Abstract
Most patients who die after traumatic brain injury (TBI) show evidence of ischemic brain damage. Nevertheless, it has proven difficult to demonstrate cerebral ischemia in TBI patients. After TBI, both global and localized changes in cerebral blood flow (CBF) are observed, depending on the extent of diffuse brain swelling and the size and location of contusions and hematoma. These changes vary considerably over time, with most TBI patients showing reduced CBF during the first 12 hours after injury, then hyperperfusion, and in some patients vasospasms before CBF eventually normalizes. This apparent neurovascular uncoupling has been ascribed to mitochondrial dysfunction, hindered oxygen diffusion into tissue, or microthrombosis. Capillary compression by astrocytic endfeet swelling is observed in biopsies acquired from TBI patients. In animal models, elevated intracranial pressure compresses capillaries, causing redistribution of capillary flows into patterns argued to cause functional shunting of oxygenated blood through the capillary bed. We used a biophysical model of oxygen transport in tissue to examine how capillary flow disturbances may contribute to the profound changes in CBF after TBI. The analysis suggests that elevated capillary transit time heterogeneity can cause critical reductions in oxygen availability in the absence of ‘classic' ischemia. We discuss diagnostic and therapeutic consequences of these predictions.
Keywords: cerebral blood flow, capillary transit time heterogeneity, ischemic injury, intracranial pressure, pericyte, traumatic brain injury
Introduction
Ninety percent of patients who die after severe traumatic brain injury (TBI) show histologic evidence of ischemic brain damage, even after excluding infarctions in relation to contusions, and brainstem infarctions, which can be attributed to increased intracranial pressure (ICP).1, 2 The ischemic lesions are typically localized in the cortex (distributed diffusely or according to either vascular territories or their boundaries), in the subcortical gray matter (basal ganglia and the hippocampus), or in the cerebellum.1, 2 These findings are consistent with extended periods of hypoxia, hypotension, or elevated ICP,2 but even after the introduction of optimized guidelines to avoid pre- and in-hospital hypoxic episodes in TBI victims, the post mortem frequency of ischemic damage has remained largely unaffected.3, 4
It has proven surprisingly difficult to demonstrate where, when, and why cerebral ischemia occurs in TBI patients. The extent to which cerebral blood flow (CBF) is reduced early after severe blunt head injury depends on the size and location of the resulting tissue injury. Adjacent and ipsilateral to large contusions or hematoma, hypoperfusion is typically more severe than in smaller lesions, whereas CBF is often globally affected by diffuse brain swelling.5 In addition to this spatial heterogeneity, CBF varies considerably over time after the injury. Within the first 12 hours after TBI, one-third of TBI patients reveal CBF values that are consistent with cerebral ischemia (<18–20 mL/100 mL/minute),5, 6 but the parallel increases in the arteriovenous oxygen concentration difference are lower than those found in cerebral ischemia.6 Positron emission tomography studies within 8–14 hours of injury show reduced CBF and global cerebral metabolic rate of oxygen (CMRO2),7 but also that brief reductions in CBF by hyperventilation (HV) are compensated by increased oxygen extraction fraction (OEF), both globally7 and regionally.8 Accordingly, the metabolic needs of brain tissue appear to be met within the first 12 hours after TBI.
After this initial hypoperfusion, the coupling between CBF and the metabolic needs of brain tissue is seemingly lost. After 12 hours, many patients develop hyperemia, with CBF values as high as twice those of normal reference tissue, whereas others maintain either low or normal CBF.6, 9, 10, 11, 12, 13, 14 Later, typically between days 4 and 15, as many as 50% of TBI patients develop signs of vasospasm and hypoperfusion.15, 16 Although these vasospasms are associated with infarction on subsequent computerized tomography images in some patients, CBF and OEF values recorded during this late phase remain higher than those recorded during the initial hypoperfusion.13, 15, 16 Because of these conflicting findings, the time at which ischemic damage occurs after TBI remains uncertain.
The apparent uncoupling of CBF and oxygen metabolism develops not only over time, but also across the brains of individual patients. Using positron emission tomography data acquired in TBI patients 9–23 hours after injury, Coles et al17 showed that the distribution of voxel OEF values after injury was much broader in TBI patients than in controls. Accordingly, some brain tissue show abnormally low OEF, consistent with either tissue damage or relative hyperperfusion, whereas a large proportion of brain tissue shows OEF values above thresholds that, in acute stroke, would characterize ischemic, infarction-prone tissue.17 Positron emission tomography studies conducted 2 to 3 days after injury show that although the CMRO2 threshold for subsequent infarction is similar to that found in acute stroke, the corresponding CBF and OEF thresholds vary greatly,18 suggesting that the uncoupling of CBF from the metabolic needs of the tissue varies across the injured brain.
The origin of the apparent flow-metabolism uncoupling remains poorly understood. Verweij et al19 reported impaired mitochondrial function in pericontusional tissue, suggesting that ATP production may be critically low, although oxygen consumption is only moderately reduced. Menon et al20 found that regional OEF showed little dependence on local tissue oxygen tension (PtO2) and changes in CBF, and proposed that diffusion from blood to tissue may be hindered by changes in capillary wall morphology. Stein et al.21 found an association between the presence of microthrombosis and selective neuronal loss, suggesting that intravascular thrombosis may contribute to the findings of widespread ischemic changes after TBI.
Capillary flow heterogeneity has been shown to reduce the extraction of diffusible solutes from the capillary bed,22, 23, 24 and animal models of elevated ICP show profoundly disturbed capillary flow patterns with evidence of ‘shunt flow'.25 In this review, we identify potential sources of capillary flow heterogeneity after TBI and examine whether the changes in CBF over time are consistent with coupling to the metabolic needs of the tissue under varying degrees of capillary flow disturbances. Then, we discuss how therapeutic interventions such as hyperventilation (HV), therapeutic hypothermia, the use of vasopressors to augment cerebral perfusion pressure (CPP), and edema management, affect brain oxygenation if capillary flow patterns are disturbed.
Changes in the Microcirculation after Traumatic Brain Injury
We first review the evidence of changes in capillary morphology and blood viscosity in the early phases after brain injury, and of changes in capillary flow patterns in conditions of elevated ICP.
