Abstract
13C Nuclear Magnetic Resonance (NMR) studies of rodent and human brain using [1-13C]/[1,6-13C2]glucose as labeled substrate have consistently found a lower enrichment (∼25% to 30%) of glutamine-C4 compared with glutamate-C4 at isotopic steady state. The source of this isotope dilution has not been established experimentally but may potentially arise either from blood/brain exchange of glutamine or from metabolism of unlabeled substrates in astrocytes, where glutamine synthesis occurs. In this study, the contribution of the former was evaluated ex vivo using 1H-[13C]-NMR spectroscopy together with intravenous infusion of [U-13C5]glutamine for 3, 15, 30, and 60 minutes in mice. 13C labeling of brain glutamine was found to be saturated at plasma glutamine levels >1.0 mmol/L. Fitting a blood–astrocyte–neuron metabolic model to the 13C enrichment time courses of glutamate and glutamine yielded the value of glutamine influx, VGln(in), 0.036±0.002 μmol/g per minute for plasma glutamine of 1.8 mmol/L. For physiologic plasma glutamine level (∼0.6 mmol/L), VGln(in) would be ∼0.010 μmol/g per minute, which corresponds to ∼6% of the glutamine synthesis rate and rises to ∼11% for saturating blood glutamine concentrations. Thus, glutamine influx from blood contributes at most ∼20% to the dilution of astroglial glutamine-C4 consistently seen in metabolic studies using [1-13C]glucose.
Keywords: glutamate, neurotransmitter, nuclear magnetic resonance spectroscopy
Introduction
Glucose, the primary source of energy in the mature brain, is mainly oxidized in neurons to support neuronal firing, and the release and recycling of neurotransmitters, glutamate, and GABA, through the neuron–astrocyte glutamate–glutamine and GABA–glutamine cycles. The metabolic pathways comprising these cycles have been revealed in vivo by 13C Nuclear Magnetic Resonance (NMR) spectroscopy combined with infusions of 13C-labeled substrates,1, 2 permitting detailed investigation of the metabolism of glucose and alternative substrates in the brain.
A consistent finding in NMR studies of rodent and human brain metabolism using [1-13C] or [1,6-13C2]glucose as precursor is the lower fractional 13C enrichment of brain glutamate and glutamine at isotopic steady state compared with blood glucose,3, 4, 5 revealing the presence of constant ‘dilutional' flows of unlabeled substrates into the respective tricarboxylic acid (TCA) cycles of neurons and astroglia. For glutamate, this dilution amounts to 25% to 30% in rodents5, 6 (∼14% in human brain7) of the theoretical maximum expected from the 13C-labeled glucose, and is thought to arise within neurons mainly from metabolism of blood-borne substrates such as lactate or ketone bodies and within the brain from glutamine produced in astrocytes through the glutamate–glutamine cycle.8, 9, 10 In addition to the glutamate-C4 dilution (relative to glucose-C1), 13C enrichment of glutamine-C4 is further ∼20% to 30% less than that of glutamate-C4 at isotopic steady state.4, 5, 11, 12, 13 As the majority of glutamine-C4 labeling in these experiments arises from neuronal glutamate via the glutamate–glutamine cycle, the lower 13C enrichment of glutamine-C4 (i.e., Gln-C4fe/Glu-C4fe<1) indicates non-neuronal inflow of unlabeled carbon8, 9, 10 atoms into glutamine. This inflow of unlabeled carbon could arise from blood-to-brain transport of unlabeled glutamine, or alternatively, astroglial oxidation of unlabeled substrates such as amino acids or short/medium-chain free fatty acids at the level of acetyl-CoA or α-ketoglutarate.8, 14, 15 Models of brain metabolism used to estimate fluxes from 13C time courses have dealt with this problem by either inclusion of a dilution flux (Vdil(Gln), Vex, or Vdil) at the level of glutamine5, 7, 12, 13, 16, 17, 18 or astroglial acetyl-CoA6 derived from nonlabeled precursors. The presence of the glutamine-C4 dilution, and its incorporation into the metabolic model was recently shown to be critical for the accurate determination of the glutamate–glutamine cycle rate in NMR studies using [1-13C]/[1,6-13C2]glucose.15 Thus, it is critical to better define the source of the astroglial glutamine dilution, allowing refinement of the metabolic models to estimate cerebral fluxes in 13C-labeling studies, while exploring alternate 13C-labeled substrates that might be exploited to study astrocytic and neuronal function in vivo.
In this study, we infused [U-13C5]glutamine into mice and measured the 13C turnover of cortical glutamine and glutamate, while fitting a metabolic model modified to include glutamine transport between blood and brain. Our findings address quantitatively the contribution of glutamine influx from blood to the maintenance of brain glutamine levels and its role in the astroglial glutamine dilution.
Materials and methods
All the experimental procedures with animals were approved by the Institutional Animals Ethics Committee of Centre for Cellular and Molecular Biology (CCMB), Hyderabad, India and were conducted in accordance with the guidelines established by Committee for the Purpose of Control and Supervision on Experiments on Animals, Ministry of Environment and Forests, Government of India. ARRIVE guidelines were followed in the preparation of the manuscript. Male C57BL6 mice (2 months old) were maintained at 12 hour/12 hour light/dark cycle at the CCMB Animal House with temperature 22±1°C and 60±5% relative humidity, and received standard chow and water ad libitum.
Animal Preparation and Infusion of [U-13C5]Glutamine
Mice were anesthetized with urethane (1.5 g/kg intraperitoneally) and the lateral tail vein catheterized for infusion of 13C-labeled glutamine. The body temperature was maintained ∼37°C with a heating pad warmed by a temperature-regulated recirculating water bath, and the respiration rate was monitored using a Biopac data acquisition system (Biopac Systems, Inc., Santa Barbara, CA, USA). Forty-five minutes after induction of anesthesia, [U-13C5]glutamine (250 mmol/L dissolved in deionized water, pH ∼7.0) was administered intravenously for 3, 15, 30, or 60 minutes, using a bolus-variable rate infusion (1,220 μmol/kg per minute (0 to 15 seconds), 244 μmol/kg per minute (15 seconds to 4 minutes), 122 μmol/kg per minute (4 to 8 minutes), and 49 μmol/kg per minute (>8 minutes). This protocol, modified from a previously described acetate infusion protocol,19 elevated the blood glutamine level to ∼4.1 mmol/L within 3 minutes from a baseline value of ∼0.6 mmol/L and maintained it at ∼1.1 mmol/L thereafter. Four mice were used for each infusion time point. In addition, mice (n=4) were also infused for 60 minutes with rates adjusted such that infusion rates at t⩾8 minutes were 20, 37, 59, and 74 μmol/kg per minute, to evaluate the steady-state brain glutamine labeling at different levels of plasma glutamine. Blood was collected from the retro-orbital sinus just before the conclusion of infusion, centrifuged at high speed, and the supernatant (plasma) collected for the measurement of concentration and 13C enrichment of glutamine in plasma. The head was frozen in situ with liquid nitrogen and stored at −80°C until further processing.
