotide, the rate-limiting step in the salvage pathway of nicotinamide adenine dinucleotide (NAD) biosynthesis.9, 10 Nicotinamide adenine dinucleotide is a major source of ATP production and also acts as the substrate for several important molecules, such as the DNA repair enzyme poly-[ADP-ribose]-polymerase 1 (PARP-1) and the transcription regulatory protein sirtuin 1. Nicotinamide phosphoribosyltransferase therefore plays a key role in the regulation of energy metabolism and stress responses. Previous studies indicated that lentivirus-mediated NAMPT overexpression is neuroprotective against ischemic brain injury,11 while genetic deletion of NAMPT exacerbates ischemic brain injury.12 Specifically, Wang and colleagues reported that NAMPT upregulation is part of a natural stress response to ischemic injury and that it protects gray matter through sirtuin 1-dependent modulation of the adenosine monophosphate-activated kinase pathway. These findings show that NAMPT is an endogenous neuroprotective molecule. However, beyond these observations, little is understood about the functions of the NAMPT molecule when overexpressed in the central nervous system (CNS) and the mechanisms underlying its protective effects, particularly in the context of ischemic injury.
In mammals, NAMPT protein can be found in both the intracellular (iNAMPT) and extracellular (eNAMPT) spaces. Whereas the function of iNAMPT as a NAD biosynthetic enzyme has been fully supported, the significance and function of eNAMPT remains unknown. Extracellular NAMPT does not exhibit classic secretory signaling. Instead, it can be secreted into the extracellular space or into the blood via a non-classical secretory mechanism13 by a number of cell types, such as adipocytes, hepatocytes, macrophages, and leukocytes.14, 15, 16 Whether neuronal cells also secrete eNAMPT and its potential function in the CNS remain unknown. In the present study, we observed that transgenic mice overexpressing NAMPT in a neuron-specific manner were highly resistant to focal cerebral ischemic brain damage. Interestingly, WMI was significantly reduced in neuronal NAMPT transgenic (Tg-NAMPT) mice compared with WT littermates. Furthermore, we detected that ischemic neuronal cells secrete NAMPT into the extracellular space and that the conditioned medium from ischemic neurons overexpressing NAMPT is protective against ischemic injury in primary cultured OLs. Finally, recombinant NAMPT protein itself was also protective against ischemia-like insults when added to the medium of primary cultured OLs. These data support a novel role for eNAMPT in protection against ischemic WMI.
Materials and Methods
Generation of Brain-Specific NAMPT Transgenic Mice
Human NAMPT cDNA tagged with hemagglutinin (HA) was PCR-amplified and inserted into pcDNA 3.1 vector (Invitrogen, Grand Island, NY, USA). Cytomegalovirus promoter was substituted with a 4.1 kb Thy-1 promoter (kindly provided by Dr Joshua Sanes, Harvard University). To enhance the expression of NAMPT, the human β-globin intron was then inserted between the Thy-1 promoter and NAMPT cDNA. The resulting construct was linearized and microinjected into zygotes collected from C57BL/6 mice. Microinjected zygotes were implanted into pseudo-pregnant C57BL/6 recipient mice. The microinjection and implantation procedures were performed by the University of Pittsburgh Transgenic Core. After birth, pups were genotyped and backbred with C57BL/6 mice. In all experiments, WT littermates backbred from the same transgenic colonies were used as controls. Only adult male mice (25 to 30 g) were used in the study.
Murine Model of Transient Focal Ischemia
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Focal cerebral ischemia was produced by intraluminal occlusion of the left middle cerebral artery (MCA) with a nylon monofilament suture, as previously described.17 Briefly, 8- to 10-week-old Tg-NAMPT or WT male littermates were anesthetized with 1.5% isoflurane in a 30% O2/70% N2O mixture under spontaneous breathing conditions. Rectal temperature was controlled at 37.0°C±0.5°C with a temperature-regulated heating pad during surgery and MCA occlusion (MCAO). Mean arterial blood pressure was monitored during MCAO through a tail cuff, and arterial blood gas was analyzed 15 minutes after the onset of ischemia. The animals underwent MCAO for 60 minutes and then reperfusion for 72 hours for infarct volume measurement or for 2 weeks for behavioral tests. In all experiments, the experimenter was masked to genotype.
Regional Cerebral Blood Flow Measurement
Changes in regional cerebral blood flow at the surface of the left CTX were recorded using laser Doppler flowmetry (PeriFlux system 5000; Jarfalla, Sweden) with a flexible optical fiber probe. The tip of the probe was affixed with glue on the skull over the core area supplied by the MCA (1 mm posterior and 5 mm lateral from bregma). Regional cerebral blood flow was measured 5 minutes before MCAO (baseline), 5 and 55 minutes during MCAO, and 5 minutes after reperfusion and expressed as a percentage of baseline values.
Measurements of Infarct Volume
At 72 hours after MCAO, brains were removed and the forebrain was sliced into 1 mm thick coronal sections. Sections were stained with 2% 2,3,5-triphenyltetrazolium chloride. Infarct volume was determined using MCID image analysis (Imaging Research, St. Catherine's, Canada). The experimenter was masked to genotype during all measurements.