Astrocytic Endfeet Swelling and Capillary Compression
Glial swelling is a prominent feature of tissue specimens obtained early after cerebral contusions in humans. In tissue removed between 3 h and 3 days after injury, disruption and gross swelling of pericapillary astrocytic endfeet is a consistent finding, and this swelling is observed to cause compression of the capillary lumen26—see Figure 1. Astrocytic endfeet swelling is limited after day 3, but microvascular compression is typically still observed within the first week.26 Astrocytic endfeet swelling has been observed as early as 1 hour after TBI in rats,27 and results in baboons further suggest that this abnormality is an early and transient phenomenon that peaks 6 hours after injury.28 In biopsies obtained the first week after injury, the capillary lumen is also affected by capillary endothelial swelling, microvascular collapse, and perivascular edema.20
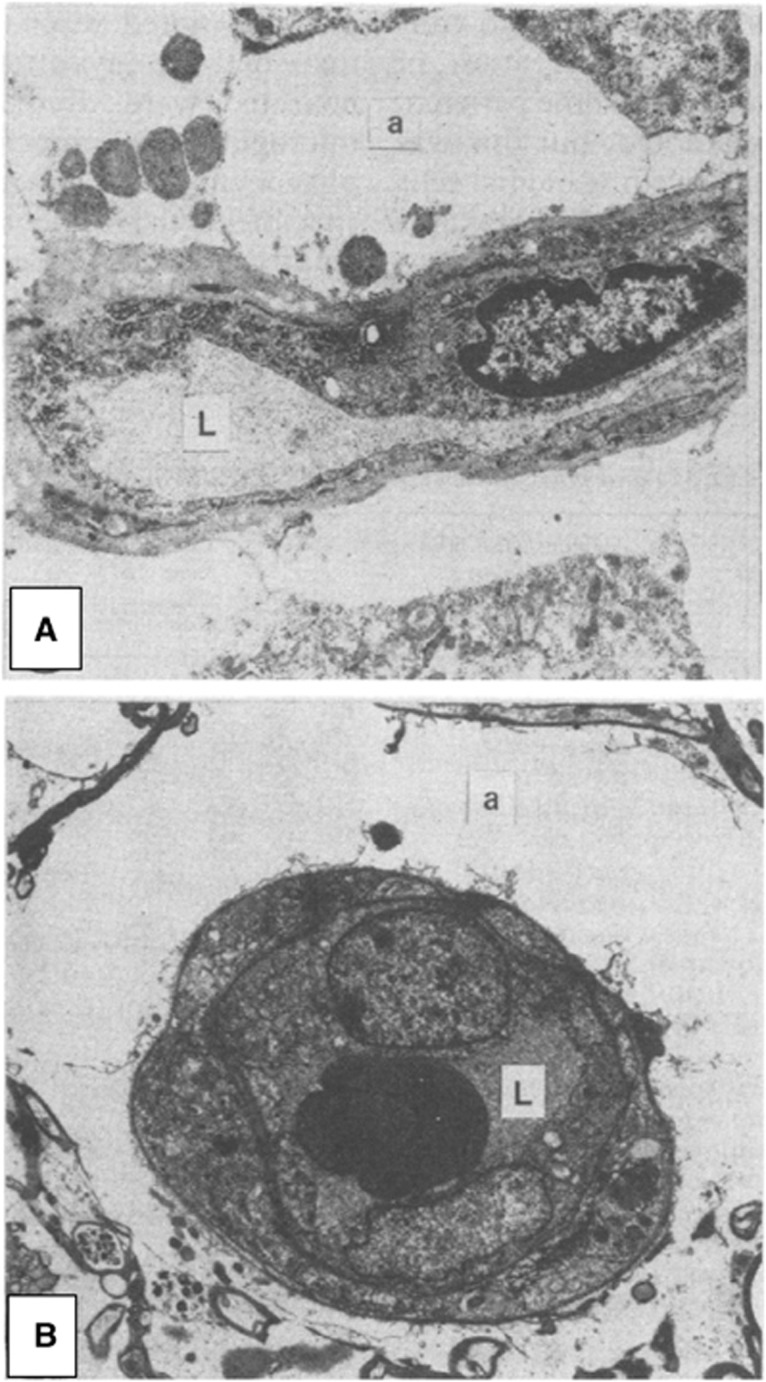
Figure 1.
Capillary compression by astrocytic endfeet swelling after cerebral contusions. Astrocytic endfeet swelling and membrane disruption is a typical finding in humans 3 hours–3 days after cerebral contusions. (A) Shows a thin section through a cortical capillary. Note the massive swelling of the perivascular astrocytic foot process (a) and the flattening of the capillary lumen (L). Magnification × 400. (B) Shows a thin section through a white matter capillary, again with gross astrocytic foot process swelling (a) and compression of the capillary lumen (L). In this case, disruptions of the membranes that limit the foot process can be observed. Magnification × 3750. Reproduced from Bullock et al,26 with permission from the publisher.
Pericyte Dysfunction
The capillary endothelium is surrounded by a basement membrane in which contractile capillary pericytes are embedded.29 Pericytes are involved in the regulation of capillary diameter during functional activation,30 in blood–brain barrier (BBB) function,31, 32 angiogenesis,33 and in aspects of the brain's immune response.34 After experimental head trauma, as many as 40% of capillary pericytes leave their pericapillary location within the first hour of the insult.35 The extent to which the loss of pericyte coverage impairs the normal control of capillary flow patterns, facilitates the formation of brain edema, or initiates inflammatory processes in the surrounding parenchyma, remains poorly understood.
Dore-Duffy et al36 recently reported widespread reductions in arteriolar and capillary diameter 4 to 8 hours after experimental TBI, coinciding with increased expression of contractile smooth muscle actin, of endothelin-1, and of its receptors (ET-A and ET-B) in pericytes. Both smooth muscle actin expression and the arteriolar and capillary constrictions were partly reversed by an ET-A antagonist.36 Importantly, the upregulation of ET-A and ET-B receptors coincided with hippocampal and sensory cortex hypoperfusion until 48 hours after TBI.37 Blockade of ET-A (but not ET-B) receptors reduced the degree of hypoperfusion in these regions at 24 hours, inducing mild hyperperfusion at 48 hours, and reducing the overall degree of neuronal damage.37
The origins of these pericyte changes remain poorly understood. Experimental cerebral ischemia has been shown to result in the constriction of cerebral pericytes, seemingly owing to the increased levels of oxidative and nitrosative stress.29, 38 Tissue that experience periods of hypoxia, critically reduced CPP, or oxidative stress at some point after TBI are therefore also likely to develop pericyte constrictions.
Intracranial Hypertension, Brain Edema, and Cerebral Hemodynamics
Increased ICP is a frequent cause of death after TBI, and the degree of brain swelling on the initial computerized tomography scan is a powerful predictor of patient outcome.39 Cerebral edema is associated with cerebral hypoperfusion, and with reductions of both cerebral blood volume (CBV) and CBF in tissue with radiologic evidence of edema.40, 41, 42 Diffusion-weighted magnetic resonance imaging has been employed in animal models of TBI to address whether the edema early after injury represents cell swelling due to acute ischemic injury (cytotoxic edema) or extravasation of fluid due to BBB leakage (vasogenic edema). Although both types of edema may cause local microvascular compression, the presence of cytotoxic edema would suggest preexisting, possibly irreversible cell injury. At 2 hours after injury (the first common time point for these studies) some studies report reduced ipsilateral apparent diffusion coefficient, which is interpreted as the presence of cytotoxic edema and a reduced extracellular space.43, 44, 45, 46, 47 Indices of fractional diffusion anisotropy and mean kurtosis (an index of non-Gaussian diffusion) agree with this interpretation.47 Other studies, however, report elevated apparent diffusion coefficient in the hours after injury.48, 49 This is believed to reflect vasogenic edema47, 50 and an enlarged extracellular space. This is supported by histology studies that show increased BBB permeability in the same period.49, 51 The BBB leakage is transient49 and these studies show apparent diffusion coefficient reductions later within the first 24 hours after TBI, indicating that cytotoxic edema is also present in these models. The etiology of edema after TBI in humans, its temporal course, and its management, however, is still not fully understood.52
In brain tissue, increased ICP and local pressure from edema or hematoma might be expected to compress capillaries and thereby disturb capillary flows: in peripheral tissue, half of the tissue capillaries are thus closed as the interstitial pressure reaches half the critical closing pressure of arterioles.53 Bragin et al54 studied the heterogeneity of capillary flow velocities during reductions in CPP by either increased ICP or reduced mean arterial pressure (MAP). For similar reductions in CPP, increased ICP caused redistribution of capillary flows to an extremely heterogeneous pattern, whereas reductions in MAP seemingly caused a reduction in capillary flow heterogeneity—see Figure 2.
Figure 2.
Capillary flow heterogeneity during reduced cerebral perfusion pressure (CPP): Increased intracranial pressure (ICP) versus reduced mean arterial pressure (MAP). Bragin et al,54 recorded cortical capillary erythrocyte velocities in rats at three CPP levels. In the top row, CPP reductions were the result of increased ICP, in the bottom row of reduced MAP. Note that intracranial hypertension lead to increased flow heterogeneity, with some capillaries displaying extremely high-flow velocities. During systemic hypotension , similar CPP values lead to homogenization and reductions of capillary flows, which is predicted to provide efficient oxygen extraction. Reproduced from ref. 54 with permission from the publisher.
Vasoactive Effects of Blood Breakdown Products
Hematoma and bleedings are frequent after TBI, and erythrocytes are found outside microvessels in biopsies taken around contusions throughout the first 2 weeks after injury.26 Evidence from subarachnoid hemorrhage suggest that hemolysis of subarachnoid blood peaks after ∼1 week,55 and that the period of the most intense angiographic vasospasms thereby coincides with peaking levels of oxyhemoglobin (HgbO).55 The vasoactive properties of hemoglobin breakdown products are described in detail in refs. 55, 56 and only briefly summarized here: first, the spontaneous autooxidation of HgbO to methemoglobin, and the iron released from hemoglobin, cause the release of highly reactive superoxide radicals.55, 56 Superoxides are thought to cause vasoconstriction by depleting vascular nitric oxide (NO) levels57, 58 and to cause lipid peroxidation, which in turn causes vasoconstriction and structural damage to cerebral microvessels, including the endothelial cell layer.59 Second, the breakdown of heme into bilirubin under such oxidative conditions results in the formation of bilirubin oxidation products that change the contractility, signaling, and metabolism in large vessels—see ref. 60 for a review. Third, HgbO has very high affinity for the NO and acts as a sink for this important vasodilator.55
The release of vasoactive substances into cerebrospinal fluid (CSF) or the interstitial space is likely to have widespread effects on both arteriolar and capillary tone. Interstitial fluid and solutes such as hemoglobin from brain parenchyma are removed along the basement membranes of arteries and capillaries to the cervical lymph nodes.61 Recent studies show that after intracisternal injection, molecules the size of hemoglobin distribute rapidly along penetrating arteries into brain tissue, along the basement membranes of arterioles and capillaries after which they drain into the cervical lymph nodes.62 It is therefore likely that the exposure of smooth muscle cells and pericytes to hemoglobin and other vasoactive substances is widespread during the period of vasospasm after TBI.62 In vitro experiments suggest that NO acts as a pericyte dilator63, 64 whereas the oxidative and nitrosative stress that result from the release of superoxide radicals have been shown to cause pericyte constrictions in vitro38 and in vivo.38
Blood Viscosity
Increased count and endothelial adhesion of leukocytes are known to disturb capillary flow patterns and lead to functional ‘shunting' of erythrocytes through the capillary bed.65 In biopsies acquired from TBI patients, adhesion of both erythrocytes and leukocytes to the vascular endothelium is observed throughout the first weeks after injury, suggesting that abnormal endothelial adhesion of blood cells may indeed disturb capillary flow patterns.26 The notion that elevated blood viscosity and impaired capillary blood passage impair brain oxygenation is consistent with findings that leukocytosis is associated with poor outcome and secondary increases in ICP after TBI.66, 67, 68 Also, administration of corticosteroids, a frequent cause of leukocytosis, in the early phases after TBI is associated with poor outcome 69
Increased blood lipid levels increase blood viscosity and would be expected to increase the heterogeneity of capillary perfusion patterns. Statin use at the time of injury has been shown to correlate with better survival and functional outcome in older head-injured individuals.70 The lipid-lowering effect of statins becomes significant 2 to 4 days after initiation of treatment in normocholesterolemic subjects,71 and any benefits of early, post-injury administration of statins are therefore likely to depend on mechanisms other than their viscosity-lowering effects.
Neurovascular Coupling in the Presence of Capillary Transit Time Heterogeneity
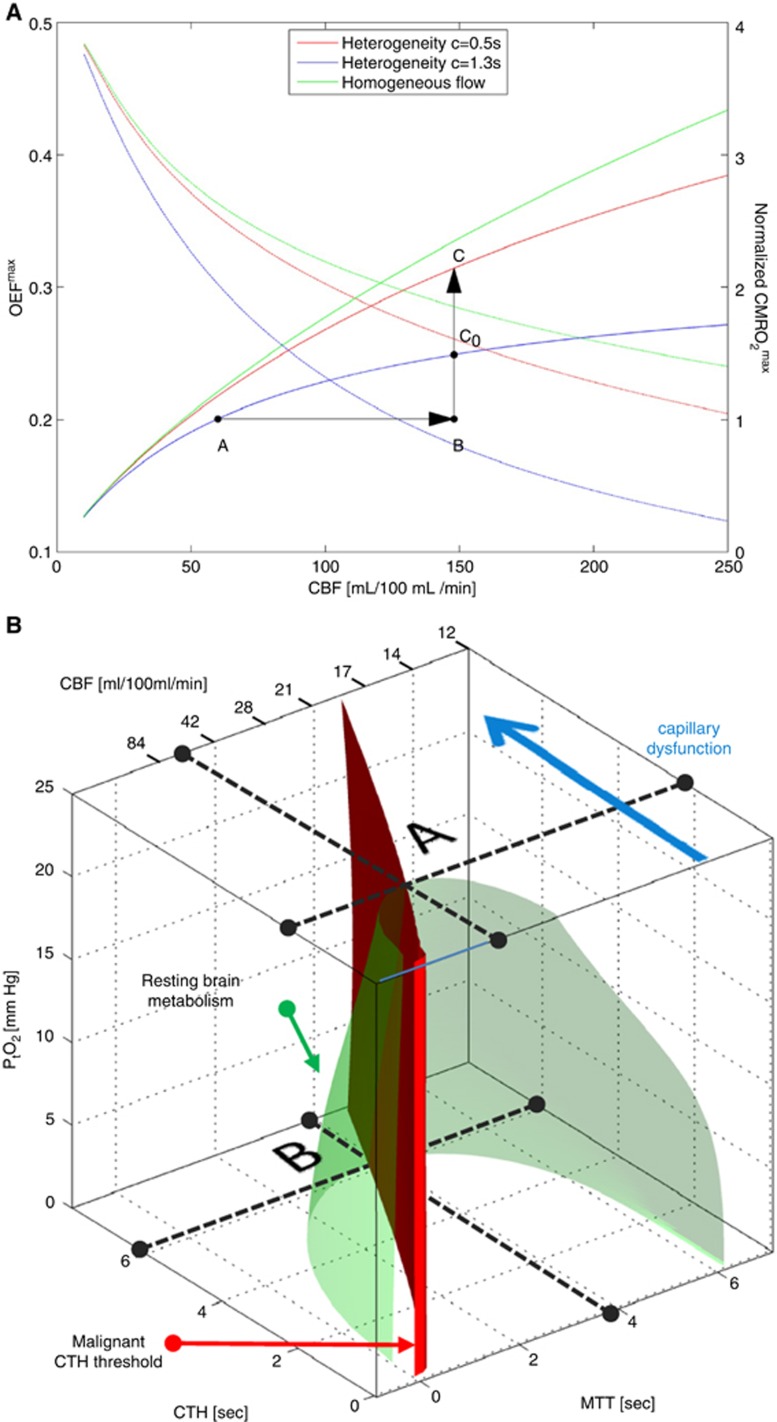
Oxygen transport in tissue is traditionally described by combining the classic flow-diffusion or Crone–Renkin equation72, 73 with accepted properties of oxygen binding to hemoglobin,74 under the assumption that OEF is identical for all tissue capillaries. In the extension of this formalism to take capillary transit time heterogeneity (CTH) into account, which is derived in ref. 22 and briefly summarized below, the Crone–Renkin formalism corresponds to the special case of negligible CTH. The maximum OEF (OEFmax) and metabolic rate of oxygen (CMRO2max) that can be supported for a given CBF in this case are shown as a green curves in Figure 3A. Note that CMRO2max measures tissue oxygenation (oxygen availability) and equals tissue CMRO2 if CBF remains perfectly coupled to the metabolic needs of the tissue. The plot was calculated for a PtO2 of 26 mm Hg75 where hemoglobin is half saturated with oxygen. The highest OEFmax value is therefore 0.5 in the plot. Note that OEFmax is high at low CBF values, whereas oxygen extraction becomes increasingly inefficient toward high CBF values.
Figure 3.
The effects of capillary transit time heterogeneity (CTH) of tissue oxygenation. In A, the green curves show, as a function of cerebral blood flow (CBF), the maximum oxygen extraction fractions (OEFmax—decreasing curves), and the maximum oxygen metabolism that can be supported (CMRO2max—increasing curve) as predicted by the flow-diffusion or Crone–Renkin equation72, 73 and accepted properties of oxygen binding to hemoglobin.74 This approach assumes that OEF is identical for all tissue capillaries. We extended this formalism to take CTH into account22 and note that the Crone–Renkin formalism corresponds to the special case of zero CTH. The blue and red curves correspond to the CTH values we previously estimated from data recorded during rest (CTH=1.3) and during functional activation (CTH=0.5) in rat brain, respectively.22, 76 Note how the combination of increased CBF and reduced CTH (A→C) efficiently increases oxygen availability during functional activation, unlike an increase in CBF without concomitant reductions in CTH (A→C0). All curves were calculated for a tissue oxygen tension (PtO2) of 26 mm Hg75 where hemoglobin is half saturated with oxygen. See text. In B, the green isocontour surface corresponds to the hemispheric metabolic rate of oxygen in resting brain.149 The red plane marks the boundary, left of which vasodilation no longer improves tissue oxygen availability (malignant CTH). The maximum value that CTH can attain at a PtO2 of 25 mm Hg, if oxygen availability is to remain above that of resting tissue, is indicated by the label A. As CTH increases further, a critical limit is reached as PtO2 approaches zero—label B. At this stage, the metabolic needs of tissue cannot be supported unless mean transit time is prolonged to a threshold of ∼4 seconds, corresponding to CBF=21 mL/100 mL/min. The blue arrow indicates how capillary dysfunction causes CTH to change, and tissue oxygen availability to approach the metabolic requirements of resting brain tissue (the green isocontour). Reproduced and modified from ref. 80.
The low OEFmax toward high flow values is the very reason why capillary flow distributions matter: if capillary flow patterns are distributed according to a certain distribution around the mean flow (CBF), then fast-flowing blood introduces a net reduction in OEFmax, and thereby CMRO2max, relative to the homogenous case assumed by the traditional Crone–Renkin equation.22, 23 The red and blue curves in Figure 3A show the relations between CBF, OEFmax, and CMRO2max, respectively, for CTH values estimated during rest (CTH=1.3) and during functional activation (CTH=0.5) in rat brain.22, 76 Note how the combination of increased CBF and reduced CTH (A→C) efficiently increases oxygen availability during functional activation, unlike an increase in CBF without concomitant reductions in CTH (A→C0).
Perhaps the most important property of the curves in 3A is that if CTH is high, and fails to decrease during increases in CBF (so-called capillary dysfunction) then vasodilation gradually fails as a means of increasing oxygen availability in tissue. Note that if CTH is maintained at the level of resting tissue, then a 250% increase in CBF (A→B) is needed to increase brain oxygenation by 50% (B→C0). If CTH increases further, our analysis shows that for a fixed PtO2, even this degree of hyperemia cannot improve tissue oxygenation sufficiently to support the metabolic needs of brain tissue.22 Instead, the extraction of sufficient oxygen to meet metabolic needs become contingent on the suppression of flow responses to (i) limit the number of erythrocytes that pass through the microvasculature too fast to exchange oxygen with the tissue, and (ii) permit oxidative metabolism to reduce PtO2 such that blood–tissue oxygen concentration gradients, and thereby OEF, increase.
If the suppression of flow responses fails, a critical condition dubbed malignant CTH develops:22 Here, high CTH and CBF combine to cause a reduction in OEFmax that outweighs the normal benefits of increased CBF. As a result, CBF increases will, paradoxically, either fail to improve tissue oxygenation, or cause it to deteriorate.22 In the brain, this would manifest itself as a paradox reduction in PtO2 during episodes of increased blood flow, and be associated with extreme risk of tissue injury. We note that such changes are characteristic of the so-called luxury perfusion syndrome, which is associated with extreme risk of infarction after cerebral ischemia.77
The Combined Effect of Cerebral Blood Flow, Capillary Transit Time Heterogeneity, and Tissue Oxygen Tension on Brain Oxygenation
Figure 3B shows how CBF, CTH, and PtO2 (along the three axes) in combination can secure sufficient oxygen to support normal brain function—see also ref. 78. In addition to CBF, capillary blood mean transit time is provided as x-axis, assuming fixed capillary blood volume. Mean transit time is given by the central volume theorem79 as the ratio between capillary blood volume and CBF. The green surface shows all combinations of mean transit time (or CBF), CTH, and PtO2 that make 2.5 mL oxygen available to each 100 mL of tissue per minute. This rate of oxygen delivery is a conservative estimate of the metabolic needs of cortical gray matter, and the interior of the green half-cone therefore represents hemodynamic conditions that can support normal brain function provided that arterial hemoglobin concentration and oxygen saturation are maintained at normal values.
The figure illustrates why, theoretically, reductions in PtO2 and in resting CBF are the only alternatives to immediate neurologic symptoms and tissue damage if CTH increases and fails to homogenize during hyperemia. Label A in Figure 3B shows the theoretical maximum for the increase in CTH (indicated by a broken line parallel to the mean transit time and CBF axes) that brain tissue can sustain at the PtO2 of normal brain tissue (25 mm Hg)75 before neuronal metabolism is predicted to become impaired. As CTH approaches this limit, oxygen availability gradually approaches the consumption by tissue, causing PtO2 to decrease. At lower PtO2, oxygen extraction is more efficient, and the green cone therefore wider. Therefore, tissue metabolism can be maintained despite increasing CTH, until oxygen tension cannot be reduced further (label B). Note that as CTH increases, CBF must be attenuated (mean transit time prolonged) in parallel to reduce the loss of oxygenated blood through ‘functional shunts' (as opposed to ‘true' anatomic shunts) through the capillary bed. When CTH approaches a critical threshold (6 seconds for the transit time distribution adapted in ref. 80), and PtO2 zero, CBF is predicted to approach 21 mL/100 mL/minute.80 If CBF is indeed attenuated to optimize brain oxygenation as capillary flow patterns become increasingly disturbed, CBF values would therefore be predicted to be close to the classic ischemic threshold of 20 to 21 mL/100 mL/minute when neurologic symptoms ensue.81 Accordingly, the classic ischemic threshold may not only be characteristic of thromboembolic events, but also of CBF adaptations to secure sufficient oxygenation in the face of critical disturbances of capillary flow patterns.78
Cerebral Blood Flow, Cerebral Blood Flow Responses, and Oxygen Extraction Fraction for Three Levels of Capillary Transit Time Heterogeneity Increase
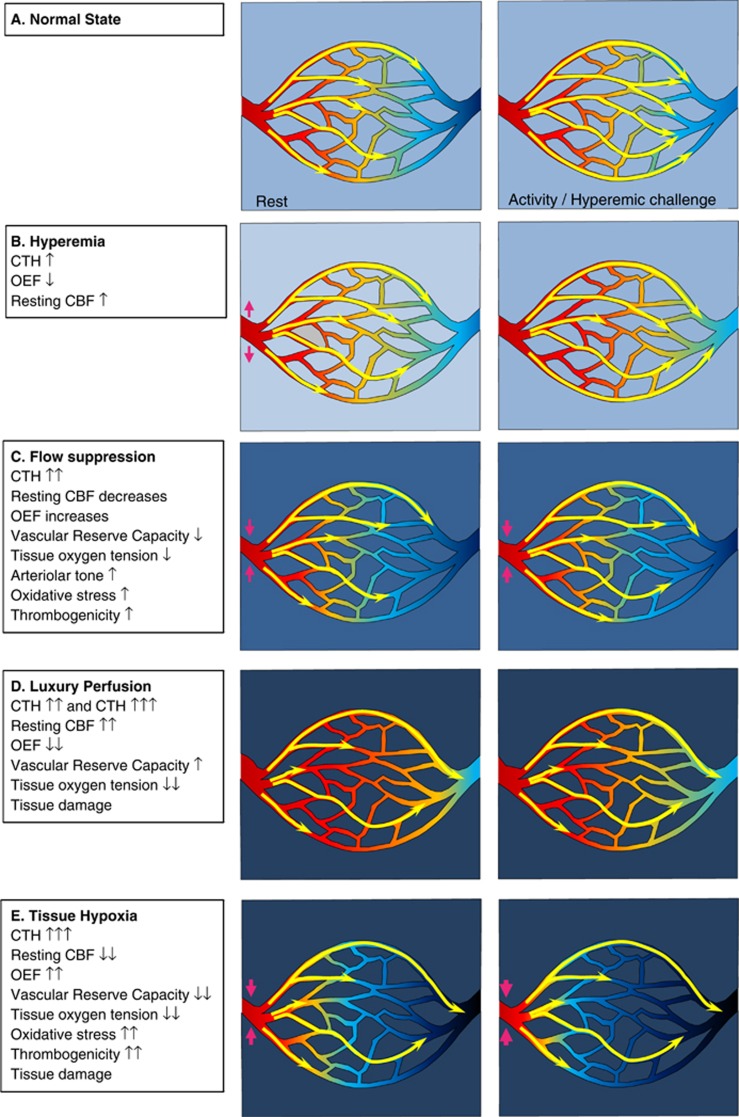
Figure 4A illustrates the dynamics of CBF and capillary flow patterns during rest and during increased metabolic needs in normal brain. The flux of erythrocytes through cortical brain capillaries is highly heterogeneous during rest, but become more homogenous during functional activation. This phenomenon maintains relatively high OEFmax during functional activation, even without changes in PtO2.
Figure 4.
Levels of capillary dysfunction (capillary transit time heterogeneity (CTH) increase). The yellow arrows indicate erythrocyte flows with different velocities through the capillary bed. The color within the vessels indicates oxygen saturation, and the background color outside indicates tissue oxygen tension. (A) The normal state, in which capillary flow patterns homogenize during hyperemia, cf. Figure 3A. (B) The hyperemic state, where slight CTH increases leads to increased cerebral blood flow (CBF). (C) The flow suppression state. (D) Luxury perfusion, a state of vasoparalysis, extreme hyperemia, and poor oxygen extraction. (E) Tissue hypoxia. Reproduced and modified from ref. 116.
Mild Capillary Dysfunction: Hyperemia
Figure 4B illustrates the metabolic consequence of mild capillary dysfunction: capillary flows are more heterogeneous than in normal tissue during rest, and cannot homogenize during hyperemia. The elevated CTH reduces the OEF that can be attained for a given PtO2, but the condition is defined by the fact that the metabolic needs of tissue can be met by slight increases in CBF, both during rest and during hyperemia (e.g., functional activation or hypercapnia). We therefore refer to states of mild CTH increases as hyperemic. The various capillary dysfunction stages, and their predicted degree of metabolic impairment, are summarized in Table 1.
Table 1. Relation between dynamic changes in CBF, PtO2, and blood oxygenation under various degrees of capillary dysfunction and neurovascular coupling.
| Capillary dysfunction | Neurovascular coupling/flow suppression | Anti-correlated | Correlated | Infarct risk |
|---|---|---|---|---|
| None | Yes | CPP versus CBF | CBF versus PtO2 CBF versus HgbO | None |
| Mild | Yes | CPP versus CBF | CBF versus PtO2 CBF versus HgbO | Low |
| Moderate | Yes | CBF versus PtO2 | Moderate | |
| Flow suppression | ←CBF versus HgbO | |||
| CPP versus CBF | ||||
| No (malignant CTH, luxury perfusion) | CBF versus PtO2 | CBF versus HgbO CPP versus CBF | High | |
| Severe | Yes Flow suppression | CBF versus PtO2 CBF versus HgbO CPP versus CBF | Very high | |
| No (malignant CTH, luxury perfusion) | CBF versus PtO2 | CBF versus HgbO CPP versus CBF | Extreme | |
CBF, cerebral blood flow; CPP cerebral perfusion pressure; CTH, capillary transit time heterogeneity; HgbO, oxygenated blood concentration; PtO2, tissue oxygen tension.
Note that the concentration of oxygenated blood is predicted to undergo a transition from being correlated with CBF to being anti-correlated at the transition from moderate to severe capillary dysfunction. See text.
Moderate Capillary Dysfunction: Flow Suppression or Luxury Perfusion and Tissue Damage
At higher CTH levels, hyperemia can no longer compensate for the parallel reduction in OEFmax during episodes of increased metabolic needs—or even during rest. Tissue function and survival in this stage depend on mechanisms that suppress normal, vasodilatory responses—see Figure 4C. As we argued above, failure to inhibit CBF or CBF responses may lead to uncontrolled hyperperfusion, tissue hypoxia, and tissue injury similar to that observed in luxury perfusion syndrome77—see Figure 4D. Note that an improvement in CTH from moderate to being only mild is expected to result in a transition from hypoperfusion to hyperperfusion and improved tissue oxygenation if CBF remains coupled to the metabolic demands of the tissue. The hyperperfusion and attenuated neuronal damage observed by Dore-Duffy et al. after ET-A blockade in their TBI model are therefore consistent with improved capillary flows and a therapeutic reduction of CTH as capillary constrictions resolve.37
Microvascular responses to a range of vasodilators are impaired after TBI in patients82 and in experimental models of TBI.83, 84, 85, 86 According to the analysis above, this phenomenon is in fact predicted to improve tissue oxygenation under conditions of elevated CTH, by suppressing flow increases that would otherwise fail to improve, or even reduce, tissue oxygenation. We have speculated that if ‘normal' vasodilation results in tissue hypoxia because of elevated CTH, this would increase hypoxia-inducible factor 1 (HIF-1) levels (see discussion of hypoxia-inducible transcription factor 1 alpha - HIF-1α - in TBI below),87 thereby upregulating nicotinamide adenine dinucleotide phosphate oxidase 2, NOX-2,88 a major source of reactive oxygen species (ROS) in the brain vasculature.89 Reactive oxygen species, in turn, react vividly with NO to attenuate vasodilation, and in this way, capillary dysfunction (elevated CTH) could attenuate microvascular flow responses via a hypoxia sensitive mechanism. Indeed, HIF-1 has been reported to upregulate NOX-2 levels as early as 1 hour after experimental TBI, and again 2 to 4 days after injury.90
If the suppression of CBF responses is coupled to the metabolic needs of the tissue, then hypoxia would be predicted to cause further attenuation of vasodilator responses to improve oxygen extraction efficacy, while reductions in the brain's oxygen metabolism would be expected to relax the need for flow suppression. Therapeutic, moderate hypothermia is reported to reduce CMRO2 by 5% to 7% per degree Celsius reduction in core temperature.91 The prediction that impaired microvascular flow responses after TBI represent adaptations to secure tissue oxygenation when CTH is high, is consistent with the finding that responses to standardized vasodilatory stimuli are reduced during hypoxia, but increased during hypothermia.92 We discuss the therapeutic effects of hypothermia further below.
Severe Capillary Transit Time Heterogeneity Increase: Hypoxia and Tissue Damage
If CTH values become very high, then uncontrolled hyperemia (luxury perfusion) leads to even more severe tissue hypoxia than outlined above, and is highly likely to cause tissue damage (see Table 1). If vasodilation is suppressed, low CBF and low PtO2 may contribute to tissue damage in several ways—see Figure 4E. First, the reduction of PtO2 is likely to cause spreading depolarizations and neuronal injury.93 Second, low PtO2 activates HIF-1α in TBI models.87 Increased HIF-1α, in turn, is a powerful stimulus for BBB opening and edema formation, possibly via the upregulation of aquaporin expression.87, 94 In addition to the detrimental effects of brain edema, pericapillary fluid is likely to further compress capillaries and thereby add to a vicious cycle of further CTH increase and hypoxia. Third, HIF-1 also upregulates nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX-2) levels, a prominent source of superoxide—a ROS—as early as 1 hour after experimental TBI, and again 2 to 4 days after injury.90 Reactive oxygen species reacts with NO to form the much more toxic peroxynitrite.95 Although NO depletion and peroxynitrite both impair smooth muscle cell relaxation and thereby offer a putative mechanism for the crucial flow suppression in this stage,96 peroxynitrite also inactivates tissue plasminogen activator, increasing thrombogenicity and thereby the risk of further tissue damage. Both platelet accumulation and widespread hypoperfusion have been observed as early as 30 minutes after TBI in rats, but their spatial distributions did not suggest that hypoperfusion is secondary to thrombosis.97 In biopsies from TBI patients, microvascular constrictions and microthrombosis have been demonstrated within hours of injury,28 consistent with the notion that flow suppression, and possibly relative tissue hypoxia, may be present at the time of astrocytic endfeet swelling early after TBI.28 The extent to which microthrombosis causes significant hypoperfusion after TBI, however, remains unclear.21 Finally, hypoxia and peroxynitrite are also known to damage mitochondria.96 Mitochondrial dysfunction, in turn, exacerbates the energy crisis by reducing the amount of ATP that can be obtained from the available oxygen, and amplifies the production of ROS.96 The generation of ROS has also been shown to alter mitochondrial dynamics and functions. Accordingly, ROS induces accelerated mitochondrial fission, increased permeability of the mitochondrial membrane, accumulation of Ca2+, and the production of even more ROS.98 Mitochondrial dysfunction is therefore likely to increase the risk of cellular damage and apoptosis in the already compromised neuronal tissue.
The hallmark of hypoxic tissue injury caused by severely disturbed capillary flow patterns is that CBF remains above the ‘classic' ischemic threshold—and even above normal CBF in cases of luxury perfusion. Another key feature of this condition is that a large proportion of blood passes through the capillary bed too fast to permit proper extraction of oxygen by the tissue. As a result, the oxygen tension of mixed venular blood is predicted to be significantly higher than that of tissue. Therefore, conditions of high CTH mimic both a diffusion hindrance of oxygen,20 and partial shunting of blood through anatomic shunts.54
Is the evolution of Cerebral Blood Flow and Oxygen Extraction Fraction after Traumatic Brain Injury Consistent with Flow-Metabolism Coupling During Various Degrees of Capillary Dysfunction?
Regional brain metabolism after TBI is affected by the patient's medication and level of consciousness, and by the extent of neuronal death in relation to both the initial injury and subsequent hypoxic episodes. With this caveat, we will briefly discuss whether the evolution of CBF, OEF, and their relation to outcome, can be understood in terms of changes in capillary perfusion patterns, accompanied by either successful (although perhaps futile) coupling of CBF to the metabolic needs of the tissue, or by insufficient flow suppression (luxury perfusion).
Based on the time course of the capillary changes reviewed above, we hypothesize that CTH is high during the initial hours after injury owing to increased ICP and glial swelling—see Figure 5. We hypothesize that regional CBF is suppressed to near-ischemic levels in the affected tissue, securing optimal tissue oxygenation under these conditions. In this state, the metabolic reserve is predicted to be either exhausted or low, and accompanied by low PtO2. This is consistent with findings that low tissue oxygen is a strong, independent predictor of poor outcome.99 The prediction that the early stage of hypoperfusion represents a stage of severe metabolic impairment is supported by the finding that during this stage, CBF is correlated with motor score and outcome, whereas no such correlation can be found beyond 12 h.6 Overgaard et al10 confirmed that outcome in patients who proceed to develop hyperemia (consistent with a milder CTH increase and either compensatory hyperemia, or luxury perfusion) is better than in those who display continued hypoperfusion (consistent with moderate or severe CTH increase and tissue hypoxia). Also, in a fluid percussion injury model of TBI in rats, contusive injury 3 occurs in regions that show severe reductions in CBF as early as 30 minutes after the injury.100
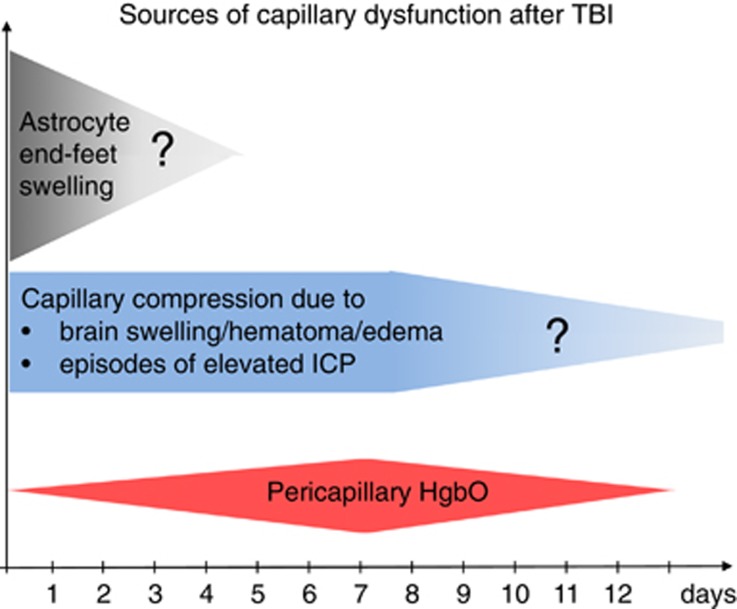
Figure 5.
Sources of capillary dysfunction after traumatic brain injury (TBI). The colored panels summarize the sources of capillary flow disturbances as identified in this review, and their estimated duration (x-axis). They include astrocyte endfeet swelling observed 3 hours to 3 days after contusions in humans (Figure 1), capillary compressions due to local swelling and increased intracranial pressure (ICP) throughout the critical period after the injury, and oxidative stress and nitric oxide depletion caused by pericapillary oxyhemoglobin. The extent to which structural damage to the capillary wall is permanent is crucial because permanent changes in capillary transit time heterogeneity (CTH) are potential sources of long-term, neurodegenerative changes.150 Reproduced and modified from ref. 116.
The prediction that the ‘optimal CBF' for brain oxygenation in the injured brain may be lower than that of normal brain is supported by studies that have suggested unwanted effects of augmenting CPP and thereby CBF: Coles et al101 demonstrated that an increase in CPP from 70 to 90 mm Hg increased both CBF and cerebral blood volume, while reducing OEF, CMRO2, and neuronal electrical function. The prediction that CBF-increases may result in paradox reductions in brain oxygenation due to malignant CTH is consistent with findings by Budohoski et al102, who recorded spontaneous changes in MAP, arterial flow velocities, PtO2, and blood oxygenation in TBI patients. In 46% of their recorded cases, PtO2 changed in proportion to blood flow, as one would expect if oxygen delivery were limited by CBF alone. In the remaining 54 %, however, PtO2 was anti-correlated with MAP and flow, i.e., increases in MAP and blood flow reduced PtO2, or vice versa,102 consistent with a state of elevated CTH in which increases in CBF no longer improves tissue oxygenation as it would be predicted by the classic Crone–Renkin equation. In these cases, some degree of flow suppression may be present, and the observed reductions in PtO2 may increase OEF sufficiently to meet the metabolic needs of the tissue, in which case, these events would be accompanied by reductions in blood oxygenation at the site of PtO2 measurement. This correlation was observed in only 60% of cases, suggesting that malignant CTH and insufficient suppression of flow may be a frequent finding after TBI.102 The proposed, conceptual algorithm for interpreting dynamic autoregulation data in terms of degree of capillary dysfunction, luxury perfusion, and metabolic impairment, is summarized in Table 1.
Glucose Extraction and Metabolism after Traumatic Brain Injury
Under aerobic conditions, oxidative phosphorylation generates 29 to 30 ATP molecules per glucose molecule, whereas lactate production under anaerobic conditions generates only two. Knudsen et al103 used indicator dilution data and mathematical analysis to show that the extraction of glucose and glucose analogs in the brain is limited by CTH, and that efficient glucose extraction during hyperemia depends on homogenization of capillary transit times, similar to the properties of oxygen extraction.22 The extended flow-diffusion equation predicts how CTH affects the extraction efficacy of any freely diffusible solute.22 Although we have yet to refine the model to take into account the kinetics of glucose transporters and glucose metabolism, our preliminary analysis suggests that elevated CTH tends to favor the extraction of glucose over oxygen, and hence aerobic glycolysis.104 Because of this differential extraction, increases in CTH would be expected to cause the conversion of some glucose into lactate, even though CBF remains at or above standard ischemic threshold. This is consistent with animal studies by Ginsberg et al,105, who showed unusually high levels of glucose analog extraction in areas of only moderately altered CBF, and with reports of elevated lactate levels in the CSF of patients who show no signs of elevated OEF.106 The prediction that glucose extraction is better preserved than oxygen extraction at high CTH—where OEF is expected to be high and the energy crisis most severe—is consistent with findings by Abate et al,107 who found the net clearance of glucose analogs, but not CMRO2, to be preserved at high OEF in TBI patients, by Robertson et al108, who found that the combination of elevated lactate production and reduced CMRO2 predicted subsequent infarction in TBI patients with no signs of ischemia, and by Ginsberg et al105, who showed that neuronal necrosis in a rat TBI model occur in areas that show the aforementioned acute CBF–glucose metabolism uncoupling. A thorough analysis of the differential effects of CTH and tissue metabolism on the net extraction of glucose and glucose analogs must be undertaken to understand how capillary flow disturbances affect the so-called Lumped Constant, which relates glucose analog uptake to actual glucose metabolism,109, 110 and thereby the conclusions of tracer-based glucose metabolism studies after TBI.
Discussion
This review identifies four major sources of capillary flow disturbances early after TBI. First, animal studies and biopsies from TBI victims show that astrocytic endfeet swelling is a source of capillary compression immediately after injury, lasting for up to 2 to 3 days in humans. Second, animal studies suggest that elevated ICP is the source of capillary compression and considerable, functional shunting of oxygenated blood through the capillary bed, although CPP and CBF is maintained. Third, widespread pericyte constriction and dislocation are likely to disturb capillary flow patterns early (hours) after brain injury. Finally, blood from hematoma, contusions, and early exudation of erythrocytes is likely to act as a sink for NO, counteracting local vasodilation in a pattern that resembles the course of subarachnoid hemorrhage over the first 2 weeks after injury—see Figure 5. The long-term effects of permanent capillary damage and flow disturbances are discussed in ref. 111.
The most important effect of elevated CTH is that it causes some blood to pass through the capillary bed at transit times too short to permit efficient extraction of its oxygen. Therefore, tissue survival becomes contingent on the attenuation of CBF and CBF responses to limit functional ‘shunting' under conditions of high CTH. Studies of dynamic autoregulation and pressure reactivity in TBI patients show that ‘passive' changes in ICP and cerebral flow velocities in response to changes in MAP are associated with high mortality and poor functional outcome.112, 113, 114, 115 We speculate that such vasoparalysis reflect the failure of metabolic vasomotor control mechanisms—including those who block ‘futile' vasodilation and luxury perfusion in states of high CTH. Note that hyperemia that is coupled to brain metabolism (Figure 4B) carries a much better prognosis than luxury perfusion (Figure 4D), which represent an important exception from the flow-metabolism coupling otherwise assumed in Figure 5. See Table 1.
The temporal course of capillary changes observed after TBI suggests that successive stages of capillary flow disturbances may exist—see Figure 5. First, an initial, dramatic increase in regional CTH, during which CBF is suppressed, PtO2 is low, and OEF is high. Second, when CTH improves as a result of reduced capillary compression, the less severe reduction of OEFmax can ultimately be compensated by elevated CBF alone, giving rise to a hyperemic state. Meanwhile, areas in which CTH remains elevated are predicted to remain hypoperfused, and show little benefits from augmented CBF. Finally, the clearance of blood breakdown products through the perivascular space is predicted to cause widespread capillary and arteriolar vasoconstrictions for a period of time, coinciding with the observation of radiographic vasospasms.116
Our review suggests that invasive monitoring of PtO2 and blood oxygenation responses to spontaneous fluctuations in MAP and flow velocities may provide means of identifying malignant CTH and the presence neurovascular coupling, albeit locally. We speculate that dynamic autoregulation data and patient outcome data can provide means of testing our interpretation of such physiologic data and possibly inform their use in individualized TBI therapy. For global measurements of brain oxygenation by the formalism presented here, we recently demonstrated that CTH can be estimated as part of routine computerized tomography perfusion imaging,117 providing a means of noninvasively assessing the extent of capillary dysfunction across the whole brain as part of the initial patient assessment.
Which Cerebral Perfusion Pressure, Cerebral Blood Flow, and Intracranial Pressure Provide Optimal Tissue Oxygenation after Traumatic Brain Injury?
When portions of the closed cranial cavity become occupied by brain edema and hematoma, even small changes in the volume of intracranial blood vessels, brain parenchyma, or of CSF, can lead to dramatic changes in ICP that may interfere not only with CTH, but with the brain's blood supply.118, 119 The CPP is the difference between the mean arterial blood pressure and ICP, and therefore the pressure difference that drives CBF. Under the assumption that CBF determines cerebral oxygenation, it is therefore rational to maintain CPP within the normal range in patients with elevated ICP to maintain brain oxygenation. Accordingly, current guidelines recommend a CPP between 50 and 70 mm Hg and further suggest that patients with intact autoregulation may tolerate higher CPP.120 In clinical practice, CPP is managed with infusion of fluids and vasopressors.
Although elevated ICP certainly interferes with cerebral oxygenation if CBF becomes hindered, this review posits that oxygen extraction may be impaired by distortions of capillary flow patterns at CPP and ICP thresholds lower than those at which CBF becomes impaired—to an extent that exceeds the benefits of maintaining normal CBF in TBI patients. Below, we first discuss whether the maintenance of CPP within the range of normal autoregulation is a necessary and sufficient condition for avoiding brain damage, and then whether therapies that target both a lower ICP and a slightly lower CBF in fact optimize cerebral oxygenation.
In normal brain, cerebral autoregulation adjusts arterial and arteriolar tone to maintain relatively constant CBF across a wide range of MAP values.121, 122, 123 It is well known, however, that brain function is preserved at CPP values far below the autoregulatory range (MAP as low as 30 mm Hg) in resting, normal subjects124 and in animal models of hypotension.125 Homogenization of capillary flow patterns may indeed facilitate oxygen extraction (normal brain OEF is a modest 30%) under these conditions: in animal models of experimental hypotension, it has been reported that capillary flow heterogeneity is reduced as CPP falls.25, 126 We have previously reanalyzed flow heterogeneity data from a study in which CPP was reduced below the normal autoregulatory range of 70 to 115 mm Hg in rats:22, 126 Using estimates of CBF, CTH, and PtO2, we found that tissue oxygenation was in fact similar to that of normotensive animals at a CPP of 50 mm Hg, and reduced by a modest 13% at a CPP as low as 30 mm Hg.22
Similarly, the maintenance of CPP within the normal autoregulatory range does not appear to be a sufficient condition for the maintenance of cerebral oxygenation after TBI if ICP is elevated. A classic study of experimental reductions in CPP by either hypotension or elevated ICP showed that, whereas autoregulation is lost at a CPP of 80 mm Hg in systemic hypotension, autoregulation and cerebral oxygen metabolism was maintained for CPP as low as 30 mm Hg in conditions of elevated ICP.125 Bragin et al25 studied the microvascular circulation under similar conditions and found that elevated ICP is associated with a high degree of microvascular shunting, and that the ‘preservation' of CBF at low CPP is in fact associated with severe tissue hypoxia, tissue damage, and blood–brain barrier breakdown.,54
Managing Capillary Dysfunction
Taken together, reduction in CPP caused by reduced outward pressure (reduced MAP) and increased inward pressure (elevated ICP) on the microvasculature may have very different effects on CTH, and thus on brain oxygenation. Indeed, the use of ICP targeted127 or (mainly) CPP targeted approaches120 to manage patients with elevated ICP remain controversial,128 particularly in view of the reductions in CBF that accompany therapeutic vasoconstriction to reduce ICP.
Below, we briefly discuss outcomes of therapeutic strategies that would be expected to reduce CTH or to ameliorate the metabolic effects of capillary dysfunction by reducing CBF or the metabolic needs of the tissue.
Hyperventilation (HV) is a powerful means of reducing intracranial vessel diameter, and thereby ICP. The accompanying reduction in CBF, we predict, provides efficient oxygen extraction for both normal and elevated CTH. This is consistent with studies by Diringer et al7, who examined nine patients 11.2 hours after TBI, before and after 30 minutes of HV to a PaCO2 of 30 mm Hg. Although HV reduced global CBF and cerebral blood volume, OEF increased from 31% to 45%, and CMRO2 remained unchanged, suggesting that the metabolic needs of the tissue were met.7 In a follow-up study, they performed regional analysis to assess the effects of 30 minutes of moderate and severe HV on regions with low CBF values:8 Even in regions with CBF less than 10, 15, and 20 mL/100 mL/minute, OEF increased in proportion to the reduction in CBF, maintaining unaltered CMRO2 in what is likely to have been partly injured tissue.
Although brief periods of HV are considered safe, prolonged HV is currently discouraged on grounds that low PaCO2 levels may cause critical vasoconstriction and reduce CSF bicarbonate levels, reducing its pH buffering capacity. In the normal brain, the pH of CSF is tightly regulated via secretion of HCO3− by the epithelium of the choroid plexus, but the capacity of this mechanism is poorly understood.129 The lack of buffering capacity is thought to cause large changes in CBF and ICP in response to small changes in pH, for example in response to the PaCO2 changes during HV.130 To examine these potentially harmful side effects, Muizelaar et al130 conducted a randomized trial in which 36 patients received HV, 36 received HV and THAM (a substance that prevent the loss of pH buffer capacity in the CSF), and 41 controls. The number of patients with favorable outcome was significantly lower in the HV group compared with the HV+THAM group after 3 and 6, but not after 12 months. Notably, CBF values were significantly lower in the HV+THAM group (who showed alkalization of the CSF) than in the HV group, and their arteriovenous oxygen concentration difference values did not suggest ischemia. After this study, prolonged HV was abandoned, although several studies appear to prove its benefits.131 One observation that has been taken to argue against the safety of HV in TBI is the accompanying reduction in PtO2. It should be kept in mind that such reductions improve oxygen extraction by creating higher blood–tissue oxygen concentrations differences. So while low PtO2 per se indicates impaired tissue oxygenation, we propose that HV-induced reductions in PtO2 within certain boundaries may not be contraindicative as suggested by some.131 In addition to the benefits of reduced ICP, HV is known to reduce CTH in normal brain.132
Indomethacin is a cyclooxygenase inhibitor that acts as a powerful vasoconstrictor, reducing CBF uniformly by one-third in both awake and anesthetized normal brain while maintaining CMRO2.133, 134 Several studies in TBI patients have shown that an indomethacin bolus followed by continuous infusion can cause prompt and prolonged reduction of ICP and CBF,135, 136 without causing reductions in CMRO2135 or subsequent radiographic signs of ischemic damage.137 See detailed discussions in refs. 138, 139. Subsequent studies have demonstrated beneficial effects of indomethacin in the treatment of TBI patients,140, 141 and we speculate that these may be attributed in part to reductions in both ICP (and hence CTH) and CBF.
Mannitol is frequently used in the management of elevated ICP—allegedly because of its ability to reduce the degree of brain edema. Interestingly, mannitol also causes an acute reduction in blood viscosity, and thereby, one would expect, in CTH. In normal brain, the accompanying increase in OEFmax would be expected to cause rapid vasoconstriction when neurovascular coupling compensates for the more efficient oxygen extraction by reducing CBF. Indeed, mannitol (1 g/kg) acutely reduces blood viscosity by 23%, vessel diameter by 12%, and ICP by 28% in the intact rat brain.142 For comparison, HV to achieve a similar reduction in vessel diameter generates a similar reduction in ICP,142 suggesting that the acute effects of mannitol may be explained in part to improved oxygenation and compensatory vasoconstriction in tissue with intact vascular regulation—rather than reductions in edema.
In animal models of TBI, mild to moderate hypothermia has proven neuroprotective in a large number of studies—See Dietrich et al143 for a review of experimental considerations and putative protective mechanisms. Although the effects of mild hypothermia on clinical outcome in human TBI remain unclear,144, 145 the treatment is considered superior in the management of refractory intracranial hypertension.146 In addition to the neuroprotection conferred by reduced oxygen metabolism (above) and mechanisms described elsewhere,91, 143 we speculate that reductions in CBF and thereby more efficient oxygen extraction under conditions of elevated CTH, may represent an important protective mechanism. Position emission tomography studies in normal pig brain showed reductions of CBF to 20 mL/100 mL/minute, and an increase in OEF to almost 90%, during gradual cooling to 32 °C.147 Although some might fear that such CBF reduction would cause ischemic damage, we note that biophysically, net oxygen extraction appears to be at its maximum for any CTH at this CBF value,80 whereas the concurrent reduction in CMRO2 would be expected to protect the brain from hypoxic damage in the event of fluctuations in CBF, blood viscosity, or blood oxygenation (saturation and hemoglobin concentration).
Conclusion
Future studies should examine whether capillary flow disturbances are prevalent after TBI, and whether they, in combination with CBF and PtO2, predict tissue oxygenation. We propose that studies of pericyte and astrocyte endfeet function, of the effects of elevated ICP and blood viscosity on capillary flow patterns, and of edema formation, in relation to TBI may improve our understanding of hypoxic tissue injury after TBI, and lead to the identification of novel strategies to improve patient outcome. Our analysis suggest that maintenance of low CBF (just above the ischemic threshold) as well as low ICP, in combination with suppression (pharmacological and by hypothermia) of cerebral oxygen metabolism optimizes the oxygen supply–demand balance in brain tissue. Reports of acute changes in capillary morphology after TBI suggest that early intervention is critical. Dynamic autoregulation measurements may allow indirect clinical evaluation of the degree of CTH increase, and guide both the therapies above, and patient prognosis. Studies should address whether permanent capillary damage persist after both mild and severe injury, given the potential for elevated CTH to cause long-term neurodegenerative changes.111 Neuroimaging based methods to study CTH,148 or routine evaluation of CBF responses to standardized vasodilatory stimuli, may prove useful in this effort.
The authors declare no conflicts of interest.
Footnotes
This study was supported by the Danish National Research Foundation (CFIN; LØ, RA, IKM, and BH), the Danish Ministry of Science, Innovation, and Education (MINDLab; LØ, RA, NKI, KRD, JUB, AT, IKM, and BH), and the VELUX Foundation (ARCADIA, LØ, BH).
References
- Graham DI, Adams JH. Ischaemic brain damage in fatal head injuries. Lancet. 1971;1:265–266. doi: 10.1016/s0140-6736(71)91003-8. [DOI] [PubMed] [Google Scholar]
- Graham DI, Adams JH, Doyle D. Ischaemic brain damage in fatal non-missile head injuries. J Neurol Sci. 1978;39:213–234. doi: 10.1016/0022-510x(78)90124-7. [DOI] [PubMed] [Google Scholar]
- Graham DI, Ford I, Adams JH, Doyle D, Teasdale GM, Lawrence AE, et al. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52:346–350. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, Graham DI. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma. 2011;28:701–709. doi: 10.1089/neu.2010.1733. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992;77:360–368. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- Diringer MN, Yundt K, Videen TO, Adams RE, Zazulia AR, Deibert E, et al. No reduction in cerebral metabolism as a result of early moderate hyperventilation following severe traumatic brain injury. J Neurosurg. 2000;92:7–13. doi: 10.3171/jns.2000.92.1.0007. [DOI] [PubMed] [Google Scholar]
- Diringer MN, Videen TO, Yundt K, Zazulia AR, Aiyagari V, Dacey RG, Jr, et al. Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg. 2002;96:103–108. doi: 10.3171/jns.2002.96.1.0103. [DOI] [PubMed] [Google Scholar]
- Obrist WD, Gennarelli TA, Segawa H, Dolinskas CA, Langfitt TW. Relation of cerebral blood flow to neurological status and outcome in head-injured patients. J Neurosurg. 1979;51:292–300. doi: 10.3171/jns.1979.51.3.0292. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Tweed WA. Cerebral circulation after head injury. 1. Cerebral blood flow and its regulation after closed head injury with emphasis on clinical correlations. J Neurosurg. 1974;41:531–541. doi: 10.3171/jns.1974.41.5.0531. [DOI] [PubMed] [Google Scholar]
- Bruce DA, Langfitt TW, Miller JD, Schutz H, Vapalahti MP, Stanek A, et al. Regional cerebral blood flow, intracranial pressure, and brain metabolism in comatose patients. J Neurosurg. 1973;38:131–144. doi: 10.3171/jns.1973.38.2.0131. [DOI] [PubMed] [Google Scholar]
- Obrist WD, Langfitt TW, Jaggi JL, Cruz J, Gennarelli TA. Cerebral blood flow and metabolism in comatose patients with acute head injury. Relationship to intracranial hypertension. J Neurosurg. 1984;61:241–253. doi: 10.3171/jns.1984.61.2.0241. [DOI] [PubMed] [Google Scholar]
- Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, Shalmon E, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- Kelly DF, Kordestani RK, Martin NA, Nguyen T, Hovda DA, Bergsneider M, et al. Hyperemia following traumatic brain injury: relationship to intracranial hypertension and outcome. J Neurosurg. 1996;85:762–771. doi: 10.3171/jns.1996.85.5.0762. [DOI] [PubMed] [Google Scholar]
- Martin NA, Doberstein C, Zane C, Caron MJ, Thomas K, Becker DP. Posttraumatic cerebral arterial spasm: transcranial Doppler ultrasound, cerebral blood flow, and angiographic findings. J Neurosurg. 1992;77:575–583. doi: 10.3171/jns.1992.77.4.0575. [DOI] [PubMed] [Google Scholar]
- Martin NA, Doberstein C, Alexander M, Khanna R, Benalcazar H, Alsina G, et al. Posttraumatic cerebral arterial spasm. J Neurotrauma. 1995;12:897–901. doi: 10.1089/neu.1995.12.897. [DOI] [PubMed] [Google Scholar]
- Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24:202–211. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- Cunningham AS, Salvador R, