Abstract
Traumatic brain injury (TBI)-induced elevated intracranial pressure (ICP) is correlated with ensuing morbidity/mortality in humans. This relationship is assumed to rely mostly on the recognition that extremely elevated ICP either indicates hematoma/contusions capable of precipitating herniation or alters cerebral perfusion pressure (CPP), which precipitates global ischemia. However, whether subischemic levels of elevated ICP without hematoma/contusion contribute to increased morbidity/mortality remains unknown. To address this knowledge gap, we utilized a model of moderate diffuse TBI in rats followed by either intraventricular ICP monitoring or manual ICP elevation to 20 mm Hg, in which CPP was above ischemic levels. The effects of ICP elevation after TBI on acute and chronic histopathology, as well as on behavioral morbidity, were evaluated. ICP elevation after TBI resulted in increased acute neuronal membrane perturbation and was also associated with reduced neuronal density at 4 weeks after injury. Somatosensory hypersensitivity was exacerbated by ICP elevation and was correlated to the observed neuronal loss. In conclusion, this study indicates that morbidity and increased neuronal damage/death associated with elevated ICP can occur without concurrent global ischemia. Therefore, understanding the pathologies associated with subischemic levels of elevated ICP could lead to the development of better therapeutic strategies for the treatment and management of TBI patients.
Keywords: behavioral morbidity, histopathology, intracranial pressure, membrane poration, traumatic brain injury
Introduction
Traumatic brain injury (TBI) remains a significant healthcare problem resulting in billions of dollars spent annually and innumerable physical and/or emotional consequences owing to the mortality and chronic morbidity.1 A major contributing factor of TBI-associated morbidity and negative outcomes in the human population is elevated intracranial pressure (ICP) to levels above 20 mm Hg.2, 3, 4 The most well-studied causes of elevated ICP-related brain damage after TBI are hematoma/contusion expansion and reduced blood flow to the brain.5 While experimental studies of hematoma and contusional TBI utilizing decompressive craniectomies showed reduced ICP and brain pathology,6, 7, 8 the Decompressive Craniectomy trial demonstrated low efficacy of this aggressive therapy on diffuse TBI.9 The findings of this level I clinical trial highlight the lack of knowledge regarding the ICP-mediated pathologies associated with diffuse TBI. In addition, despite the well-known relationship between elevated ICP and ensuing global ischemia, due to reduction in cerebral perfusion pressure (CPP10, 11, 12) little is known about the consequences of more modest ICP elevations, to the clinical threshold of 20 mm Hg, independent of potential global ischemia. These questions assume greater clinical relevance in light of recent debates regarding the potential risks of ICP monitoring after TBI.13, 14, 15
Several experimental studies have shown that mechanical forces associated with TBI precipitate membrane perturbation that is potentially associated with cell death as well as acute changes in circuitry/cell excitability.16, 17, 18, 19, 20, 21 We have recently demonstrated that neuronal membrane perturbation in layers V and VI of the somatosensory cortex of rats was exacerbated acutely in the face of naturally elevated ICP after TBI.22 However, whether such exacerbated membrane perturbation results in increased TBI-associated morbidity over time remains unknown. Moreover, we cannot exclude the possibility that the exacerbated membrane perturbation and elevated ICP observed in our previous model occurred independently.
In the current communication, we explore the acute and chronic consequences of modestly elevated ICP after TBI utilizing a model of moderate diffuse TBI in rats, followed by either routine intraventricular ICP monitoring alone or manual ICP elevation to 20 mm Hg, at which level, the CPP remained above ischemic levels (50 mm Hg).23, 24, 25 Acute neuronal pathology was investigated via analysis of axonal injury and neuronal membrane perturbation within layers V and VI of the somatosensory cortex 6 hours after TBI. The potential for chronic neuronal loss in this cortical region was also evaluated 4 weeks after injury. Finally, behavioral morbidity, in the form of somatosensory hypersensitivity, was assessed using the whisker nuisance task (WNT), as changes in sensitivity to whisker stimulation are associated with damage in the somatosensory cortex.26, 27 Through these multifaceted approaches, modest elevations of ICP were found to result in exacerbated acute neuronal membrane perturbation and reduced neuronal density chronically after TBI. Behavioral morbidity was also exacerbated with ICP elevation in a fashion that correlated to the reduction in neuronal density.
Materials and Methods
Animals
Experiments were conducted in accordance with the Virginia Commonwealth University institutional ethical guidelines concerning the care and use of laboratory animals (Institutional Animal Care and Use Committee, Virginia Commonwealth University), which adhere to regulations including, but not limited to, those set forth in the “Guide for the Care and Use of Laboratory Animals: 8th Edition” (National Research Council). Thirty adult (12 to 16-week-old) male Sprague–Dawley rats weighing 350 to 450 g were used for this study. Animals were housed in individual cages on a 12-hour light–dark cycle, with free access to food and water. To investigate the pathologic and behavioral effects of ICP elevation after diffuse moderate TBI, animals were randomly divided into one of three injury groups: sham, TBI alone, or TBI+ICP elevation, with the animals killed and histologically evaluated at either 6 hours or 4 weeks. The experimental paradigm utilized is depicted in Figure 1.
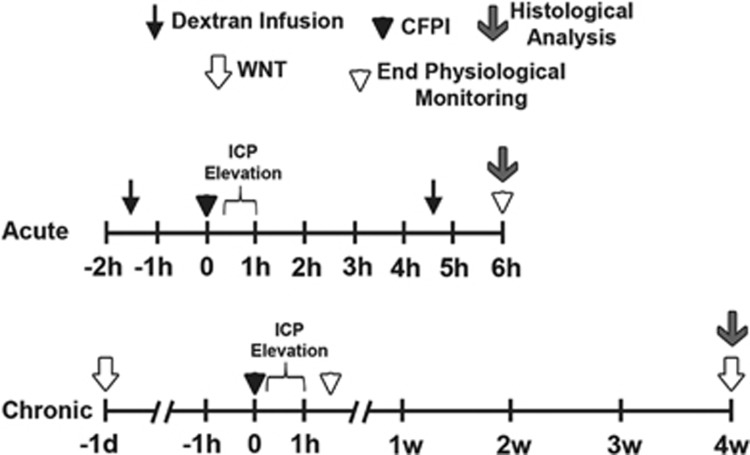
Figure 1.
Schematic representation of the timelines for the experimental procedures used in this study. Top timeline represents acute studies whereas the bottom timeline refers to chronic procedures. A cFPI (black arrowhead) was used to induce a moderate diffuse traumatic brain injury (TBI) that was followed in a subset of animals by manual intracranial pressure (ICP) elevation via intraventricular infusion of sterile saline 15 minutes to 1 hour after injury. Control animals were subjected to the same procedures except for the cFPI and ICP elevation (manually induced changes in ICP were only observed after injury, data not shown). For all conditions, animals were maintained under anesthesia throughout the duration of physiologic monitoring, until they were either transcardially perfused for histologic analysis (acute procedures; gray arrow) or allowed to recover from anesthesia (chronic procedures; white arrowhead). Note that infusions of different fluorescently conjugated dextrans (black arrow) took place both before and 4.5 hours after cFPI/sham injuries in the acute studies. Similarly, the whisker nuisance task (WNT; white arrows) was assessed 1 day before and 4 weeks after TBI or sham injury in the chronic studies.