Neurologic Deficits and Behavioral Tests
Three neurobehavioral tests were performed by an observer masked to the experiments, as previously reported by us.18 First, neurologic deficits were scored on a 0 to 5 scale as follows: no neurologic deficit (0); failure to extend the right forepaw fully (1); circling to the right (2); falling to the right (3); unable to walk spontaneously (4); or dead (5). Second, the corner test was performed on days 0, 1, 3, 5, 7, 10, and 14 after ischemia. A mouse was placed between two angled (30°) boards, facing the corner. Rearing and subsequent turning behavior to either side was recorded. The non-ischemic mouse turns either left or right whereas the ischemic mouse turns preferentially toward the left side. The numbers of left turns were counted in 10 trials. For the third test, the rotarod, animals were placed on an accelerating rotating rod (from 4 to 40 r.p.m. over 300 seconds) and their latency to fall was recorded. Preoperative training was performed for three trials a day for 3 days and the last trial served as preoperative baseline. Postoperative testing was performed on day 1, 3, 5, 7, 10, and 14 postinjury. The data are expressed as the mean duration of time on the rotarod per day as a percentage of the presurgery control value.
Generation of Lentiviral Vectors
Based on our experience, the phosphoglycerate kinase promoter mediates better gene expression than the ubiquitin promoter in neurons. Therefore, we modified the lentiviral shuttle vector FUEW (kindly provided by Dr Carlos Lois from the Massachusetts Institute of Technology) in which green fluorescent protein (GFP) was driven by the ubiquitin promoter and generated two lentiviral vectors: lenti-GFP and lenti-NAMPT. Lenti-GFP was produced by substitution of the ubiquitin promoter in FUEW by the phosphoglycerate kinase promoter. Lenti-NAMPT was produced by substitution of GFP in lenti-GFP with NAMPT cDNA containing the HA tag. Lentiviral vectors were generated by CaPO4 precipitation transfection in 293 cells, concentrated by sucrose ultracentrifugation, and further purified by ion exchange chromatography using Mustang Q Acrodisc Units (PALL Corporation, Port Washington, NY, USA). The titer of the purified lentiviral vector was 1.2 × 109/transfection units/ml.
Generation of NAMPT Protein
His6-tagged NAMPT cDNA was generated by PCR, inserted into pET-30a (Novagen, Darmstadt, Germany), and transformed into BL21 Escherichia coli. Recombinant protein was purified using superflow Ni-NTA agarose column (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions and dialyzed in 300 NaCl containing 10 mmol/l Tris-HCl (pH 8.0).
In Vitro Models of Ischemia-Like Insults
Primary cultures of cortical neurons were prepared from 17 day-old Sprague Dawley rat embryos (Charles River Laboratories, Horsham, PA, USA), as previously described.17 Experiments were conducted at 8 to 10 days in vitro, when cultures consisted primarily of neurons (97%). Cortical neurons were pretreated at 8 days in vitro with 1 × 107 transduction units/ml lenti-NAMPT or lenti-GFP virus for 72 hours, and then subjected to either oxygen–glucose deprivation (OGD) or N-methyl D-aspartate neurotoxicity.
Primary Oligodendrocyte Precursor Cells/Oligodendrocytes Culture and In Vitro Models of Ischemia-Like Insults
Primary OL precursor cell cultures were prepared as previously described.19 Briefly, cerebral cortices from postnatal day 1 to 3 Sprague Dawley rats were dissected and minced. Dissociated cells from 2 to 3 rats were plated in a tissue culture flask coated with poly-L-lysine (Sigma, St Louis, MO, USA). Cell cultures were maintained in DMEM/F12 (Invitrogen) containing 10% fetal bovine serum at 37°C with 5% CO2. Cultures were maintained for 10 days, with a medium change every 3 days. On day 10, flasks were sealed and rotated at 200 r.p.m. at 37°C for 1 hour to shake off microglia. Flasks were then rotated again overnight. On the next morning, the medium containing the detached OL precursor cells was collected and plated on tissue culture dishes coated with Poly-DL-ornithine (Sigma) in basal culture medium containing 10 ng/ml platelet-derived growth factor and 10 ng/ml basic fibroblast growth factor for OL precursor cells. For differentiation experiments, cells were kept in basal culture medium containing 15 nmol/l triiodothyronine and 10 ng/ml ciliary neurotrophic factor for 3 to 7 days.
For the OGD model, OLs were subjected to OGD for 180 minutes, and returned to normal culture media and conditions. To induce alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) excitotoxicity, OLs were challenged with 50 μmol/L of AMPA for 5 minutes and then returned to normal culture media and conditions. Purified recombinant NAMPT was added (5 μg/ml) to primary oligodendrocytic cultures before, during, and after insults. Twenty-four hours after insults, cell death was quantified by Hoechst 33258 (Invitrogen) nuclear staining and lactate dehydrogenase release.
In a parallel study, neurons were transduced with lenti-NAMPT or control lenti-GFP virus and subjected to OGD treatment for 60 minutes. Conditioned medium was collected 24 hours after OGD, coincubated with OLs, and then subjected to OGD treatment for 180 minutes. Cells were incubated for 24 hours with conditioned medium and cell death was analyzed as above. To deplete NAMPT released into culture medium, conditioned medium was incubated with NAMPT antibodies (10 μg/ml) and agarose A/G beads, followed by centrifugation.
Immunofluorescent Staining
After blocking with 5% bovine serum albumin in phosphate-buffered saline for 1 hour, sections were incubated with primary antibodies at 4°C overnight followed by the appropriate secondary antibodies for 1 hour at room temperature. The primary antibodies used in this study include: mouse anti-HA (1:100, Cell Signaling, Danvers, MA, USA), rabbit anti-NAMPT (1:500, Bethyl Laboratories, Montgomery, TX, USA), or mouse anti-NAMPT (1:300, Enzo Life Sciences, Farmingdale, NY, USA), mouse anti-NeuN (1:500, Millipore, Billerica, MA, USA), rabbit anti-GFAP (1:500, Dako, Carpinteria, CA, USA), rabbit anti-Iba1 (1:2000, Wako, Richmond, VA, USA), mouse anti-APC (1:400, Millipore), rat anti-CD31 (1:200, Abcam, Cambridge, MA, USA), and rabbit anti-myelin basic protein (MBP, 1:500, Abcam).
Luxol Fast Blue Stain
Brain sections were deparaffinized with xylene and dehydrated with alcohols, and then immersed in a 0.1% alcoholic solution containing Luxol fast blue (Sigma) at 56°C for 2 hours, followed by washing with distilled water. Sections were then incubated in 0.05% lithium carbonate, dehydrated through graded alcohols, mounted with Permount (Sigma), and examined by light microscopy. The quantitative analysis for remyelination in Luxol fast blue stained section was performed as described previously.20 The area of reduced Luxol fast blue staining was measured and expressed as a percentage of the contralateral hemisphere.
Statistical Analysis
Results are reported as mean±s.e.m. The difference between means was assessed by the Student's t-test for single comparisons or by analysis of variance followed by post hoc Bonferroni–Dunn tests for multiple comparisons. P⩽0.05 was deemed statistically significant.
Results
Neuron-Specific Overexpression of NAMPT is Protective Against Cerebral Ischemic Injury in a Transgenic Mouse Line
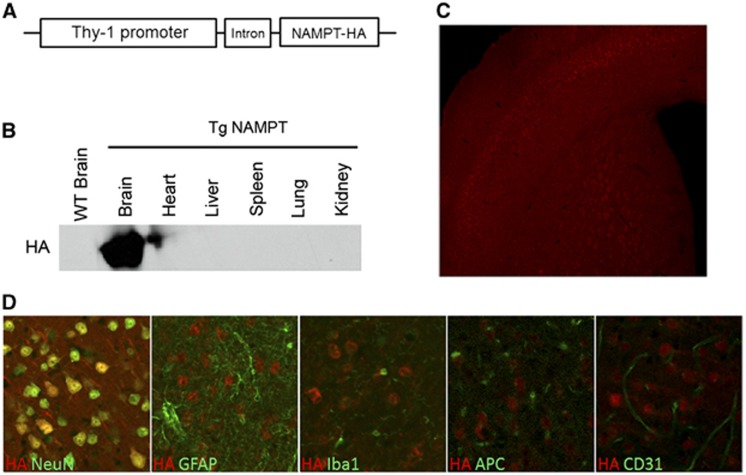
Previous reports indicated that lentiviral-mediated NAMPT overexpression decreases cerebral ischemic infarct size in vivo.11 To better regulate the overexpression of NAMPT for further study in vivo, we developed a mouse line overexpressing the NAMPT transgene. Because NAMPT regulates NAD/ATP production, pan-organ overexpression may complicate the interpretation of its role in the brain. Thus, we decided to overexpress NAMPT specifically within neurons by generating a brain-specific NAMPT transgenic mouse line. Nicotinamide phosphoribosyltransferase cDNA tagged with HA was placed under control of the neuron-specific promoter Thy-1, and an intron was inserted to enhance the expression of NAMPT (Figure 1A). After extensive breeding on a C57BL/6 background, we successfully generated a transgenic mouse line that overexpresses NAMPT exclusively in the brain (Figure 1B). Transgenic NAMPT protein (HA-tagged) was found throughout the whole brain, but with particularly high expression in pyramidal cell layers 5 and 6 in the CTX and within striatal fiber bundles (Figure 1C). Furthermore, the majority of HA-positive cells were colabeled with the neuronal marker NeuN, indicating that the transgene was primarily expressed in neurons (Figure 1D). No expression of HA–NAMPT was detected in other cell types, such as astrocytes, microglia, OLs, and endothelial cells (Figure 1D). No alterations in body weight or blood glucose levels were found in neuron-targeted Tg-NAMPT mice (data not shown), and endogenous NAMPT remained normally expressed in other tissues at similar levels as WT mice (data not shown).
Figure 1.