Extraction of Metabolites from Brain Tissue
The brain was dissected in a cryo-chamber maintained at −20°C to isolate the cerebral cortex tissue. Metabolites were extracted from the frozen tissue as described previously.20 Briefly, weighed tissue was powdered with ice-cold 0.1 N HCl/Methanol, and [2-13C]glycine was added as an internal concentration reference. Tissue was homogenized in 60% ethanol and centrifuged at 14,000 g. The supernatant was lyophilized and powder was dissolved in 550 μL of phosphate-buffered (25 mmol/L, pH=7) deuterium oxide (D2O) containing 0.25 mmol/L sodium 3-(trimethylsilyl)-2,2',3,3'-tetradeuteropropionate for NMR analysis.
Analysis of Plasma Glutamine
The plasma was mixed with D2O containing sodium formate (0.5 mmol/L) and passed through a centrifugal filter (10 kDa cutoff) to remove macromolecules. A 1H-[13C]-NMR spectrum of filtered plasma was acquired at 600 MHz NMR spectrometer to measure the concentration and 13C enrichment of glutamine (Bruker AVANCE II, Karlsruhe, Germany).3 The concentration of glutamine was determined from the resonance intensity at 2.46 p.p.m. in the non-edited spectrum, relative to the formate resonance at 8.45 p.p.m. The percent enrichment of glutamine-C4 was quantified from the intensity ratio of the 2.46-p.p.m. resonances of the difference spectrum (13C only) and nonedited spectrum (12C+13C), respectively.
Nuclear Magnetic Resonance Spectroscopy of Cortical Extracts
1H-[13C]-NMR spectroscopy of cortical tissue extracts was performed at 600 MHz NMR spectrometer using a triple resonance probe. All the pulses were replaced with adiabatic half or full passage as described earlier by de Graaf et al.3 A typical 1H-[13C]-NMR experiment involves acquisition of two sets of spin-echo 1H NMR spectra with an alternating ‘ON'/‘OFF' 13C inversion pulse. The parameters used for acquisition of NMR spectra were repetition time=5.5 seconds, echo time=8 ms, number of points in free induction decay=16,384, spectral width=7,212 kHz, number of averages 512 (ref. 21). The free induction decays thus obtained were zero filled to 128 K data points, apodized (Lorentzian line broadening 0.75 Hz), Fourier transformed, and phase corrected. 13C-edited 1H NMR spectrum (1H{2 × 13C}) was obtained by subtracting a subspectrum obtained with 13C inversion pulse ‘ON' (1H{12C–13C}) from that acquired under ‘OFF' condition (1H{12C+13C}). The peak intensity of different metabolites measured from the spectrum obtained from ‘OFF' condition of C-13 inversion pulse (1H{12C+13C}) using the inbuilt integration tool in Topspin 2.1 was used for concentration measurement. The intensity of the labeled metabolites was obtained from the 13C-edited (1H{2 × 13C}) spectrum. The loss of NMR signal intensity due to rapid pulsing was corrected using a correction factor obtained from data on a single sample acquired with above-mentioned parameters and also fully relaxed acquisitions (repetition time=20 seconds). The concentration of metabolites was calculated relative to [2-13C]glycine (obtained from the edited 13C spectrum). The isotopic 13C enrichments of amino acids at different carbon positions were calculated from the ratios of the areas in the difference spectrum (13C only) and the nonedited spectrum. To reduce the variation due to plasma glutamine labeling, the measured 13C enrichments of cortical amino acids were normalized with the plasma glutamine 13C labeling.
Description of the Metabolic Model and Determination of Metabolic Rates
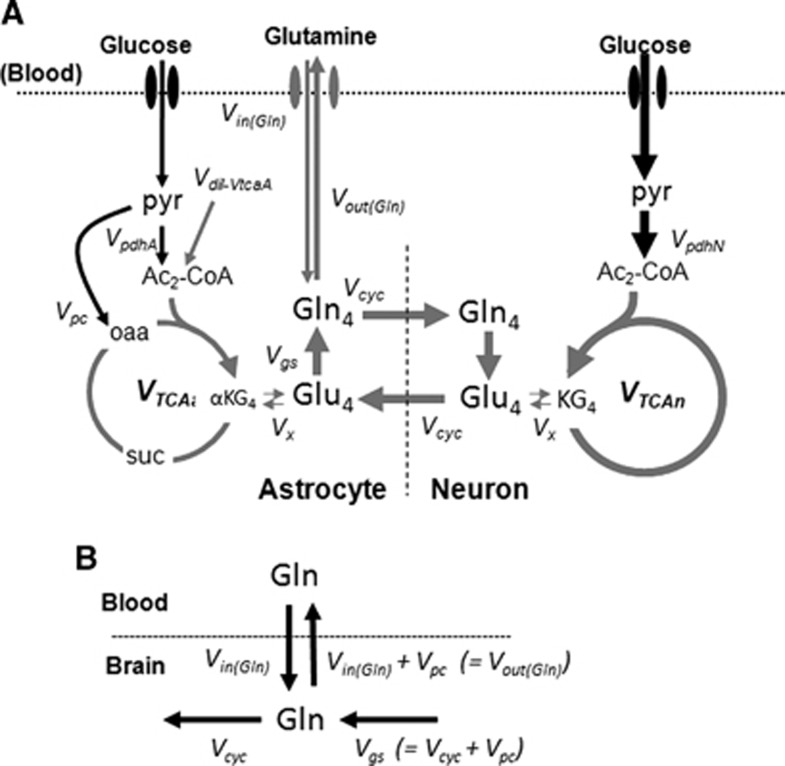
The time courses of 13C labeling of cortical amino acids were fitted with a metabolic model that incorporates glutamine influx and efflux between blood and brain (Figure 1; Supplementary Table S1). The metabolic model consists of three compartments (blood, astrocytes, and neurons) allowing for transport and metabolism of glutamine (13C-labeled) and glucose (unlabeled) in the two neural cell types. In this model, [U-13C5]glutamine enters the brain, mixes with the endogenous (unlabeled) glutamine, which can then provide the precursors for neurotransmitter glutamate synthesis in neurons through glutamate–glutamine cycling. Glutamate released during neuronal activity is taken up by astrocytes and converted into glutamine, by the enzymatic action of glutamine synthetase, completing the cycle. Although glutamine is isotopically labeled with 13C in all five carbon atoms, only carbon 4 is depicted for glutamine and glutamate. The continuous metabolism of unlabeled glucose in the TCA cycles of neurons and astrocytes will introduce unlabeled carbons (dilution) in glutamine and glutamate at the level of acetyl-CoA produced from pyruvate. The mass balance and isotope rate equations describing the model appear in Supplementary Information (Supplementary Table S1).