Surgical Preparation and Injury Induction
Animals were intubated and ventilated with 1.5% to 2.5% isoflurane, 30% O2, and 70% N2O throughout the duration of the surgery and physiologic monitoring. Body temperature was maintained at 37 °C with a rectal thermometer connected to a feedback-controlled heating pad (Harvard Apparatus, Holliston, MA, USA). For acute survival animals (6 hours), a PE50 polyethylene tube connected to a closed pressure system (Becton Dickinson, Franklin Lakes, NJ, USA) was placed into the right femoral artery to monitor the mean arterial blood pressure (MABP) before and for 6 hours after injury. All animals were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). A midline incision was made between the bregma and lambda, and a 4.8 mm diameter circular craniotomy was made along the sagittal suture midway between the bregma and lambda for injury induction. A 2 mm diameter burr hole was also drilled into the left parietal bone overlying the left lateral ventricle (0.8 mm posterior, 1.3 mm lateral, and 2.5 to 3 mm ventral relative to bregma) through which a 25-gauge needle, connected to a pressure transducer and a micro infusion pump 11 Elite syringe pump (Harvard Apparatus) via PE50 tubing, was placed into the left ventricle. Appropriate placement was verified via a 3 μl/minute infusion of sterile saline within the closed fluid pressure system during needle placement.22, 28 The needle was held in the lateral ventricle for at least 5 minutes to record preinjury ICP, then the needle was slowly removed. Bone wax was used to seal the burr hole used for the ICP measurements before preparation for sham or central fluid percussion injury (cFPI). The procedures used to induce cFPI were consistent with those described previously.29 Briefly, a Luer-Loc syringe hub was affixed to the craniotomy site with dental acrylic (methyl methacrylate; Hygenic, Akron, OH, USA) that was applied around the hub, including the area overlying the sealed burr hole. Animals were removed from the stereotaxic frame and injured at a magnitude of 2.05±0.05 atmospheres with a pressure pulse measured by a transducer affixed to the injury device and displayed on an oscilloscope (Tektronix, Beaverton, OR, USA). Immediately after the injury, animals were reconnected to the ventilator and physiologic monitoring devices. The hub, dental acrylic, and bone wax were removed en bloc and Gelfoam was placed over the craniotomy/injury site. The animal was then replaced in the stereotaxic device, and the ICP probe was reinserted into the lateral ventricle, as described above, for postinjury ICP monitoring. Identical surgical procedures were followed for sham-injured animals, without the release of the pendulum to induce the injury. For animals sustaining TBI followed by ICP elevation, ICP was manually elevated to 20 mm Hg via infusion of sterile normal saline at 3 to 10 μl/minute (typically <40 μl used) using the micro infusion Pump 11 Elite syringe pump controlled by the experimenter. Once 20 mm Hg was achieved, ICP was maintained for 1 hour after injury (Figure 1). After initial ICP elevation, the majority of animals did not require supplemental saline infusion. Manually induced changes in ICP were only achievable after TBI (data not shown). Animals surviving chronically (4 weeks) were allowed to recover 30 minutes after ICP monitoring only or ICP elevation and then were returned to clean home-cages (Figure 1).
Tracer Infusion
In the acute (6 hours) survival animals, two Alexa-conjugated dextrans were infused into the lateral ventricle as described previously.22 Briefly, 15 μl of 10 kDa dextran conjugated to either Alexa Fluor (AF) 568 or 488 (40 mg/ml in 0.9% sterile saline; 2 mg/kg; Life Technologies, CA, USA) was infused into the left lateral ventricle at 0.5 to 2 μl/minute, with continuous ICP monitoring, before injury preparation (Figure 1). Half of the animals were infused with AF 568 dextran and half were infused with AF 488 dextran preinjury to avoid bias caused by differences in fluorescent signal detectability. The needle remained in the ventricle for 20 minutes after completion of the tracer infusion. It was then slowly removed and the animal was prepared for sham, TBI alone, or TBI+ICP elevation, as described above. Four and a half hours after injury, the ICP needle was reinserted into the left lateral ventricle and AF 568-conjugated or AF 488-conjugated 10 kDa dextran (opposite of the dextran infused before injury) was infused in the same fashion as the preinjury dextran (Figure 1). The tracer was allowed to diffuse for an additional hour before transcardial perfusion at 6 hours after sham or cFPI.
Physiologic Assessment
Heart rate, respiratory rate, and hemoglobin oxygen saturation were monitored via a hindpaw pulse oximetry sensor (STARR Life Sciences, Oakmont, PA, USA) for the duration of anesthesia, except during the induction of injury. Intracranial pressure was measured intraventricularly, as described above. In acutely surviving animals (6 hours), the femoral artery was cannulated for MABP monitoring and blood gas analysis (Stat Profile pHOx, Nova Biomedical, Waltham, MA, USA), which was performed before killing to confirm the ongoing physiologic measurements. All physiologic measurements (MABP, ICP, heart rate, hemoglobin oxygen saturation) were recorded using a PowerLab System (AD Instruments, Colorado Springs, CO, USA). Cerebral perfusion pressure was determined by subtracting the ICP from the MABP. All animals maintained physiologic homeostasis (i.e., MABP>60 mm Hg, CPP>50 mm Hg, oxygenation>90%, heart rate>200 b.p.m.; Table 1). To minimize stress and obviate any concern that femoral ligation could negatively influence behavioral outcomes, a femoral artery cannula was not placed and MABP was not measured in animals within the chronic survival group. Intracranial pressure and noninvasive systemic physiologic monitoring, however, were performed during surgery and for 1.5 hours after cFPI or sham injury in animals within the chronic survival groups (Figure 1).
Table 1. Physiologic parameters.
| Parameter | Group | Preinjury | Postinjury |
|---|---|---|---|
| Heart rate (BPM) | Sham | 380.70±22.63 | 362.98±12.09 |
| TBI | 337.93±12.68 | 350.43±11.27 | |
| TBI+ICP elevation | 357.72±5.95 | 365.02±7.44 | |
| Hemoglobin O2 (%) | Sham | 97.64±0.42 | 97.42±0.29 |
| TBI | 97.97±0.14 | 97.66±0.15 | |
| TBI+ICP elevation | 97.77±0.18 | 97.21±0.32 | |
| CPP (mm Hg) | Sham | 73.10±1.04 | 80.70±4.87 |
| TBI | 74.17±1.54 | 73.99±1.56 | |
| TBI+ICP elevation | 73.03±2.53 | 67.75±2.61a | |
| MABP (mm Hg) | Sham | 84.60±1.18 | 89.05±4.41 |
| TBI | 83.70±1.74 | 86.86±1.08 | |
| TBI+ICP elevation | 82.39±2.21 | 87.15±1.57 |
BPM, beats per minute; CPP, cerebral perfusion pressure; ICP, intracranial pressure; MABP, mean arterial blood pressure; TBI, traumatic brain injury.
Physiologic measurements averaged for 1 hour before and up to 6 hours after sham injury, TBI alone, or TBI+ICP elevation.
Significant differences from sham at the same measurement point (either pre or postinjury). Sham, n=3 animals; TBI, n=5; TBI+ICP elevation, n=8. All measures are reported as mean±s.e.m.
Tissue Processing
At 6 hours or 4 weeks postsham or cFPI animals were injected with 400 μl Euthasol euthanasia-III solution (Henry Schein, Dublin, OH, USA) and underwent transcardial perfusion with 0.9% saline followed by 4% paraformaldehyde/0.2% glutaraldehyde in Millonig's buffer (136 mmol/L sodium phosphate monobasic/109 mmol/L sodium hydroxide) for immunohistochemical analysis (Figure 1). After transcardial perfusion, the brains were removed and postfixed for 24 hours. As expected, little gross pathology was observed in animals sustaining TBI, either with or without additional postinjury ICP elevation. Acutely (6 hours) after TBI, subarachnoid hemorrhage and isolated petechial hemorrhage in the subcortical white matter were seen; however, these occurred without any sign of contusion, hematoma formation, or tissue loss in the cortex. Chronically (4 weeks) after TBI, neither subarachnoid hemorrhage nor cortical contusion was observed. Only one injured animal displayed evidence of tissue loss, which was confined to the superficial layers of the cortex and did not interfere with the histologic analysis.
Postfixed brains were sectioned coronally in 0.1 mmol/L phosphate buffer with a vibratome (Leica, Banockburn, IL, USA) at a thickness of 40 μm from bregma to ∼4.0 mm posterior to bregma. Sections were collected serially in 12-well plates and stored in Millonig's buffer at 4 °C. All quantitative analyses were performed at least 1 mm posterior to the needle track used for ICP monitoring/elevation, in a blinded fashion, throughout the rostral–caudal extent (1.8 mm±0.2 mm to 3.8 mm±0.2 mm posterior to bregma). A random starting well was selected (wells 1 to 12) and four serial sections, each 480 μm apart, were analyzed. All histologic analyses were restricted to layers V and VI of the lateral somatosensory neocortex extending from the area lateral to CA1 to the area lateral to CA3 of the hippocampus.
Detection and Quantification of Axonal Injury
For amyloid precursor protein (APP) immunohistochemistry sections were immunolabeled, as previously described,22 with a primary rabbit antibody against the C terminus of β-APP (Cat. #51–2700, 1:1,000, Life Technologies) followed by secondary antibody, biotinylated goat anti-rabbit IgG (Cat. #BA-1000, 1:1,000, Vector Laboratories, Burlingame, CA, USA). The sections were then incubated in avidin biotinylated enzyme complex using the Vectastain ABC kit (Vector Laboratories) followed by visualization with 0.05% diaminobenzidine/0.01% H2O2/0.3% imidazole/phosphate-buffered saline. The tissue was mounted, dehydrated, and cover-slipped. Visualization of APP-labeled axonal swellings was performed using a Nikon Eclipse 800 microscope (Nikon, Tokyo, Japan) equipped with an Olympus DP71 camera (Olympus, Center Valley, PA, USA). The region of interest within the somatosensory neocortex (a 3.75 mm × 1.55 mm rectangular area) was imaged bilaterally at × 4 magnification (n=8 images per animal), holding image acquisition settings constant for all groups analyzed. Analysis of the number of APP+ axonal swellings was performed using the particle analysis function in ImageJ software (NIH, Bethesda, MD, USA). The number of APP+ swellings per unit area was quantified for each image and averaged for each animal.
Neuronal Membrane Perturbation Analysis
Consistent with previous studies, we assessed the potential for neuronal membrane perturbation via the utilization of parenchymal 10 kDa dextrans, which are impermeable to neurons with intact membranes.18, 19, 22, 28, 29 Dextran-containing cells, indicative of membrane perturbation, could be visualized via confocal microscopy without further processing by utilizing the infused dextrans conjugated to fluorophores (AF 488 and AF 568). Sections were blocked with 10% normal goat serum, permeabilized with 1.5% Triton in normal goat serum, and immunolabeled with NeuN (Cat. #MAB377, 1:500, Millipore, Billerica, MA, USA) to identify neurons. Secondary antibody AF 633-conjugated goat anti-mouse IgG (Cat. #A21052, 1:250, Life Technologies) was then incubated and the tissue was mounted with Vectashield hardset mounting medium with DAPI (Cat. #H-1500, Vector Laboratories). Sections were analyzed by confocal microscopy using a Zeiss LSM 700 System (Carol Zeiss, Oberkochen, Germany). Quantitative analysis was performed as described previously.22 Briefly, confocal images of the left neocortical region of interest were taken at × 40 magnification in a systematically random fashion by a blinded investigator using DAPI labeling to verify focus (n=38 images per animal). Image acquisition settings were held constant for comparable regions (layer V or VI) for all groups analyzed. Analyses of neurons exhibiting preinjury dextran uptake, postinjury dextran uptake, or both pre and postinjury dextran uptake were performed using the ImageJ colocalization finder plugin (overlap coefficient⩾0.9) and traditional cell counting. Dextran containing neurons were quantified for each image and averaged for each animal.
Neuronal Density and Damage Analysis
To quantify the density of neurons as well as the percent of damaged neurons sections were stained with hematoxylin and eosin. Tissue was mounted on gelatin-coated slides before dehydration and rehydration. Rehydrated tissue was incubated in hematoxylin (Cat #4820-30-13, Trevigen, Gaithersburg, MD, USA; diluted 3:1 in H2O) followed by washes in 0.002% ammonia water and a quick dip in 0.25% eosin Y/0.005% acetic acid/95% ethanol before clearing of the sections through increasing concentrations of ethanol and cover-slipped with Permount (Cat #SP15–100 Thermo Fisher Scientific, Waltham, MA, USA). Sections were visualized using a Nikon Eclipse 800 microscope (Nikon) equipped with an Olympus DP71 camera (Olympus). The region of interest within the somatosensory neocortex was imaged bilaterally at × 20 magnification in a systematically random fashion (68 images per animal; ∼65% of the total region of interest). The number of neurons per image (882.3 μm × 658.8 μm; ∼0.58 mm2) was counted and averaged for each animal. Damaged neurons delineated by their eosinophilic cytoplasm and/or condensed nuclei were also counted and the percentage of damaged neurons per total number of neurons analyzed was quantified. Comparable to previous descriptions, quantitative analyses were performed in a blinded fashion.
Whisker Nuisance Task
One day before and 4 weeks after sham injury, TBI alone, or TBI+ICP elevation chronic survival animals were evaluated for somatosensory hypersensitivity using the WNT27 (Figure 1). Briefly, for each testing period, animals were acclimated to the open-field testing environment for 5 minutes followed by three consecutive 5-minute sessions of manual whisker stimulation of both mystacial pads using the wooden aspect of a 6-inch cotton tipped applicator stick. All tests were conducted at the same time of day and digitally video recorded for analysis. To asses the response to the sensory stimulation, the predominant behavioral responses were scored on a 0 to 2 point scale (0=absent, 1=present, 2=profound) by a blinded investigator for indications of hypersensitivity: (1) freezing, (2) cowering/aggressive stance/body position, (3) rapid and/or forced breathing, (4) retracted whisker position, (5) lack of whisking response, (6) evasiveness of stimulus, (7) fearful/aggressive response to stick, and (8) compulsive grooming. The scores for each animal were summed and averaged for the three trials with the highest possible score being 16 (higher score indicates more pronounced agitation/sensitivity). To reduce variability due to inherently hypersensitive animals, any animal with a presurgery WNT score of >5 was excluded from the study. Two animals were excluded based on this criterion.
Statistical Analysis
One-way analysis of variance (ANOVA) and Bonferroni's post hoc tests were performed for all between group histologic and physiologic analyses. Paired t-tests were done to analyze pre and postinjury differences within each group. A Kruskal–Wallis nonparametric analysis followed by a Mann–Whitney U-test with a Bonferroni correction for multiple comparisons was used to analyze behavioral data. Statistical significance was set at a P value <0.05. Data are presented as mean±s.e.m.
Results
Physiology
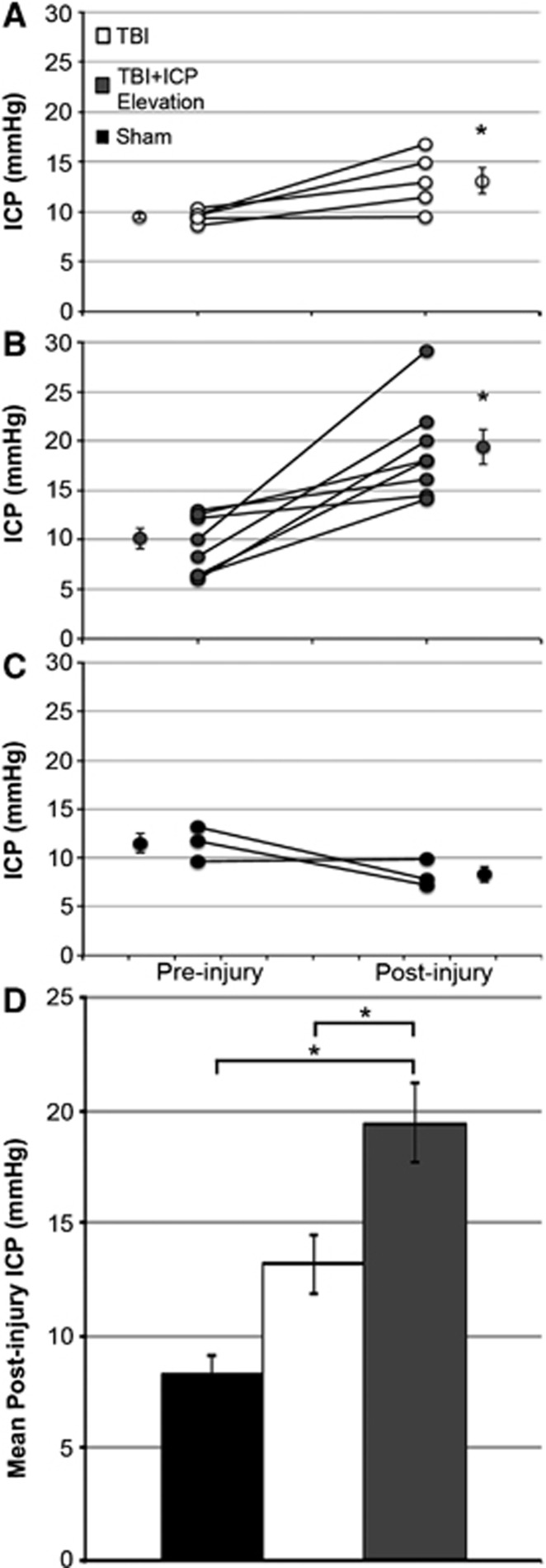
To explore the physiologic changes associated with TBI and/or manual ICP elevation, ICP, MABP, CPP, heart rate, and hemoglobin oxygen saturation were assessed for 1 hour before and up to 6 hours after injury. Averages of the pre and postinjury assessed values for these physiologic parameters are depicted in Table 1. Heart rate and hemoglobin oxygen saturation were comparable across all groups (one-way ANOVA, preinjury heart rate F2,10=2.48, P=0.133; postinjury heart rate: F2,10=0.66, P=0.539; preinjury hemoglobin oxygen saturation: F2,13=0.44, P=0.650; postinjury hemoglobin oxygen saturation: F2,13=0.59, P=0.568; Table 1). Figures 2A and 2B illustrate that the postinjury ICP levels increased in both the TBI alone and the TBI+ICP-elevation groups compared with preinjury levels (paired t-test, TBI: P=0.038; TBI+ICP elevation: P=0.002). Postinjury ICP levels after TBI alone, however, did not significantly differ from postsham levels and remained within the normal range of 5 to 15 mm Hg30 (one-way ANOVA, F2,13=9.57, P=0.003, Bonferroni post hoc P=0.370; Figure 2D). Importantly, manual ICP elevation to 20 mm Hg resulted in a significant increase in the 6-hour mean postinjury ICP level above postinjury levels found with sham or TBI alone, although manual manipulation was only conducted from 15 minutes to 1 hour after injury (Bonferroni post hoc, P=0.004 compared with sham, P=0.048 compared with TBI; Figure 2D). As expected, after sham injury, ICP remained consistent with presham values (paired t-test, P=0.212; Figure 2C). Likewise, before sham or cFPI, the average ICP was comparable for all groups (one-way ANOVA, F2,13=0.726, P=0.503).
Figure 2.
Manual modest intracranial pressure (ICP) elevation to 20 mm Hg from 15 minutes to 1 hour after traumatic brain injury (TBI) consistently elevates mean postinjury ICP. (A–C) Plots of the mean pre and postinjury ICP for (A) TBI, (B) TBI+ICP elevation, and (C) sham-injured animals. Each pre/postinjury pair represents a single animal. For each group, the pre and postinjury mean ICP of all animals is depicted next to the individual values. Legend in (A) is applicable for the entire figure. (D) Bar graph depicting the mean postinjury intracranial pressure (ICP) for the sham (black), TBI (white), and TBI+ICP elevation groups (dark gray). Averages are for the duration of physiologic monitoring (15 minutes to 6 hours post TBI or sham injuries). Sham n=3 animals, TBI n=5, TBI+ICP elevation n=8. Error bars represent ±s.e.m. *P<0.05.
It is important to note that for all groups, the CPP remained well above levels associated with signs of ischemia (50 mm Hg23, 24, 25). Before injury all groups displayed comparable CPP levels (one-way ANOVA, F2,13=0.067, P=0.935; Table 1). Moreover, after injury, the CPP of the TBI+ICP-elevation group was comparable to that of the TBI alone group and all the preinjury CPP readings; however, it was significantly less than the postsham levels (one-way ANOVA, F2,13=4.43, P=0.034, Bonferroni post hoc, P=0.570 compared with TBI, P=0.039 compared with sham; Table 1).
The MABP for all groups was equivalent before and following sham or cFPI and also remained well above detrimental levels of 60 mm Hg for all groups.23, 24 Therefore, the significant decrease in CPP levels observed in the TBI+ICP-elevation group was primarily due to the changes in ICP within both the sham and TBI+ICP-elevation groups and not due to alterations in the MABP after injury. The persistence of the modest ICP elevation after limited manual manipulation and the lack of MABP reactivity to TBI makes this an ideal model for studying the long-term effects of modest ICP elevation after TBI.
Neuronal Membrane Perturbation, but not Axonal Injury, is Exacerbated Acutely with Manual Modest Intracranial Pressure Elevation after Traumatic Brain Injury
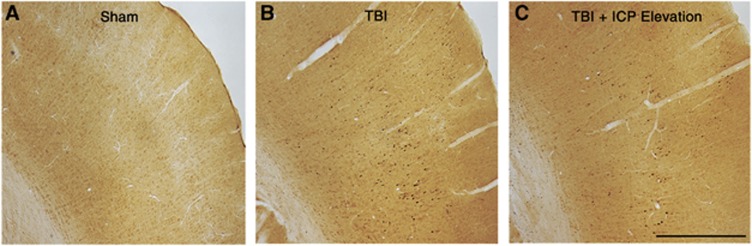
Based on our previous findings that neuronal membrane perturbation, but not axotomy, was exacerbated acutely in the face of naturally elevated ICP,22 we probed the possibility that similar results would be observed with manual ICP elevation post TBI. As the amount of axotomy is commonly used to evaluate the magnitude of diffuse TBI,31, 32 we first analyzed the number of inured axons, visualized via APP accumulation within axonal swellings, 6 hours after TBI (sham n=2 animals, TBI n=4, TBI+ICP elevation n=5). In accordance with the previous reports, no APP+ axonal swellings were seen in sham-injured controls (Figure 3A), whereas TBI alone and TBI+ICP-elevation showed numerous APP+ swellings within layers V and VI of the somatosensory cortex (Figure 3). However, there was no difference in the number of axonal swellings between TBI alone and TBI+ICP elevation (TBI alone=32.53±12.92 swellings/5.8 mm2; TBI+ICP elevation=44.50±22.26 swellings/5.8 mm2; t-test, P=0.678; Figures 3B and 3C). This is consistent with our previous finding that secondary elevation of ICP does not amplify the extent of axotomy acutely after TBI.22
Figure 3.
Axonal injury was not aggravated by modest manual intracranial pressure (ICP) elevation after traumatic brain injury (TBI). Representative photomicrographs of amyloid precursor protein immunoreactivity within the somatosensory cortex from (A) sham-injured animals and animals sustaining (B) TBI alone or (C) TBI+ICP elevation. Scale bar, 1 mm.
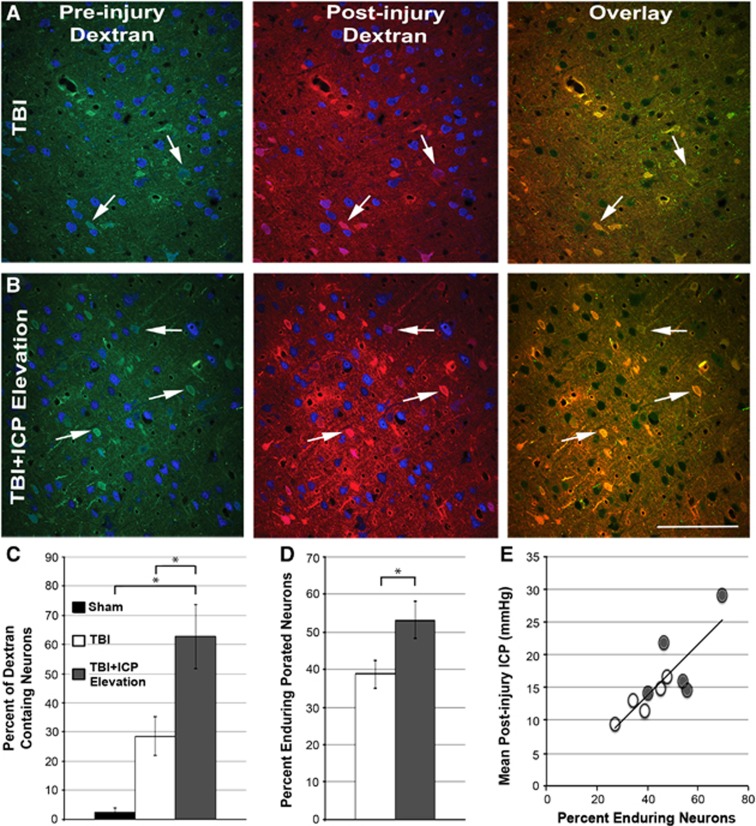
It has been well established, by ourselves and others, that fluorescently labeled 10 kDa dextrans, normally excluded from neurons with intact plasma membranes, can be reliably utilized to identify neurons sustaining membrane perturbation after injury both in vitro and in vivo.18, 19, 22, 28, 29 Based on our previous finding that naturally occurring ICP elevation was associated with exacerbated TBI-induced neuronal membrane perturbation,22 we next explored whether modest manual ICP elevation to 20 mm Hg would result in a similar exacerbation. To this end, the percentage of NeuN+ neurons sustaining injury-induced membrane disruption, reflected by the uptake of fluorescently labeled dextrans infused intraventricularly before or 4.5 hours after TBI was evaluated acutely after injury (Figure 4). In accordance with previous studies, sham-injured animals had virtually no neuronal membrane perturbation within the somatosensory neocortex (Figure 4C). Animals undergoing TBI alone or ICP elevation after TBI, however, exhibited neuronal membrane perturbation in layers V and VI of the somatosensory neocortex (Figures 4A and 4B). Although the percentage of neurons exhibiting acute membrane perturbation in the TBI alone group was greater than that observed in the sham group, this difference did not reach statistical significance (one-way ANOVA, F2,10=10.97, P=0.003, Bonferroni post hoc P=0.234; Figure 4C). In contrast, however, the percentage of membrane-perturbed neurons in animals sustaining manually elevated ICP after TBI was increased over twenty-fold compared with sham-injured animals and was doubled compared with animals sustaining TBI alone (Bonferroni post hoc P=0.003 compared with sham and P=0.041 compared with TBI; Figure 4C). Of the 62.62% (±10.91) of membrane-perturbed neurons identified with TBI+ICP elevation, 25.32% (±6.33) were flooded with the preinjury-administered dextran and 37.32% (±7.18) were flooded with the postinjury-administered dextran. These findings indicate that manual modest ICP elevation exacerbates neuronal membrane perturbation.
Figure 4.
Neuronal membrane perturbation is exacerbated by manual modest intracranial pressure (ICP) elevation after traumatic brain injury (TBI). Representative photomicrographs of neurons within layers V and VI of the somatosensory cortex from animals sustaining (A) TBI alone or (B) TBI+ICP elevation, infused with the preinjury (green; left column) and postinjury (red; middle column) administered dextrans. Neurons are in blue (NeuN). Overlays (right column) depict neurons containing both pre and postinjury-administered dextrans (enduring membrane-perturbed neurons; white arrows). (C) Bar graph depicting the mean percentage of dextran-containing neurons (pre or postinjury infusions) per total number of neurons analyzed for sham animals (black) and animals sustaining TBI alone (white) or TBI+ICP elevation (gray). Legend in (C) is applicable for the entire figure. (D) Bar graph depicting the mean percentage of enduring membrane-perturbed neurons per total membrane-perturbed neurons for animals sustaining TBI alone (white) or TBI+ICP elevation (gray). (E) Scatter plot illustrating the significant correlation between the mean postinjury ICP and the percentage of enduring membrane porated neurons. Each point represents an individual animal sustaining either TBI alone (white) or TBI+ICP elevation (gray). Sham n=3 animals, TBI n=5, TBI+ICP elevation n=5. Error bars represent ±s.e.m. *P<0.05. Scale bar, 20 μm.
The administration of fluorescently tagged dextrans before and after injury also allowed for the visualization of subpopulations within the greater membrane-perturbed population. These subpopulations include resealing (neurons containing only the preinjury-administered dextran), delayed membrane perturbation (neurons containing only the postinjury-administered dextran), and enduring membrane perturbation (neurons containing both the pre and postinjury-administered dextrans). As we recently showed that enduring and/or delayed membrane-perturbed neurons could have intensified pathologic consequences,22 a subpopulation analysis was done. Our results showed that the incidence of enduring perturbed neurons increased with TBI+ICP elevation as compared with TBI alone (t-test, P=0.048; Figure 4D). Interestingly, the average postinjury ICP was positively correlated to the increase in enduring membrane-perturbed neurons (Pearson's correlation coefficient=0.82, R2=0.68, P=0.002; Figure 4E). However, the percentage of membrane-perturbed neurons that demonstrated either membrane resealing or delayed membrane perturbation was not different between the TBI alone and TBI+ICP elevation groups (TBI versus TBI+ICP elevation; resealing: 32.53±12.03% versus 15.24±5.40%, t-test, P=0.226; delayed perturbation: 27.68±10.44% versus 31.67±8.18, t-test, P=0.777). Taken together, this indicates that the increase in membrane perturbation seen with manual ICP elevation after TBI is due to an increase in neurons that fail to reseal their membranes, possibly precipitating chronic neuronal damage or death.
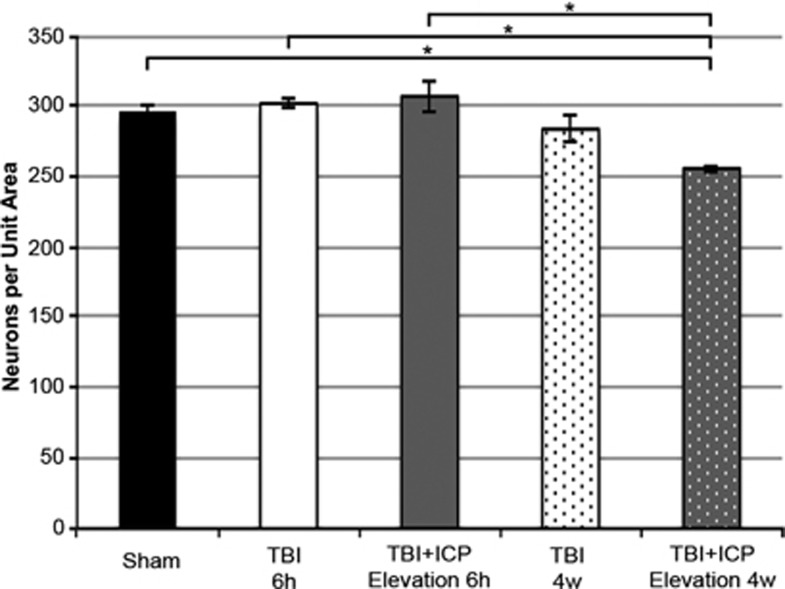
Manual Modest Intracranial Pressure Elevation is Associated with Neuronal Loss 4 Weeks after Traumatic Brain Injury
Our previous study indicated that membrane poration was associated with acute neuronal damage.22 To explore whether the acute exacerbation in neuronal membrane perturbation induced by ICP elevations after TBI was associated with long-term neuronal loss, the density of total neurons was assessed in the somatosensory cortex of sham and injured animals 6 hours and 4 weeks after injury (Figure 5). No homogenous cellular damage/loss, indicative of a global ischemic event, was observed in the tissue at either time point. While the density of total neurons per unit area was equivalent between sham, TBI alone at 6 hours, TBI+ICP elevation at 6 hours, and TBI alone at 4 weeks (one-way ANOVA, F2,16=9.152, P=0.000, Bonferroni post hoc P=1.00 for all comparisons), animals sustaining TBI+ICP elevation had significantly fewer neurons at 4 weeks after injury compared with sham-injured animals or either injury group at 6 hours after injury, a reduction of ∼14% (Bonferroni post hoc P=0.004 compared with sham, P=0.002 compared with TBI 6 hours, P=0.001 compared with TBI+ICP elevation 6 hours; Figure 5). To explore whether the neuronal loss observed was associated with ongoing pathologic changes, the percent of damaged neurons was assessed at 4 weeks. The percent of damaged neurons, identified by their eosinophilic cytoplasm and/or a condensed nucleus was comparable across all groups at 4 weeks (sham=16.11±0.51%, TBI=18.66±1.62%, TBI+ICP elevation=16.79±1.53% one-way ANOVA, F2,7=0.65, P=0.551). This reduction in neuronal density without corresponding increases in overt neuronal morphologic pathology at 4 weeks after injury indicates that the pathologic progression associated with acute neuronal membrane perturbation occurred between 6 hours and 4 weeks after injury.
Figure 5.
Modest manual intracranial pressure (ICP) elevation after traumatic brain injury (TBI) is associated with a decrease in neuronal density 4 weeks after injury. Bar graph depicting the mean number of neurons per unit area (0.58 mm2) in the somatosensory cortex (layers V and VI) of sham-injured animals (black) and animals sustaining either TBI alone at 6 hours (white), TBI+ICP elevation at 6 hours (gray), TBI alone at 4 weeks (white with black dots), or TBI+ICP elevation at 4 weeks (gray with white dots). Sham n=5 animals, TBI 6 hours n=4, TBI+ICP elevation 6 hours n=4, TBI 4 weeks n=4, TBI+ICP elevation 4 weeks n=4. Error bars represent±s.e.m. *P<0.05.
Modest Intracranial Pressure Elevation after Traumatic Brain Injury is Associated with Increased Behavioral Morbidity
Elevated ICP after TBI resulted in the exacerbation of acute diffuse neuronal membrane perturbation and chronic neuronal loss within the somatosensory cortex. As damage to the somatosensory cortex is correlated to changes in the response to whisker stimulation, the WNT26, 27 was performed 4 weeks after sham or TBI to investigate whether the exacerbated neuronal pathology in the face of elevated ICP translates to exaggerated TBI-induced behavioral changes (Figure 6).
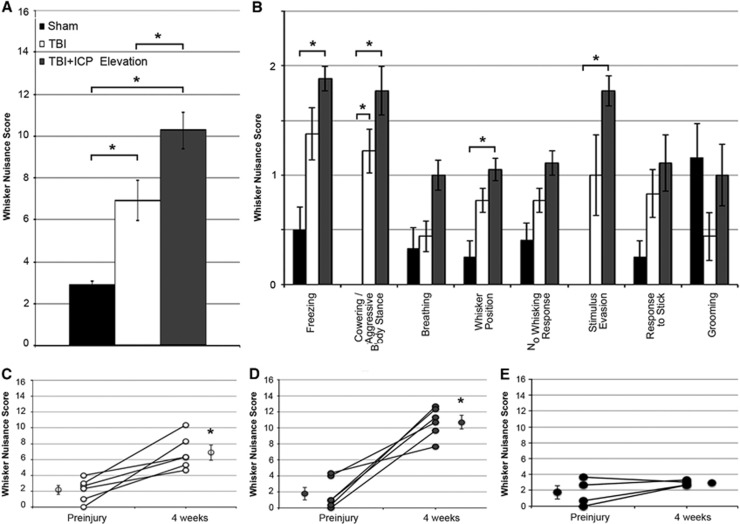
Figure 6.
Somatosensory hypersensitivity is intensified in animals sustaining traumatic brain injury (TBI) followed by modest intracranial pressure (ICP) elevation. (A) Bar graph depicting the mean total score for the whisker nuisance task (WNT). Legend in (A) is applicable for the entire figure. (B) Mean scores for each behavioral measure used to calculate the total WNT score (see methods) for sham (black), TBI alone (white), and TBI+ICP elevation (gray). (C–E) plots of the pre and postinjury WNT scores for (C) TBI, (D) TBI+ICP elevation and (E) sham-injured animals. Each pre/postinjury pair represents a single animal. For each group, the mean of all animals is depicted next to the individual values. Sham n=4 animals, TBI n=6, TBI+ICP elevation n=6. Error bars represent±s.e.m. *P<0.05.
Animals sustaining TBI alone displayed increased agitation in response to whisker stimulation, indicative of increased somatosensory sensitivity27 (Kruskal–Wallis test, P=0.005, Mann–Whitney U-test with Bonferroni correction P=0.030 compared with sham; Figure 6A). This difference was observed across the majority of criteria analyzed (Figure 6B). Interestingly, animals sustaining TBI+ICP elevation after injury displayed greater hypersensitivity and agitation to the stimulation as compared with animals sustaining either sham injury or TBI alone (Mann–Whitney U-test with Bonferroni correction P=0.030 compared with sham, P=0.045 compared with TBI; Figure 6A). The increased hypersensitivity observed in the TBI+ICP elevation group was also discernible across the majority of the behavioral criteria compared with sham or TBI alone (Figure 6B). Consistent with previous studies, sham-injured animals were not agitated by the somatosensory whisker stimulation26, 27 (Figure 6A).
Because the WNT was performed 1 day before injury as well as at 4 weeks after injury (Figure 1), a direct comparison of the behavioral changes associated with sham, TBI alone, and TBI+ICP elevation could be made (Figures 6C–E). While TBI alone and TBI+ICP elevation displayed a significant increase in hypersensitivity compared with preinjury measures (paired t-tests, TBI: P=0.006; TBI+ICP elevation: P=0.001), the degree of change in animals sustaining TBI+ICP elevation appears much greater than that of TBI alone (Figures 6C and 6D). As expected, no change was observed for sham-injured animals (paired T-test, P=0.212; Figure 6E). Moreover, somatosensory hypersensitivity was negatively correlated to neuronal density (Pearson's correlation coefficient=−0.86, R2=0.74, P=0.013), indicating that neuronal loss in the somatosensory cortex is associated with increased somatosensory sensitivity after TBI. Altogether these results indicate that ICP elevation after TBI, without concurrent ischemia, is associated with exacerbated behavioral morbidity.
Discussion
The current study demonstrates that elevated ICP after diffuse TBI can have profound detrimental short and long-term consequences without the added confound of global ischemia. While axonal injury remained unaltered by manual ICP elevation after TBI, neuronal membrane perturbation was exacerbated acutely. Moreover, the level of postinjury ICP was directly correlated to the degree of enduring neuronal membrane perturbation. Manual ICP elevation was also associated with a decrease in neuronal density and an increase in somatosensory hypersensitivity chronically. Taken together, this study points to a critical role of subischemic ICP elevation on the deleterious behavioral and pathologic consequences that are induced by diffuse TBI.
Our current model, which utilizes well-controlled ICP elevations achieved by limited manual manipulation, enables for a more causative link between ICP elevation after diffuse TBI and acute neuronal membrane perturbation than could be achieved in our previous model of naturally occurring ICP elevation.22 Nevertheless, the fact that for both studies the percent of neurons containing either the pre or postinjury-administered dextrans in the face of ICP elevation was similar reinforces the idea that the increase in ICP after diffuse TBI is associated with acute neuronal pathologies, regardless of the manner in which ICP is elevated (manually or naturally). Importantly, in the current study, the degree of ICP elevation was directly correlated to the level of membrane perturbation. Specifically, animals with higher mean postinjury ICP showed a greater percentage of neurons with enduring membrane perturbation. The effect of modestly elevated ICP on neuronal plasma membranes precipitating this finding is poorly understood and requires more investigation. Increased localized tissue damage owing to the exacerbated microcirculatory compromise in the face of modestly elevated ICP could increase perilesional damage in a contusional model of TBI.33, 34 However, because of the lack of contusion and/or square wave tissue damage in this diffuse model of TBI, enhanced microcirculatory issues are unlikely to cause this correlation. A more plausible possibility is that the modest elevation in ICP causes a direct pressure effect on the neuronal plasma membrane that reduces the neuron's ability to reseal, thus pushing it to an enduring phenotype of membrane perturbation. This direct pressure effect could be transduced through the activation of the TRPV2 Ca2+ channel, which is both temperature and mechanosensitive and is found in layer V and VI neurons of the rat neocortex.35, 36 The activation (or over activation) of this channel could lead to calcium-mediated increased protease and/or phospholipase activity, which in turn could overwhelm the mechanisms promoting membrane resealing in these neurons leading to secondary plasmalemmal poration and an enduring phenotype of membrane perturbation, as suggested by the LaPlaca lab.18
While the long-term pathologic progression of sustained neuronal membrane perturbation remains unknown, our findings suggest that this most likely accounts for the neuronal loss observed 4 weeks after injury. Similar to the relationship observed acutely for the incidence of membrane perturbation, the TBI+ICP-elevation group displayed a reduction in neuronal density at 4 weeks after injury compared with animals that sustained either sham injury or TBI alone. Thus, membrane perturbation may lead to neuronal death, which in turn could be intensified by ICP elevation.
In fact, previous studies have demonstrated that membrane perturbation is associated with an influx of calcium ions16, 37, 38 capable of triggering calpain-mediated cell death.39, 40 Alternatively, as previously shown, chronically membrane-perturbed neurons, including neurons sustaining enduring membrane perturbation, demonstrate a redistribution of the lysosomal protease cathepsin-B,22 which can precipitate cell death via the cleavage of Bid leading to the mitochondrial release of AIF and cytochrome c.41, 42
Neuronal death, however, may not be the only pathologic consequence of membrane perturbation associated with TBI. It has been demonstrated that a subset of membrane-perturbed neurons maintain the potential for repair and membrane resealing.18, 20, 28 The fact that the percent of neuronal loss observed in the TBI+ICP-elevation group 4 weeks after injury was less (∼62% of neurons being membrane perturbed at 6 hours versus an ∼13% reduction in neuronal density at 4 weeks post TBI+ICP elevation) than the membrane-perturbed values seen acutely supports this scenario. In addition, we observed membrane-perturbed neurons containing only the preinjury infused dextran acutely after TBI, demonstrating that there is membrane resealing in this model. Some neurons classified as enduring membrane perturbed at 6 hours after injury could undergo membrane resealing at a later time point and thus might not progress to cell death. These persisting neurons, however, may be dysfunctional, which could compound the hypersensitive response to somatosensory stimulation observed with elevated ICP after injury.
The described exacerbated behavioral morbidity is reminiscent of the clinical observation that ICP elevation above 20 mm Hg after TBI is associated with increased morbidity in the human population.2, 3, 4 In addition, hypersensitivity to sensory stimulus is a common phenomenon in patients suffering from TBI.43, 44 Thus, information regarding the effects of ICP elevation after TBI is not only scientifically, but also clinically relevant to our understanding of the neuropathological and neuropsychological changes that occur in response to brain injury.
Unfortunately, current clinical therapies for elevated ICP after TBI focus on the worst case scenario, aiming to ameliorate the impact of global ischemia due to the effect of severely elevated ICP on CPP.10, 11 These approaches have proven to have limited success in overcoming the predisposition for negative outcomes associated with elevated ICP after TBI45, 46 sparking debate over the efficacy of such ICP/CPP strategies and the cost benefit of invasive ICP monitoring.13, 14, 15, 30, 47, 48, 49 The current study, however, suggests that global ischemia is not the only threat of elevated ICP contributing to morbidity after TBI and speaks to a need for additional basic research exploring the pathologies exacerbated by elevated ICP after TBI.
In conclusion, this study indicates that modest elevations in ICP after diffuse TBI, without attendant reductions in CPP capable of triggering global ischemia, exacerbate behavioral morbidity and neuronal pathology. Understanding the mechanisms by which ICP elevation aggravate these outcomes could lead to the development of novel therapeutic interventions to alleviate the propensity for negative outcomes associated with elevated ICP after TBI.
Acknowledgments
The authors sincerely thank Dr Scott Henderson for his microscopy and image analysis expertise, Dr Robert Hamm for assistance with statistical analysis, Dr Jonathan Lifshitz for his direction and invaluable assistance with the behavioral analysis, Dr Melissa McGinn and Dr Anders Haanell for helpful scientific discussions, Sue Walker, C Lynn Davis and Jesse Sims for instrumental technical assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by NIH grants NS045824 and 5P30NS047463. This work was supported by NIH grant NS045824. Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS center core grant, 5P30NS047463.
References
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, et al. Surveillance for traumatic brain injury-related deaths—United States, 1997-2007. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503–516. doi: 10.3171/jns.1977.47.4.0503. [DOI] [PubMed] [Google Scholar]
- Treggiari MM, Schutz N, Yanez ND, Romand JA. Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: a systematic review. Neurocrit Care. 2007;6:104–112. doi: 10.1007/s12028-007-0012-1. [DOI] [PubMed] [Google Scholar]
- Sheth KN, Stein DM, Aarabi B, Hu P, Kufera Ja, Scalea TM, et al. Intracranial pressure dose and outcome in traumatic brain injury. Neurocrit Care. 2013;18:26–32. doi: 10.1007/s12028-012-9780-3. [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Maas AIR. Traumatic intracranial hypertension. N Engl J Med. 2014;370:2121–2130. doi: 10.1056/NEJMra1208708. [DOI] [PubMed] [Google Scholar]
- Tomura S, Nawashiro H, Otani N, Uozumi Y, Toyooka T, Ohsumi A, et al. Effect of decompressive craniectomy on aquaporin-4 expression after lateral fluid percussion injury in rats. J Neurotrauma. 2011;28:237–243. doi: 10.1089/neu.2010.1443. [DOI] [PubMed] [Google Scholar]
- Zweckberger K, Stoffel M, Baethmann A, Plesnila N. Effect of decompression craniotomy on increase of contusion volume and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2003;20:1307–1314. doi: 10.1089/089771503322686102. [DOI] [PubMed] [Google Scholar]
- Moody Ra, Ruamsuke S, Mullan SF. An evaluation of decompression in experimental head injury. J Neurosurg. 1968;29:586–590. doi: 10.3171/jns.1968.29.6.0586. [DOI] [PubMed] [Google Scholar]
- Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- Marin-Caballos AJ, Murillo-Cabezas F, Cayuela-Dominguez A, Dominguez-Roldan JM, Rincon-Ferrari MD, Valencia-Anguita J, et al. Cerebral perfusion pressure and risk of brain hypoxia in severe head injury: a prospective observational study. Crit care. 2005;9:R670–R676. doi: 10.1186/cc3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat P, Sala N, Payen J-F, Oddo M. Beyond intracranial pressure: optimization of cerebral blood flow, oxygen, and substrate delivery after traumatic brain injury. Ann Intensive Care. 2013;3:23. doi: 10.1186/2110-5820-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin DE, Bush RC, Nemoto EM. Effect of cerebral perfusion pressure on cerebral cortical microvascular shunting at high intracranial pressure in rats. Stroke. 2013;44:177–181. doi: 10.1161/STROKEAHA.112.668293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut RM. Intracranial pressure monitoring in brain-injured patients is associated with worsening of survival. J Trauma. 2008;65:500–501. doi: 10.1097/TA.0b013e31818020b3. [DOI] [PubMed] [Google Scholar]
- Cremer OL, van Dijk GW, van Wensen E, Brekelmans GJ, Moons KG, Leenen LP, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med. 2005;33:2207–2213. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- Alali AS, Fowler Ra, Mainprize TG, Scales DC, Kiss A, de Mestral C, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the american college of surgeons trauma quality improvement program. J Neurotrauma. 2013;30:1737–1746. doi: 10.1089/neu.2012.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes DM, LaPlaca MC, Cargill RS., 2nd Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol. 2003;184:420–427. doi: 10.1016/s0014-4886(03)00254-1. [DOI] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp Neurol. 2008;212:422–430. doi: 10.1016/j.expneurol.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Vernekar VN, LaPlaca MC. Trauma-induced plasmalemma disruptions in three-dimensional neural cultures are dependent on strain modality and rate. J Neurotrauma. 2011;28:2219–2233. doi: 10.1089/neu.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo AM, Liu J, Lam CK, Dvorak M, Tetzlaff W, Oxland TR. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurg Spine. 2007;6:255–266. doi: 10.3171/spi.2007.6.3.255. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Dalkara T, You Z, Qiu J, Bermpohl D, Mehta N, et al. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:490–505. doi: 10.1038/sj.jcbfm.9600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JE, Povlishock JT, Jacobs KM. Electrophysiological abnormalities in both axotomized and nonaxotomized pyramidal neurons following mild traumatic brain injury. J Neurosci. 2012;32:6682–6687. doi: 10.1523/JNEUROSCI.0881-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenaye AD, McGinn MJ, Povlishock JT. Increased intracranial pressure after diffuse traumatic brain injury exacerbates neuronal somatic membrane poration but not axonal injury: evidence for primary intracranial pressure-induced neuronal perturbation. J Cereb Blood Flow Metab. 2012;32:1919–1932. doi: 10.1038/jcbfm.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24 (Suppl 1:S59–S64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- Dennis AM, Haselkorn ML, Vagni VA, Garman RH, Janesko-Feldman K, Bayir H, et al. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotrauma. 2009;26:889–899. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JC, Pillai S, Cherian L, Garcia R, Grill RJ, Robertson CS. Histopathological and behavioral effects of immediate and delayed hemorrhagic shock after mild traumatic brain injury in rats. J Neurotrauma. 2012;29:322–334. doi: 10.1089/neu.2011.1979. [DOI] [PubMed] [Google Scholar]
- Thomas TC, Hinzman JM, Gerhardt GA, Lifshitz J. Hypersensitive glutamate signaling correlates with the development of late-onset behavioral morbidity in diffuse brain-injured circuitry. J Neurotrauma. 2012;29:187–200. doi: 10.1089/neu.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara KC, Lisembee AM, Lifshitz J. The whisker nuisance task identifies a late-onset, persistent sensory sensitivity in diffuse brain-injured rats. J Neurotrauma. 2010;27:695–706. doi: 10.1089/neu.2009.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas O, Lifshitz J, Povlishock JT. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury. J Neurosci. 2006;26:3130–3140. doi: 10.1523/JNEUROSCI.5119-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton RH, Povlishock JT. Identification and characterization of heterogeneous neuronal injury and death in regions of diffuse brain injury: evidence for multiple independent injury phenotypes. J Neurosci. 2004;24:3543–3553. doi: 10.1523/JNEUROSCI.5048-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ieva A, Schmitz EM, Cusimano MD. Analysis of intracranial pressure: past, present, and future. Neuroscientist. 2013;6:592–603. doi: 10.1177/1073858412474845. [DOI] [PubMed] [Google Scholar]
- Hellewell SC, Yan EB, Agyapomaa DA, Bye N, Morganti-Kossmann MC. Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J Neurotrauma. 2010;27:1997–2010. doi: 10.1089/neu.2009.1245. [DOI] [PubMed] [Google Scholar]
- Fujita M, Oda Y, Wei EP, Povlishock JT. The combination of either tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J Neurotrauma. 2011;28:1209–1218. doi: 10.1089/neu.2011.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Bell JD, Siddiq IP, Baker AJ. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:575–584. doi: 10.1038/jcbfm.2008.151. [DOI] [PubMed] [Google Scholar]
- Schwarzmaier SM, Kim S-W, Trabold R, Plesnila N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma. 2010;27:121–130. doi: 10.1089/neu.2009.1114. [DOI] [PubMed] [Google Scholar]
- Folgering JHa, Sharif-Naeini R, Dedman A, Patel A, Delmas P, Honoré E. Molecular basis of the mammalian pressure-sensitive ion channels: focus on vascular mechanotransduction. Prog Biophys Mol Biol. 2008;97:180–195. doi: 10.1016/j.pbiomolbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci. 2005;22:825–834. doi: 10.1111/j.1460-9568.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- Lusardi TA, Wolf JA, Putt ME, Smith DH, Meaney DF. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J Neurotrauma. 2004;21:61–72. doi: 10.1089/089771504772695959. [DOI] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp Neurol. 2009;219:553–561. doi: 10.1016/j.expneurol.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MJ, Kelley BJ, Akinyi L, Oli MW, Liu MC, Hayes RL, et al. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J Neuropathol Exp Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czogalla A, Sikorski AF. Spectrin and calpain: a “target” and a “sniper” in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62:1913–1924. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub A, Park MA, Hanna D, Hong Y, Mitchell C, Pandya AP, et al. OSU-03012 promotes caspase-independent but PERK-, cathepsin B-, BID-, and AIF-dependent killing of transformed cells. Mol Pharmacol. 2006;70:589–603. doi: 10.1124/mol.106.025007. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhong C, Shi L, Guo Y, Fan Z. Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to Necroptosis. J Immunol. 2009;182:6993–7000. doi: 10.4049/jimmunol.0802502. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Twijnstra A, Wijnen G, Jolles J. Tolerance for light and sound of patients with persistent post-concussional symptoms 6 months after mild head injury. J Neurol. 1991;238:443–446. doi: 10.1007/BF00314651. [DOI] [PubMed] [Google Scholar]
- Waddell PA, Gronwall DM. Sensitivity to light and sound following minor head injury. Acta Neurol Scand. 1984;69:270–276. doi: 10.1111/j.1600-0404.1984.tb07812.x. [DOI] [PubMed] [Google Scholar]
- Ward JD, Becker DP, Miller JD, Choi SC, Marmarou A, Wood C, et al. Failure of prophylactic barbiturate coma in the treatment of severe head injury. J Neurosurg. 1985;62:383–388. doi: 10.3171/jns.1985.62.3.0383. [DOI] [PubMed] [Google Scholar]
- Curley G, Kavanagh BP, Laffey JG. Hypocapnia and the injured brain: more harm than benefit. Crit Care Med. 2010;38:1348–1359. doi: 10.1097/CCM.0b013e3181d8cf2b. [DOI] [PubMed] [Google Scholar]
- Romner B. Intracranial pressure monitoring in traumatic. Nat Rev Neurol. 2013;9:185–186. doi: 10.1038/nrneurol.2013.37. [DOI] [PubMed] [Google Scholar]
- Hutchinson P, Kolias A. Intracranial pressure monitoring in severe traumatic brain injury. BMJ Br Med J. 2013;346:f1000. doi: 10.1136/bmj.f1000. [DOI] [PubMed] [Google Scholar]
- De Bonis P, Anile C, Honeybul S. Intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2013;368:1749–1750. doi: 10.1056/NEJMc1301076. [DOI] [PubMed] [Google Scholar]