Generation of neuron-specific nicotinamide phosphoribosyltransferase (NAMPT) transgenic (Tg-NAMPT) mice. (A) Schematic map of NAMPT transgenic construct. (B) Western blotting using HA antibodies shows that transgenic NAMPT–HA is only expressed in the brain of Tg-NAMPT mice. (C) Immunofluorescent staining illustrates that NAMPT is predominantly expressed in the cortex and fiber bundles in the striatum. (D) Immunofluorescent double-labeling for HA tag with the neuronal marker NeuN, the astrocyte marker GFAP, the microglia marker Iba1, the oligodendrocyte marker APC, and the endothelial marker CD31; HA, hemagglutinin.
Using these novel neuron-specific Tg-NAMPT mice, we sought to determine if neuronal overexpression of NAMPT alone would be adequate to confer protection against focal ischemic injury. Neuronal Tg-NAMPT mice and WT littermates were subjected to 60 minutes MCAO and infarct volume and neurologic deficits were assessed 72 hours after reperfusion. Functional outcomes were evaluated by the corner and rotarod tests for 2 weeks. Neuronal NAMPT transgenic overexpression significantly reduced infarct volume by 65% compared with WT littermates (P=0.018, Figures 2A and 2B) at 72 hours after MCAO, suggesting that NAMPT overexpression exerts robust tissue protective effects. Compared with WT littermates, Tg-NAMPT mice also exhibited significantly lower neurologic deficit scores (Figure 2C, P≤0.05), turned less frequently to the left side in the corner test (Figure 2D, P≤0.05), and remained longer on the rotarod test (Figure 2E, P≤0.05). Importantly, the functional improvements lasted for 2 weeks after ischemia, indicating that NAMPT transgenic overexpression provides long-term functional improvement against ischemic brain injury. Physiologic parameters (blood pressure, blood gases, and blood glucose; data not shown) and regional cerebral blood flow (Table 1) were not affected by transgene expression. Together, these data indicate that neuronal targeting of NAMPT is sufficient to confer potent neuroprotection against focal cerebral ischemic injury.
Figure 2.
Nicotinamide phosphoribosyltransferase (NAMPT) overexpression protects against ischemic brain injury. (A) Representative photographs of triphenyltetrazolium chloride (TTC) stained coronal brain sections from NAMPT transgenic (Tg-NAMPT) mice and wild-type (WT) littermates at 72 hours after 60-minute middle cerebral artery occlusion (MCAO). (B) Quantitative measurements of infarct volume. (C) Neurologic deficit scores. (D) Corner test. (E) Rotarod test. Data are mean±s.e.m., n=10/group. *P⩽0.05 versus WT littermates; #P⩽0.05 versus sham.
Table 1. Changes of rCBF before, during, and after MCAO in Tg-NAMPT mice and WT littermates.
| WT | Tg-NAMPT | |
|---|---|---|
| 5 Minutes before MCAO | 100±6.69 | 100±6.64 |
| 5 Minutes during MCAO | 10.95±1.23* | 10.4±1.21* |
| 55 Minutes during MCAO | 11.6±1.25* | 10.5±0.75* |
| 5 Minutes after reperfusion | 57.6±9.5* | 56.5±8.2* |
MCAO, middle cerebral artery occlusion; rCBF, regional cerebral blood flow; Tg-NAMPT, NAMPT transgenic; WT, wild-type. rCBF at the surface of the left cortex at 5 minutes before MCAO, 5 and 55 minutes during MCAO, and 5 minutes after reperfusion. Data represent mean±s.e.m. n=10, *P⩽0.05 versus before MCAO. There is no significant difference in rCBF changes between Tg-NAMPT mice and WT littermates.
Nicotinamide Phosphoribosyltransferase Transgenic Overexpression Attenuates White Matter Injury after Ischemia
Previous studies using lentiviral vectors in vivo or NAMPT deletion have already indicated a neuroprotective role for NAMPT against ischemic injury.11, 12 Thus, the finding that neuronal overexpression of NAMPT was neuroprotective against cerebral ischemic injury was not surprising. Even though the preservation of neurons is beneficial, focal ischemic injury affects both gray matter and WM and unresolved WMI can result in delayed axonal or Wallerian degeneration. Thus, in addition to the gross neural protection afforded by neuronal NAMPT transgenic expression (Figures 2A and 2B), we also examined the state of WM. Focal ischemia significantly reduced myelin expression in striatal fibers and corpus callosum in WT mice 72 hours after reperfusion, as reflected by lighter and smaller areas of Luxol fast blue staining (Figure 3A, top panels and Figure 3B). Notably, neuronal NAMPT transgenic overexpression significantly increased the area of myelin expression after ischemia compared with WT littermates (Figure 3A, bottom panels and Figure 3B). This indicates that WMI was either prevented or repaired in mice overexpressing neuronal NAMPT. Since the NAMPT transgene was constructed to be under the control of the neuron-specific promoter Thy-1, we hypothesized that neuronally expressed NAMPT may be functioning in a novel extracellular manner to protect WM.
Figure 3.
Nicotinamide phosphoribosyltransferase (NAMPT) overexpression attenuates white matter injury (WMI). (A) Representative Luxol fast blue (LFB) staining of contralateral and ipsilateral striatum and corpus callosum (CC) in NAMPT transgenic (Tg-NAMPT) mice and wild-type (WT) littermates. (B) Quantitative analysis of LFB staining. Data are expressed as a percentage of the contralateral hemisphere. *P⩽0.01 versus WT littermates.
Ischemic Injury Induces Nicotinamide Phosphoribosyltransferase Expression and Secretion from Neurons
Extracellular NAMPT has been found both locally in non-CNS systems and circulating systemically in the blood. Extracellular NAMPT can be distinguished from iNAMPT by its larger molecular weight arising from posttranslational modifications.13 Whether the CNS also has eNAMPT and its function in the ischemic brain remains unknown. Both eNAMPT and iNAMPT protein expression was detectable with two different NAMPT antibodies in WT C57BL/6 mice. Although iNAMPT remained unchanged, eNAMPT was dramatically induced at 24 hours after ischemia, peaking at 72 hours but remaining at high levels even after 168 hours (Figure 4A). These findings suggest that eNAMPT is selectively induced after ischemia, perhaps as part of a long-term stress response pathway. Interestingly, NAMPT expression was induced in both neuronal cell bodies and WM regions, including striatal fiber bundles (Figure 4B, top panel) and the corpus callosum (Figure 4B, bottom panel) in WT mice after ischemia.
Figure 4.
Extracellular nicotinamide phosphoribosyltransferase (eNAMPT) is expressed in the brain and induced after ischemia. (A) Western blot analysis of NAMPT expression in the ischemic penumbra after middle cerebral artery occlusion (MCAO) in wild-type (WT) C57BL/6 mice using two different NAMPT antibodies for the intracellular (iNAMPT) and extracellular forms of NAMPT. (B) Immunofluorescent staining of brain slices with antibodies against NAMPT and myelin basic protein (MBP) shows that higher NAMPT level in fiber bundles of the striatum and corpus callosum (CC) in the ipsilateral (Ipsi) hemisphere. CTX: cortex. (C) Enzyme-linked immunosorbent assay (ELISA) for NAMPT release into extracellular medium from primary neurons subjected to oxygen–glucose deprivation (OGD). The ELISA was performed 0, 1, 2, 4, 8, and 24 hours after OGD. Data are mean±s.e.m. from three independent experiments. *P⩽0.05 versus untreated neurons.
To confirm that ischemic neurons are capable of secreting NAMPT protein into the extracellular space, we subjected neuronal cultures to OGD and measured NAMPT levels in the medium using ELISA. Consistent with the in vivo results, we found that the presence of NAMPT was robustly increased in the culture medium, peaking at 4 hours after OGD, and then decreasing gradually, indicating that ischemic neurons are indeed capable of secreting NAMPT into the extracellular medium (Figure 4C). These novel findings suggest that eNAMPT is present in the CNS, and that ischemic neurons are able to secrete eNAMPT into the extracellular space.
Secretion of NAMPT can be Induced by Forced Overexpression in Neurons and Confers WM Protection
Given our in vitro observations, transgenically overexpressed NAMPT may also be secreted into the extracellular space in both CTX and striatum in vivo. Thus, we hypothesized that NAMPT overexpression in neurons may lead to protection against WMI via the heightened secretion of eNAMPT, based on the following observations: (1) ischemia-induced NAMPT protein expression appears fairly diffuse in the corpus callosum (a region with relatively few neuronal cell bodies, Figure 4B), (2) neuronal overexpression of NAMPT exerts ischemic protection of WM in vivo (Figure 3), and (3) ischemic neurons possess the ability to secrete NAMPT in vitro (Figure 4C).
To directly address the extent of NAMPT secretion when overexpressed in neurons, we measured NAMPT levels in the culture medium of primary neurons transduced with either lenti-NAMPT–HA or a control lenti-GFP vector. Forced NAMPT overexpression in neurons significantly increased NAMPT secretion into the medium even without OGD, as detected by ELISA (Figure 5A), as well as by western blot (Figure 5B). Notably, the levels of protein found in the culture medium of transduced neurons exceeded the levels found from ischemic neurons by ∼50-fold. As the endogenous levels of NAMPT secreted by ischemic neurons appear to be insufficient to confer full protection, exogenously delivered NAMPT protein may be necessary to effectively protect the brain against ischemic injury. We also observed that forced overexpression of NAMPT in Tg-NAMPT mice increased the levels of HA-tagged NAMPT within neuronal cell bodies as well as in the extracellular space under non-ischemic conditions (Figure 5C, upper panel). The expression of eNAMPT in the CTX and striatum of Tg-NAMPT mice was confirmed by western blot analysis. In contrast, no eNAMPT was detected in the brain of WT mice (Figure 5C, lower panel).
Figure 5.
Secretion of nicotinamide phosphoribosyltransferase (NAMPT) from neurons can be induced by forced overexpression and confers white matter (WM) protection. (A) Enzyme-linked immunosorbent assay (ELISA) for NAMPT release into medium from primary neurons 72 hours after lentiviral transduction. ***P⩽0.001 versus untreated neurons. (B) Western blot analysis of NAMPT from neurons transduced with lenti-NAMPT. (C) Upper panel: HA staining shows NAMPT secretion into the extracellular space in the cortex (CTX) and striatum (STR) in NAMPT transgenic (Tg-NAMPT) mice, but not in wild-type (WT) littermates. Yellow arrows showing extracellular HA staining and white arrows showing HA staining in striatal fiber bundles. Lower panel: Western blot analysis of extracellular NAMPT (eNAMPT) and intracellular NAMPT (iNAMPT) in both CTX and STR in Tg-NAMPT and WT mice. (D and E) Oligodendrocytic death induced by oxygen–glucose deprivation (OGD) in the presence or absence of conditioned media (CM) from neurons transduced with lenti-NAMPT. Conditioned media from neurons transfected with lenti-GFP served as a control. Secreted NAMPT in CM was depleted by specific NAMPT antibody. Cell death was analyzed 24 hours later by Hoechst staining, D or LDH release, E. Data are mean±s.e.m. from three independent experiments. *P⩽0.05 versus OGD alone/CM from lenti-GFP. CC, corpus callosum; GFP, green fluorescent protein; HA, hemagglutinin.
Because the presence of eNAMPT in transgenic mice was correlated with WM protection in vivo, we sought to directly assess if the protective effects of neuronal NAMPT overexpression were because of neuronal secretions or because of indirect effects stemming from neuronal protection. In the latter scenario, the WM protection might be secondary to direct protection of NAMPT-overexpressing neurons and independent of NAMPT secretion. To assess whether NAMPT overexpression in neurons directly leads to OL protection against ischemia-like insults via extracellular factors, cultured cortical neurons were transduced with lenti-NAMPT or a control lenti-GFP vector and subjected to OGD for 60 minutes. Twenty-four hours after OGD, conditioned medium was collected from the ischemic neuronal cultures and coincubated with mature oligodendrocytic cultures that have been subjected to OGD for 3 hours. As expected, conditioned medium from ischemic neuronal cultures transduced with lenti-NAMPT, but not lenti-GFP, significantly inhibited OGD-induced OL cell death (Figures 5D and 5E). However, the protective effects of conditioned medium were abolished by antibody-mediated NAMPT depletion, strongly suggesting that the protective effect is mediated specifically by NAMPT protein secreted into the medium.
To more directly assess the role of eNAMPT on WM ischemic protection, we incubated primary oligodendrocytic cultures with recombinant NAMPT protein (5 μg/ml) before, during, and after 180 minutes of OGD. The addition of recombinant NAMPT protein significantly inhibited ischemic oligodendrocytic cell death at 24 hours after OGD (Figure 6A). Similarly, recombinant NAMPT protein reduced oligodendrocytic cell death induced by another ischemia-like insult, AMPA neurotoxicity (Figure 6B), suggesting that NAMPT delivered into the extracellular compartment—either via secretion by neurons or via exogenous administration of recombinant protein—effectively confers oligodendrocytic protection against ischemia-like insults.
Figure 6.
Exogenous nicotinamide phosphoribosyltransferase (NAMPT) protein reduces oxygen–glucose deprivation (OGD)- and alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA)-induced oligodendrocytic death. Primary oligodendrocytes (OLs) were pretreated with NAMPT protein (5 μg/ml) and then subjected to OGD for 180 minutes (A) or AMPA (50 μmol/L) for 5 minutes (B). Cell death was analyzed by Hoechst staining or lactate dehydrogenase (LDH) release 24 hours later. Data are mean±s.e.m. from three independent experiments. *P⩽0.05 versus OGD/AMPA alone; #P⩽0.05 versus untreated control cells.
Together, these observations indicate that neuronal overexpression of NAMPT leads to secretion of NAMPT into the extracellular space, which directly protects WM independent of the presence of neurons. As addition of NAMPT antibodies significantly reduced the protective effects of conditioned medium and the sole addition of recombinant NAMPT to oligodendrocytic cultures was sufficient to confer protection, we conclude that eNAMPT is responsible for WM protection against ischemic injury.
Discussion
The role of NAMPT in both non-CNS and neural systems has focused primarily on its intracellular function in NAD biosynthesis. Although this function is extremely important in ischemic contexts, we have discovered a new role for NAMPT in the present report. We are the first group to show that NAMPT confers protection against ischemic WMI in vivo. Previous reports in stroke models had only showed protection of gray matter by NAMPT. The second novel finding of the present study is that neuronal overexpression of NAMPT leads to its secretion into the extracellular space after ischemia-like insults. Third, our in vitro data strongly suggest that secreted, eNAMPT can protect OLs from ischemia-like insults. Fourth, delivery of exogenous NAMPT protein into the culture medium is sufficient to protect OLs against ischemia-like insults. The potential for NAMPT to function in an extracellular manner leads to the exciting possibility of using the recombinant protein in a therapeutic setting. In considering its translational value, it is also important to note that NAMPT can cross the blood–brain barrier (unpublished findings). Thus, recombinant proteins such as NAMPT represent a clinically feasible tool that can be generated and delivered under controlled settings.
In our transgenic mouse model, NAMPT was driven by the Thy-1 promoter, which has been widely used for neuronal overexpression of transgenes. We did not observe the presence of HA-tagged NAMPT within non-neuronal cells in the mouse brain (Figure 1D), although Thy-1 has been reported to be expressed in endothelial cells and in white blood cells such as T lymphocytes,21, 22 two cell types that are intimately involved in the pathogenesis of ischemic stroke. However, T-cell infiltration into the brain usually occurs 3 days after cerebral ischemia,23 whereas NAMPT already exerted WM protection effect within this timeframe in our studies. Therefore, it is unlikely that NAMPT functions through or is affected by NAMPT expression in T cells, at least not at the early time points observed in this study.
Nicotinamide phosphoribosyltransferase has been best characterized in terms of its function as the rate-limiting enzyme in the NAD biosynthetic pathway. As the availability of NAD is a critical determinant for neuronal ischemic sensitivity, the neuroprotective function of NAMPT has historically centered on the role of iNAMPT in NAD production. Consistent with the NAD biosynthetic function of NAMPT, inhibition of enzymatic activity of NAMPT by intraperitoneal administration of FK866 significantly reduced brain nicotinamide mononucleotide levels and substantially enlarged MCAO-induced infarction.11 NAMPT has also been shown to protect against stroke by increasing the activity of sirtuin 1 through an increase in NAD.11 However, our data suggest that a novel, extracellular function of NAMPT may also contribute to ischemic WM protection. On the other hand, NAMPT protection of WM could be distinct from NAD-dependent protection, especially given its extracellular localization. The function of NAMPT in the extracellular milieu could be either via NAD biosynthesis in the extracellular compartment, or via an unknown capacity, such as binding to a receptor and activating pro-survival signaling pathways. In line with this, we have found that incubation of neurons/OLs with recombinant NAMPT protein can activate several pro-survival signaling pathways, such as PI3K/Akt, ERK1/2, and Stat5a (unpublished data), indicating that these molecules might mediate the protective role of NAMPT. In other words, it seems likely that NAMPT binds to an unrecognized receptor on cell membranes and then exerts its protective effect via downstream pro-survival signaling pathways. We are currently attempting to identify the potential receptor for NAMPT on neurons/OLs. Further exploration into the mechanism of NAMPT and its protection of ischemic WMI may yield novel insights into both the function of NAMPT and the process of WMI under ischemic settings.
In addition to its function in NAD synthesis, NAMPT also has been characterized as a putative cytokine that can promote inflammation in peripheral systems. Nicotinamide phosphoribosyltransferase, also called pre-B-cell colony-enhancing factor 1, was first isolated from a human peripheral blood lymphocyte cDNA library and was shown to synergize with the pre-B-cell colony formation activity of stem cell factor and interleukin 7.21 Nicotinamide phosphoribosyltransferase also regulates the activity of monocytes and neutrophils in response to inflammatory stimuli.22, 23 The role of inflammation is well established in cerebral ischemic research and appears to exert a powerful influence over brain injury and recovery. As such, the acute overactivation of inflammatory processes likely contributes to the injured state, whereas a more subdued prolonged inflammatory response appears to assist in neural remodeling and repair, for which WM is critical. Our finding that NAMPT is secreted into the ischemic brain leads us to speculate that eNAMPT influences not only OLs, but also cells such as microglia, the resident inflammatory cells in the CNS, and contributes to the regulation of inflammatory processes after ischemia (data not shown). Further studies examining the role of exogenous NAMPT on inflammatory processes with longer survival periods may yield additional new insights into the effects of NAMPT on ischemic recovery. In addition to inflammation, oxidative stress has also been shown to propagate injury after ischemia. A recent study showed that NAMPT is essential for the benefits of calorie restriction against oxidative stress,24 indicating that eNAMPT may also exert antioxidant effects. Thus, future studies to examine whether eNAMPT protects OLs by inhibiting oxidative stress are highly warranted.
As mentioned above, the significant contribution of WM to ischemic outcomes has been historically overlooked, and largely assumed to be secondary to neuronal or axonal injury. However, recent work underscores a unique role for WM health in determining ischemic severity. Several drugs that protect against ischemic injury are now being explored in their effects on WM, including the only FDA (Food and Drug Administration) approved therapeutic, tissue plasminogen activator2 as well as delayed administration of the histone deacetylase inhibitor valproic acid.25 Similar to the novel means (secretion) by which NAMPT appears to promote WM integrity, tissue plasminogen activator may also promote WM protection and recovery independent of its classic proteolytic activity, acting instead as a novel cytokine-like molecule. Although it is tempting to speculate that NAMPT may exert WM protection via its NAD biosynthetic activity, the mechanisms of WMI and recovery after cerebral ischemia are not well understood and may respond to NAMPT in a novel manner. Further exploration into both the process by which WM responds to ischemic injury as well as the mechanism of WM protection by NAMPT will serve to further the development of effective therapeutics for stroke recovery.
Several animal models, such as ET-1 injection and chronic hypoperfusion models,26, 27 have been developed to specifically examine WMI after stroke and do not involve significant gray matter injury. However, our initial findings that neuronal NAMPT confers ischemic WM protection and that neurons readily release NAMPT in vitro under ischemic contexts led us to hypothesize that NAMPT is released from damaged neurons and subsequently protects OLs from ischemic insults. Therefore, the classic tMCAO model, which involves both gray matter and WM damage, is more suitable for this study. Further investigation on the role of NAMPT in diffuse WMI states will be a logical subsequent extension of the current study.
In conclusion, the present study shows that NAMPT may not function simply in a restricted intracellular manner during stroke recovery and that it protects against ischemic injury in both gray matter and WM. We have presented multiple lines of evidence supporting the extracellular secretion of NAMPT by neuronal cells either overexpressing transgenic NAMPT or subjected to ischemic insults. This secretion is correlated with the protection of OLs from ischemic injury. Furthermore, the addition of recombinant NAMPT itself is protective against ischemic WMI. These findings promote the concept that NAMPT is an endogenous protective molecule that may circulate throughout neural tissue and exert beneficial effects on a variety of cell types, likely via distinct mechanisms. The increase of NAMPT in ischemic tissue from non-transgenic mice also supports the notion that NAMPT is part of a natural stress response, consistent with previous reports.11 Thus, the preservation and recovery of WM by molecules such as NAMPT may lead to improvements in axonal repair and function and promote long-term behavioral recovery after cerebral ischemia.
Acknowledgments
The authors thank Carol Culver for editorial assistance, Pat Strickler for secretarial support, Dr Joshua Sanes from Harvard University for providing Thy-1 promoter, and Dr Carlos Lois from Massachusetts Institute of Technology for providing the lentivirus system.
The authors declare no conflict of interest.
Footnotes
This project was supported by National Institutes of Health/NINDS grants NS079345, (to GC), VA Merit Review grants RX000199 and BX002346 (to GC), and Chinese Natural Science Foundation grants 81371306 (to Yanqin Gao).
References
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, et al. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA.Cerebral white matter is highly vulnerable to ischemia Stroke 1996271641–1646.discussion 1647. [DOI] [PubMed] [Google Scholar]
- Ho PW, Reutens DC, Phan TG, Wright PM, Markus R, Indra I, et al. Is white matter involved in patients entered into typical trials of neuroprotection. Stroke. 2005;36:2742–2744. doi: 10.1161/01.STR.0000189748.52500.a7. [DOI] [PubMed] [Google Scholar]
- Desmond DW. Cognition and white matter lesions. Cerebrovasc Dis. 2002;13 (Suppl 2:53–57. doi: 10.1159/000049151. [DOI] [PubMed] [Google Scholar]
- Leys D, Englund E, Del Ser T, Inzitari D, Fazekas F, Bornstein N, et al. White matter changes in stroke patients. Relationship with stroke subtype and outcome. Eur Neurol. 1999;42:67–75. doi: 10.1159/000069414. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Leys D, Fazekas F, Longstreth WT, Jr., Inzitari D, Wallin A, et al. Role of white matter lesions in cognitive impairment of vascular origin. Alzheimer Dis Assoc Disord. 1999;13 (Suppl 3:S49–S54. [PubMed] [Google Scholar]
- Arai K, Lo EH. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp Transl Stroke Med. 2009;1:6. doi: 10.1186/2040-7378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S. The NAD World: a new systemic regulatory network for metabolism and aging—Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S.Enzymology of NAD+ synthesis Adv Enzymol Relat Areas Mol Biol 199973135–182.xi. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Tian WW, Viollet B, Rui YC, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, et al. Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1962–1971. doi: 10.1038/jcbfm.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- Friebe D, Neef M, Kratzsch J, Erbs S, Dittrich K, Garten A, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54:1200–1211. doi: 10.1007/s00125-010-2042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Petzold S, Barnikol-Oettler A, Korner A, Thasler WE, Kratzsch J, et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem Biophys Res Commun. 2010;391:376–381. doi: 10.1016/j.bbrc.2009.11.066. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Xing J, Jing Z, Stetler RA, Zhang F, Luo Y, et al. Mutant erythropoietin without erythropoietic activity is neuroprotective against ischemic brain injury. Stroke. 2012;43:3071–3077. doi: 10.1161/STROKEAHA.112.663120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, et al. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–1051. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- Schubert K, Gutknecht D, Koberle M, Anderegg U, Saalbach A. Melanoma cells use Thy-1 (CD90) on endothelial cells for metastasis formation. Am J Pathol. 2013;182:266–276. doi: 10.1016/j.ajpath.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Ades EW, Zwerner RK, Acton RT, Balch CM. Isolation and partial characterization of the human homologue of Thy-1. J Exp Med. 1980;151:400–406. doi: 10.1084/jem.151.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Song J, Ke SF, Zhou CC, Zhang SL, Guan YF, Xu TY, et al. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J Gerontol A Biol Sci Med Sci. 2014;69:44–57. doi: 10.1093/gerona/glt122. [DOI] [PubMed] [Google Scholar]
- Schilling E, Wehrhahn J, Klein C, Raulien N, Ceglarek U, Hauschildt S. Inhibition of nicotinamide phosphoribosyltransferase modifies LPS-induced inflammatory responses of human monocytes. Innate Immun. 2012;18:518–530. doi: 10.1177/1753425911423853. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. An animal model of capsular infarct: endothelin-1 injections in the rat. Behav Brain Res. 2006;169:206–211. doi: 10.1016/j.bbr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]