Figure 1.
(A) Metabolic model of brain glucose and glutamine transport/metabolism. Glutamine is transported from blood-to-brain and brain-to-blood at rates VGln(in) and VGln(out), respectively. Glutamine is assumed to mix completely with astroglial glutamine upon transport. Metabolism of unlabeled glucose leads to extensive dilution of metabolites and amino acids along the depicted pathways. The metabolic model was fitted to the kinetic and steady-state data (Glutamine-C4, Glutamate-C4, and Glutamate-C3) with iteration of VGln(in) and Vpdh(N). Values of Vgln, VGln(out), VTCA(a) (=0.21 × VTCA(N)), and Vcyc (=0.39 × VTCA(N)) were calculated parameters, with Vpc set equal to 0.02 μmol/g per minute. (B) Schematic depiction of balance of mass flows between blood and brain glutamine pools with interconnecting pathways.
The driver input consisted of the time courses of blood plasma glutamine concentrations and 13C percentage enrichments. The target data to be fitted consisted of the time courses of cortical Glu-C4, C3 and Gln-C4 13C enrichments (Figure 1A) for the estimation of glutamine inflow VGln(in). Metabolic modeling was performed using the CWave software, version 3.0 (ref. 16) running in MATLAB (Natick, MA, USA) version R2011b with Microsoft Windows XP. Certain metabolic parameters were constrained to fixed values taken from a previous study of urethane anesthetized C57Bl6 mouse cortex22 including the ratio, Vcyc/Vtca(N), of 0.39, and pyruvate carboxylase flux, Vpc, of 0.02 μmol/g per minute; these and additional parameters are listed in Supplementary Information (Supplementary Table S1). The astroglial glutamate pool was assumed to be 16% of total glutamate based on the previous studies in mice.22 Parameters that were iterated include the rate of glutamine influx, VGln(in), and neuronal pyruvate dehydrogenase flux, Vpdh(N).
Statistics
Results consisting of concentrations and 13C enrichments are reported as group averages±standard deviations (SDs). The uncertainties in the model parameters, VGln(in) and Vgln, and their distributions were determined by Monte Carlo simulations with 1,000 iterations using CWave, as described in Patel et al.19 Briefly, 1,000 simulated noisy data sets were generated by adding the standard deviation of the scatter about the least square fit, and the data sets were fitted with the model to generate 1,000 values for each parameter. These values were used to estimate the distributions of uncertainty of each parameter. Results are reported as mean±SD.
Results
Concentration and 13C Enrichment of Plasma Glutamine
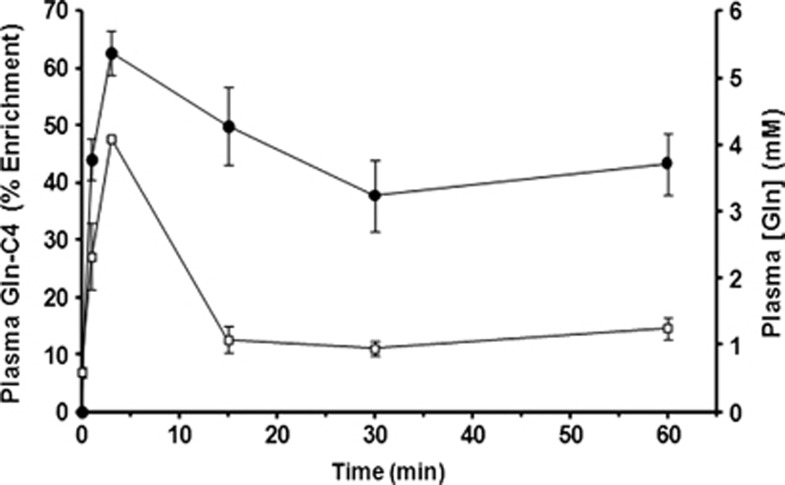
The baseline blood plasma glutamine concentration was 0.59±0.08 mmol/L, as measured in a separate group of noninfused mice (n=7). Intravenous infusion of [U-13C5]glutamine led to a rapid increase in plasma levels to 4.1 mmol/L in 3 minutes, which declined to 1.1 mmol/L within 15 minutes and remained approximately constant throughout the remainder of the 60 minutes study duration (Figure 2; Table 1). The 13C enrichment of plasma glutamine was 62.8±4.0% (above the natural abundance of 1.1%) at 3 minutes, and it remained elevated (37% to 50%) throughout the infusion. In mice subjected to prolonged infusions (60 minutes) of [U-13C5]glutamine to achieve quasi isotopic steady-state conditions, a range of elevated and constant plasma glutamine levels were produced by adjusting the rate of infusion. For steady-state infusion rates (t⩾8 minutes) of 20, 37, 59, and 74 μmol/kg per minute, plasma glutamine concentrations and percentage enrichments were increased, covering a range of values from 0.8 to 2.9 mmol/L and from 30% to 61%, respectively (Table 2).
Figure 2.
Plasma glutamine concentration and 13C percentage enrichment measured at different times during the intravenous infusion of [U-13C5]glutamine. Filled symbols, Gln-C4 enrichment (%), left vertical axis; open symbols, glutamine concentration in mmol/L, right vertical axis. These data were used as the plasma glutamine driver in the fitting of the time courses. Data are plotted as mean±SD.
Table 1. Concentrations (mmol/L) and 13C enrichments (%) of plasma and brain amino acids during intravenous [U-13C5]glutamine infusion.
| Infusion Time (minutes) |
Plasma glutamine |
Cortical amino acids enrichment (%) |
||||
|---|---|---|---|---|---|---|
| Concentration (mmol/L) | 13C Enrichment (%) | GluC4 | GABAC2 | GlnC4 | GluC3 | |
| 3 | 4.09±0.02 | 62.8±4.0 | 0.2±0.1 | 0.2±0.1 | 2.0±0.3 | 0.1±0.1 |
| 15 | 1.08±0.20 | 49.9±6.8 | 1.3±0.3 | 1.1±0.3 | 3.5±0.2 | 0.7±0.1 |
| 30 | 0.95±0.12 | 37.8±6.2 | 2.3±0.8 | 1.6±0.9 | 4.4±0.7 | 1.4±0.4 |
| 60 | 1.26±0.16 | 43.3±5.4 | 3.9±0.5 | 3.4±0.4 | 6.2±0.3 | 2.9±0.4 |
The 13C enrichment of amino acids was normalized as: 50 × (Metabolitesbrain/GlnC4blood). Values represent mean±SD of n=4 mice per group. Concentrations (mmol/L) and 13C percentage enrichments (% excess above the 1.1% natural abundance) of plasma and brain glutamine were calculated from 1H-[13C]-Nuclear Magnetic Resonance spectra as described in Materials and Methods.
Table 2. Steady-state 13C enrichment of cortical amino acids for different concentrations of plasma glutamine during [U-13C5]glutamine infusion.
| Infusion rate (μmol/kg per minute) |
Plasma glutamine |
Cortical amino acids
13C enrichment (%) |
||||
|---|---|---|---|---|---|---|
| Concentration (mmol/L) | 13C Enrichment (%) | GluC4 | GABAC2 | GlnC4 | GluC3 | |
| 20 | 0.8±0.2 | 29.5±2.0 | 1.4±0.5 | 1.0±0.5 | 2.7±0.4 | 1.2±0.3 |
| 37 | 1.0±0.1 | 45.1±6.3 | 2.4±0.5 | 2.1±0.3 | 5.0±0.2 | 2.1±0.3 |
| 49 | 1.3±0.2 | 43.3±5.4 | 3.9±0.5 | 3.4±0.4 | 6.2±0.3 | 2.9±0.4 |
| 59 | 1.4±0.1 | 54.5±0.7 | 4.4±1.3 | 3.7±0.9 | 7.1±0.8 | 3.4±0.7 |
| 74 | 2.9±0.2 | 60.7±2.4 | 4.7±0.4 | 3.9±0.5 | 8.2±0.3 | 3.6±0.3 |
The 13C enrichment of amino acids was normalized as: 50 × (Metabolitesbrain/GlnC4blood). Values represent mean±SD of n=4 mice per group. Concentrations (mmol/L) and 13C percentage enrichments (% excess above the 1.1% natural abundance) of plasma and brain glutamine were calculated from 1H-[13C]-Nuclear Magnetic Resonance spectra as described in Materials and Methods.
Effect of Plasma Glutamine Elevation on Concentrations of Cortical Metabolites
The concentrations of cortical metabolites were measured in the extract from the 1H-[13C+12C]-NMR spectrum (Figure 3A). The time course of metabolite data revealed that compared with baseline (∼5.6±0.4 μmol/g22) the average cortical glutamine concentration rose slightly during the course of the [U-13C5]glutamine infusion (Supplementary Table S2). The steady-state cortical glutamine concentrations were found to be similar over the plasma glutamine concentration range of 0.8 to 2.9 mmol/L (Supplementary Table S3). The levels of metabolites were found to be unaltered during 13C-glutamine infusion at different time points or at steady state (60 minutes) with different infusion rates. Thus, plasma glutamine elevations (0.8 to 2.9 mmol/L) had minimal to no effect on cerebral cortical metabolite levels.
Figure 3.
Representative 1H-[13C]-Nuclear Magnetic Resonance (NMR) spectra of cortical tissue extracts of mouse cerebral cortex after timed infusions of [U-13C5]glutamine. (A) 1H{12C+13C} NMR depicts the total metabolite intensities after a 15-minute infusion of [U-13C5]glutamine. (B) 1H{13C} NMR represents the difference spectra depicting 13C-labeled metabolites for different infusion times of [U-13C5]glutamine. Spectra were increased 16 times for clarity. Peak labels: Asp3, aspartate-H3; Cre, creatine+phosphocreatine; Cho, choline; GABA2,3, γ-amino butyric acid-H2,3; Gln4, glutamine-H4; Glu4, glutamate-H4; Glu3, glutamate-H3; Glx3, (glutamate+glutamine)-H3; Gly2, glycine-H2 (reference); NAA, N-acetyl aspartate; Tau, taurine.
Effect of Plasma Glutamine Elevations on the Time Course of Brain Metabolite Enrichments
The infusion of [U-13C5]glutamine led to a significant 13C enrichment of brain amino acids, as shown in 1H-[13C]-NMR difference spectra of cortical extracts measured ex vivo for different infusion times (Figure 3B). The 13C labeling of cortical glutamate and glutamine was observed at 3 minutes, the earliest infusion time point, whereas 13C labeling of aspartate and GABA was seen clearly 15 minutes onwards.
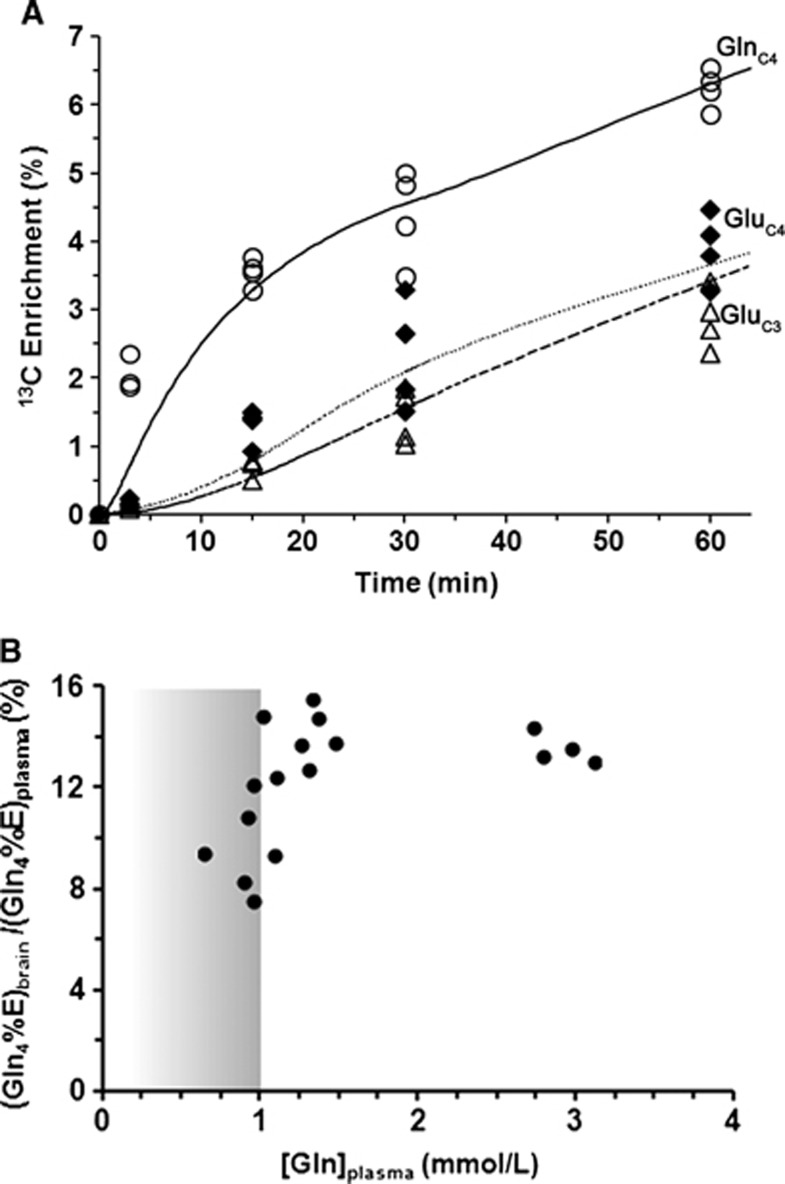
The time courses of 13C enrichment of cortical glutamine-C4 and glutamate-C4/C3 are shown in Figure 4A. Glutamine-C4 13C enrichment rose within 3 minutes to ∼2% (percentage enrichment normalized to blood enrichment) followed by a more gradual rise to ∼6% at 60 minutes. Glutamate-C4 13C enrichment lagged glutamine-C4 at all infusion time points reaching to ∼4% at 60 minutes, consistent with blood glutamine uptake in astroglia and metabolism to glutamate in neurons. Glutamate-C3 enrichment rose slower than glutamate-C4 (Figure 4A), despite the use of uniformly labeled glutamine precursor, suggesting an initial processing of glutamine (glutamate) destined for neurotransmitter glutamate synthesis in the neuronal TCA cycle where dilution with glucose-derived acetyl-CoA occurs.
Figure 4.
(A) Time courses of 13C labeling of cortical glutamine-C4 (○) and glutamate-C4 (♦), C3 (Δ) measured ex vivo after intravenous infusion of [U-13C5]glutamine with best fits of the transport/metabolic model. Data points represent individual values as measured in the cortical extracts. The line represents the best fit of the transport/metabolic model depicted in Figure 1 with iteration of the unidirectional glutamine influx (VGln(in)). The target data for the fit included the time courses of glutamine-C4, glutamate-C4,C3 enrichments. (B) Quasi-steady state cortical glutamine-C4 enrichment (%) in relation to plasma glutamine concentration. The normalized cortical glutamine-C4 enrichment (100 × {GlnC4brain/GlnC4blood}) showed saturation kinetics with increasing plasma glutamine concentration, attaining a maximum enrichment of ∼13.6% for plasma glutamine concentrations ⩾1.0 mmol/L. The threshold for plasma glutamine saturation, as shown by vertical line, was determined where the brain glutamine percentage enrichment decreased below 3 standard deviations of the mean 11.8% (13.6±0.59% 13.6−(3 × 0.59)=11.8%) for [Gln]plasma from the high infusion rate (2.90 mmol/L).
In another experiment, 13C labeling of brain glutamine was measured at 60 minutes (steady state) for different plasma glutamine levels achieved by adjustment of the [U-13C5]glutamine infusion rate. A plot of the normalized steady-state brain glutamine-C4 13C enrichment against increasing plasma glutamine concentration revealed a steep dependence between 0 and ∼1 mmol/L followed by the saturation of glutamine enrichment at ∼11.8% for plasma glutamine concentration ⩾1.0 mmol/L (Figure 4B). Extrapolating the plasma glutamine concentration proportionally downward to the baseline (physiologically normal) value of 0.59 mmol/L, we estimate that brain glutamine-C4 enrichment would be ∼7% (11.8% × 0.59 mmol/L/1.0 mmol/L). Considering that the ‘dilution' of glutamine-C4 enrichment relative to glutamate-C4 at isotopic steady state during infusion of [1-13C] or [1,6-13C2]glucose amounts to ∼30% (see Discussion), for plasma glutamine at physiologic concentration, glutamine influx from blood then could account for ∼20% of this dilution. This is likely an upper limit considering that glutamine in plasma would compete with other nonpolar amino acids for transport into the brain.23
Blood-to-Brain Glutamine Influx
The rate of glutamine influx from blood, VGln(in), was determined by modeling the time courses of 13C enrichment of brain glutamine-C4 and glutamate-C4,C3 from [U-13C5]glutamine resulting in a quasi-constant plasma glutamine level of ∼1.8 mmol/L, which was above the concentration at which transport was saturated. This approach treated VGln(in) as an independent iterated parameter (Figure 1A). The best fit of this model to the time course data was achieved with a value of 0.036±0.002 μmol/g per minute for VGln(in). The initial rate of glutamine-C4 labeling was also estimated by fitting a single exponential function to the brain glutamine-C4 concentration time course using nonlinear least squares regression and found a similar rate, 0.041±0.006 μmol/g per minute (Supplementary Figure S1). This indicates that the metabolic model captured the essential quantitative features of blood-to-brain glutamine transport and turnover.
The value of VGln(in) at saturating (>1.0 mmol/L) plasma glutamine level was 10.7% of the rate of cortical glutamine synthesis (Vgln, 0.33 μmol/g per minute), which corresponds to ∼5.3% of Vgln for a physiologic level (0.59 mmol/L) of plasma glutamine. Comparing this with the 25% dilution of glutamine-C4 (relative to glutamate-C4) measured in mouse cerebral cortex from [1-13C]glucose at isotopic steady state,22 glutamine influx from blood could account for ∼20% of this dilution for physiologic blood glutamine level. Thus, ∼80% of the glutamine-C4 dilution must arise through other sources, most likely fed through preceding step(s) in astroglial metabolism.
Discussion
In this study, we have shown that blood-borne labeled glutamine enters into brain in a concentration and time-dependent manner, reaching saturation for plasma glutamine concentration above ∼1.0 mmol/L. Cortical glutamate and GABA were labeled from plasma [U-13C5]glutamine in the order (glutamine-C4>glutamate-C4>GABA-C2), consistent with glutamine flow from astrocytes to neurons in the glutamate/GABA cycle. Further, glutamine influx from blood at physiologic blood glutamine level can account at most for ∼20% of the previously observed steady-state isotopic dilution of glutamine-C4 (relative to glutamate-C4) from [1-13C]glucose.
Comparison of Glutamine Influx with Previously Reported Values
The rate of glutamine influx (VGln(in), 0.036±0.002 μmol/g per minute), as measured under near constant and saturating levels of plasma glutamine (1.8 mmol/L), is similar to the blood-to-brain maximum transport rate, Vmax, of 0.043 μmol/g per minute reported in anesthetized rats in vivo by Smith et al.23 Values of Vmax measured from the uptake of glutamine incubated in vitro using brain slices vary substantially. For example, the Vmax reported by Benjamin et al24 for nonstimulated rat cortical slices maintained at 37°C of 0.2 and 0.5 μmol/g over 12 minutes (corresponding to 0.017 and 0.042 μmol/g per minute, respectively) from uptake of L-[U-14C]glutamine over a range of glutamine concentrations between 67 μmol/L and 2 mmol/L is from 6 to 13 times lower than the Vmax of 0.23 μmol/g per minute reported by Balcar and Johnston25 for slices of cerebral gray matter at 25°C for a much lower range of medium glutamine levels (5 to 60 μmol/L).
Assuming that VGln(in) scales proportionally with plasma glutamine concentration for levels below saturation (≤1.0 mmol/L; Figure 4B), the estimated VGln(in) for physiologic blood glutamine (0.59 mmol/L) would be at most ∼0.021 μmol/g per minute (=0.036 × 0.59/1.0). This value is considerably higher than previously reported rates of glutamine influx for physiologic plasma glutamine concentrations (0.42 to 0.48 mmol/L) of 0.0049 μmol/g per minute26 or 0.0041 μmol/g per minute in parietal cortex23 as measured with L-[14C]glutamine and in situ carotid artery perfusion. However, under physiologic condition, several amino acids present in blood may compete with glutamine for transport, effectively raising the apparent plasma concentration required for half maximal transport, Km', and reducing its influx as much as 22-fold.23 Because our study was conducted with elevated and saturating blood glutamine concentrations, effectively reducing the effects of other competing amino acids, our estimate of VGln(in) for physiologic blood glutamine level likely reflects an upper limit.
Compared with the in vivo estimates, reported maximum rates of glutamine transport in cultured neurons and astrocytes are much higher (e.g., Vmax of 0.7 to 5.0 μmol/g per minute assuming 100 mg protein/g),27, 28, 29 suggesting that the lower rates measured in vivo reflect mainly transport through the blood–brain barrier. Such comparisons, however, must be made with ample caution due to the incomplete understanding of transporter types involved, their physical properties (e.g., whether electrogenic or electroneutral, ionic dependencies, and potential for exchange), and the consequence of averaging over multiple transporter types and compartments reflected in the in vivo measurement.
Substrates Contributing to the Glutamine Dilution at Isotopic Steady State
Our findings indicate that blood-borne glutamine at physiologic levels provides at most 15% to 20% of the glutamine-C4 dilution observed using [1-13C]glucose as precursor. Because glutamine is synthesized in astrocytes and 70% to 80% of glutamine is derived directly from glutamate released by neurons, the remaining dilution of glutamine-C4 relative to glutamate-C4 must arise in astrocytes from unlabeled sources. Other potential sources of glutamine-C4 dilution could include influx of unlabeled amino acids arising from blood or the breakdown of endogenous proteins, which enter the TCA cycle at the level of acetyl-CoA or α-ketoglutarate. The combined inflows from these sources for several potentially relevant amino acids (glutamate, glutamine, serine, glycine, alanine, leucine, threonine, cysteine, and proline) has been estimated to be at most ∼0.11 μmol/g per minute,8 although a large fraction of this is ascribed to leucine influx, the oxidation of which may not occur in astrocytes due to the absence of branched-chain ketoacid dehydrogenase.30 Other unlabeled amino acids that can enter the astroglial TCA cycle at succinyl-CoA or oxaloacetate (methionine and aspartate) do not contribute to glutamine-C4 dilution. Another source of glutamine-C4 dilution could arise from unlabeled astrocytic glycogen to the extent that glycogen had not attained an isotopic steady state at the time of measurement and the pyruvate produced from glycogen was oxidized in astrocytes. 13C-labeled glucose infusions conducted over long periods, such as performed to measure glycogen turnover,31 could address this question.
In addition to the above substrates, a number of non-glucose substrates that can be metabolized to acetyl-CoA are supplied from blood, as well as acetyl groups arising through turnover of endogenous membrane lipids and proteins. A significant amount of free acetate is present in mouse blood (∼0.2 to 0.9 mmol/L),32 which can be readily oxidized in brain, mainly by astrocytes.33 In the cerebral cortex of anesthetized rats, blood acetate is oxidized at a rate of 0.04 μmol/g per minute19 for physiologic blood acetate level (0.94 mmol/L), contributing ∼13% of the rate of glutamine synthesis. The 13C labeling of glutamine-C4 from [2-13C]acetate, an astroglial specific substrate, has been shown to be higher than glutamate-C4.19, 34 Hence the dilution of astroglial acetyl-CoA from unlabeled acetate will result in additional dilution of glutamine-C4 when compared with that at glutamate-C4. The relevant dilution in glutamine-C4 relates to the dilution of astroglial glutamate-C4 (precursor of glutamine-C4) from astroglial sources relative to the much larger pool of neuronal glutamate which directly supports 70% to 80% of glutamine synthesis (and C4 labeling from C1-glucose). Both neurons and astrocytes can oxidize fatty acids to acetyl-CoA through the β-oxidation pathway, although short-chain free fatty acids (e.g., butyrate and octanoate) are metabolized mainly by astrocytes. Ebert et al14 have shown that in anesthetized rats octanoate infused intravenously to a blood level of 0.25 mmol/L could supply ∼20% of brain metabolism, or nearly all of astrocyte oxidation. Considering that the rates of astrocyte oxidation and glutamine synthesis are comparable (Vtca(A)/Vgln=∼0.50), we suggest that under physiologic conditions the availability of non-glucose substrates appears to be sufficient to account for the additional dilution in glutamine-C4 labeling (relative to glutamate-C4) from 13C-glucose.
In the metabolic model used to evaluate the transport properties, glutamine efflux (VGln(out)) provides mass balance by including an efflux path for net anaplerosis (pyruvate carboxylase flux), which in the present study was 0.056 μmol/g per minute. This value exceeds the maximum transport rate reported by Smith et al23 and our measurement of the unidirectional transport maximum in the brain (VGln(in), 0.033±0.002 μmol/g per minute). We note that mass balance can also be achieved without the need to expel glutamine if the glutamate produced by anaplerosis replaces glutamate which is lost by oxidation,17, 35 effectively reducing the rate of glutamine efflux to the same degree. Although considerable in vitro evidence exists for glutamate oxidation in astrocytes,36 studies conducted in vivo in rodents under euglycemic conditions did not show a significant glutamate oxidation,19 emphasizing the need for new approaches to resolve this issue. Alternatively, considerable numbers of glutamine transporters may be bound at the luminal side of epithelium of the blood–brain barrier, which will reduce the available capacity for glutamine inflow, although the extracellular glutamine concentration of ∼0.39 mmol/L37 is similar to plasma levels under physiologic conditions and therefore not saturating.
Limitations of the Study and Other Observations
There are some limitations in the present study that impact interpretation. First, glutamine transport between blood and brain involves several transporter proteins, all of which belong to one or more families of large neutral amino-acid transporters.26, 38, 39, 40 Glutamine transport into brain and cerebrospinal fluid from the blood involves both System N, A and L-like transporters, and within brain parenchyma, glutamine transport into neurons and astrocytes involves mainly systems-A and N transporter proteins, respectively. Because the present study involved bulk cortical tissue measurements of glutamine and glutamate, and consequently did not differentiate between the different neural compartments, the measured value of VGln(in) must reflect an average of all the different transporter isoforms mediating cortical glutamine uptake and metabolism. In addition, brain glutamine levels were assumed constant in the modeling, although levels were ∼16% higher at the end of the glutamine infusion compared with a group of noninfused mice. This increase is likely due to net glutamine influx from blood, consistent with the continuing rise in cortical glutamine-C4 percentage 13C enrichment (Figure 4A), despite constant plasma glutamine enrichment and concentration. However, some of this increase might reflect the increase in glutamine levels in the brain capillaries compartment. We note that the current study does not allow determination of the magnitude of the astroglial dilution as a reference for comparison with VGln(in) because unlabeled carbon from nonglucose sources cannot be differentiated from glucose. Moreover, the high glutamine levels during the glutamine infusion would be expected to compete for uptake with other large nonpolar amino acids, reducing their contribution to astroglial acetyl-CoA production.
Finally, the current study was conducted in mice anesthetized with urethane, which produces a long-lasting steady level of anesthesia. Metabolic analysis performed under urethane and other anesthetics using [1,6-13C2]glucose shows similar differences in the labeling of glutamate-C4 and glutamine-C4 when accounting for depth of anesthesia (neural activity). Because different anesthetics do not appear to influence the difference in glutamate and glutamine-C4 13C labeling at steady state, it may be argued that anesthesia has little impact on the transport of glutamine from blood to brain, although further studies are needed to address this issue. The labeling of astroglial glutamine from [2-13C]acetate5 or [2-13C]glucose17 has been used to estimate the neurotransmitter cycling-to-TCA cycle flux ratio of glutamatergic and GABAergic neurons, Vcyc(Glu-Gln)/Vtca(Glu) and Vcyc(GABA-Gln)/Vtca(GABA). In principal, the same approach could be applied with data from 13C-labeled glutamine. However, in the present study systemic metabolism of the infused 13C-glutamine led to formation of [1-13C]glucose in the blood, which would be expected to be metabolized by neurons to glutamate-C4 (and GABA-C2) (Table 2), thus not allowing a reliable estimate of Vcyc(Glu-Gln)/Vtca(Glu) under the present experimental condition.
Conclusions
13C-Labeled glutamine is readily transported from the blood into the brain, first enriching brain glutamine and then glutamate and GABA, consistent with neuron–astrocyte neurotransmitter cycling. Glutamine influx showed saturation above blood concentrations of 1.0 mmol/L, reaching 14% enrichment with a unidirectional rate of glutamine influx of 0.036 μmol/g per minute. For a physiologic level of plasma glutamine (∼0.6 mmol/L), glutamine influx into brain may account, at most, for ∼20% of the ‘dilution' of glutamine-C4 (relative to glutamate-C4 at isotopic steady state) from [1-13C]glucose. The findings suggest that the largest fraction of the non-glucose dilution arises from glial metabolism of unlabeled precursors producing acetyl-CoA. Because 13C-labeled glutamine has direct access to neurons after reaching the interstitial space, this substrate may prove useful in the assessment of neuronal function and dysfunction in vivo.
Acknowledgments
The authors would like to thank Dr Robin A de Graaf, Yale University for providing the 1H-[13C]-NMR sequence, Mr. Bhargidhar Babu for his assistance in conducting the animal studies. All NMR experiments were performed at the NMR Facility, CCMB, Hyderabad, India.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by grants from the CSIR network project BSC0208 to ABP.
Supplementary Material
References
- de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- Lanz B, Gruetter R, Duarte JM. Metabolic flux and compartmentation analysis in the brain. Front Endocrinol (Lausanne) 2013;4:156. doi: 10.3389/fendo.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf RA, Brown PB, Mason GF, Rothman DL, Behar KL. Detection of [1,6-13C2]-glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn Reson Med. 2003;49:37–46. doi: 10.1002/mrm.10348. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL, Shulman RG, et al. Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. J Cereb Blood Flow Metab. 2004;24:972–985. doi: 10.1097/01.WCB.0000126234.16188.71. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci USA. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JM, Lanz B, Gruetter R. Compartmentalized cerebral metabolism of [1,6-13C]glucose determined by in vivo 13C NMR spectroscopy at 14.1 T. Front Neuroenergetics. 2011;3:3. doi: 10.3389/fnene.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci USA. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Exchange-mediated dilution of brain lactate specific activity: implications for the origin of glutamate dilution and the contributions of glutamine dilution and other pathways. J Neurochem. 2009;109 (Suppl 1:30–37. doi: 10.1111/j.1471-4159.2009.05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R, Novotny EJ, Boulware SD, Mason GF, Rothman DL, Shulman GI, et al. Localized 13C NMR spectroscopy in the human brain of amino acid labeling from D-[1-13C]glucose. J Neurochem. 1994;63:1377–1385. doi: 10.1046/j.1471-4159.1994.63041377.x. [DOI] [PubMed] [Google Scholar]
- Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J Cereb Blood Flow Metab. 1995;15:12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, et al. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres S, Raffard G, Franconi JM, Merle M. Close coupling between astrocytic and neuronal metabolisms to fulfill anaplerotic and energy needs in the rat brain. J Cereb Blood Flow Metab. 2008;28:712–724. doi: 10.1038/sj.jcbfm.9600568. [DOI] [PubMed] [Google Scholar]
- Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Rothman DL, Behar KL, Xu S. Determination of the glutamate-glutamine cycling flux using two-compartment dynamic metabolic modeling is sensitive to astroglial dilution. J Cereb Blood Flow Metab. 2009;29:108–118. doi: 10.1038/jcbfm.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Falk Petersen K, de Graaf RA, Kanamatsu T, Otsuki T, Shulman GI, et al. A comparison of 13C NMR measurements of the rates of glutamine synthesis and the tricarboxylic acid cycle during oral and intravenous administration of [1-13C]glucose. Brain Res Brain Res Protoc. 2003;10:181–190. doi: 10.1016/s1385-299x(02)00217-9. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, et al. In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during [2-13C]glucose infusion. J Neurochem. 2001;76:975–989. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang L, Jiang Y, Ma X, Chowdhury GM, Mason GF. Regional metabolite levels and turnover in the awake rat brain under the influence of nicotine. J. Neurochem. 2010;113:1447–1458. doi: 10.1111/j.1471-4159.2010.06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Rothman DL, Behar KL, Mason GF. Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J Cereb Blood Flow Metab. 2010;30:1200–1213. doi: 10.1038/jcbfm.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Rothman DL, Cline GW, Behar KL. Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res. 2001;919:207–220. doi: 10.1016/s0006-8993(01)03015-3. [DOI] [PubMed] [Google Scholar]
- Bagga P, Chugani AN, Varadarajan KS, Patel AB. In vivo NMR studies of regional cerebral energetics in MPTP model of Parkinson's disease: recovery of cerebral metabolism with acute levodopa treatment. J Neurochem. 2013;127:365–377. doi: 10.1111/jnc.12407. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Ambadipudi S, Patel AB. Glutamatergic and GABAergic TCA cycle and neurotransmitter cycling fluxes in different regions of mouse brain. J Cereb Blood Flow Metab. 2013;33:1523–1531. doi: 10.1038/jcbfm.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Benjamin AM, Verjee ZH, Quastel JH. Kinetics of cerebral uptake processes in vitro of L-glutamine, branched-chain L-amino acids, and L-phenylalanine: effects of ouabain. J Neurochem. 1980;35:67–77. doi: 10.1111/j.1471-4159.1980.tb12490.x. [DOI] [PubMed] [Google Scholar]
- Balcar VJ, Johnston GA. High affinity uptake of L-glutamine in rat brain slices. J Neurochem. 1975;24:875–879. doi: 10.1111/j.1471-4159.1975.tb03650.x. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Kawai N, Ren XD, Abdelkarim GE, Keep RF. Glutamine uptake at the blood-brain barrier is mediated by N-system transport. J Neurochem. 1998;71:2565–2573. doi: 10.1046/j.1471-4159.1998.71062565.x. [DOI] [PubMed] [Google Scholar]
- Nagaraja TN, Brookes N. Glutamine transport in mouse cerebral astrocytes. J Neurochem. 1996;66:1665–1674. doi: 10.1046/j.1471-4159.1996.66041665.x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Hertz L, Svenneby G, Kvamme E. Phosphate activated glutaminase activity and glutamine uptake in primary cultures of astrocytes. J Neurochem. 1979;32:943–950. doi: 10.1111/j.1471-4159.1979.tb04579.x. [DOI] [PubMed] [Google Scholar]
- Su TZ, Campbell GW, Oxender DL. Glutamine transport in cerebellar granule cells in culture. Brain Res. 1997;757:69–78. doi: 10.1016/s0006-8993(97)00139-x. [DOI] [PubMed] [Google Scholar]
- Cole JT, Sweatt AJ, Hutson SM. Expression of mitochondrial branched-chain aminotransferase and alpha-keto-acid dehydrogenase in rat brain: implications for neurotransmitter metabolism. Front Neuroanat. 2012;6:18. doi: 10.3389/fnana.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Gruetter R. In vivo 13C NMR assessment of brain glycogen concentration and turnover in the awake rat. Neurochem Int. 2003;43:317–322. doi: 10.1016/s0197-0186(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Veeraiah P, Subramaniam V, Patel AB. Differential effects of ethanol on regional glutamatergic and GABAergic neurotransmitter pathways in mouse brain. J Neurochem. 2013;128:628–640. doi: 10.1111/jnc.12508. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz B, Xin L, Millet P, Gruetter R. In vivo quantification of neuro-glial metabolism and glial glutamate concentration using 1H-[13C] MRS at 14.1T. J Neurochem. 2014;128:125–139. doi: 10.1111/jnc.12479. [DOI] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, Maciejewski PK, Behar KL. Is there in vivo evidence for amino acid shuttles carrying ammonia from neurons to astrocytes. Neurochem Res. 2012;37:2597–2612. doi: 10.1007/s11064-012-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Hertz E. Cataplerotic TCA cycle flux determined as glutamate-sustained oxygen consumption in primary cultures of astrocytes. Neurochem Int. 2003;43:355–361. doi: 10.1016/s0197-0186(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Kanamori K, Ross BD. Quantitative determination of extracellular glutamine concentration in rat brain, and its elevation in vivo by system A transport inhibitor, alpha-(methylamino)isobutyrate. J Neurochem. 2004;90:203–210. doi: 10.1111/j.1471-4159.2004.02478.x. [DOI] [PubMed] [Google Scholar]
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Cangiano C, Cardelli-Cangiano P, James JH, Rossi-Fanelli F, Patrizi MA, Brackett KA, et al. Brain microvessels take up large neutral amino acids in exchange for glutamine. Cooperative role of Na+-dependent and Na+-independent systems. J Biol Chem. 1983;258:8949–8954. [PubMed] [Google Scholar]
- Matsuo H, Tsukada S, Nakata T, Chairoungdua A, Kim DK, Cha SH, et al. Expression of a system L neutral amino acid transporter at the blood-brain barrier. Neuroreport. 2000;11:3507–3511. doi: 10.1097/00001756-200011090-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.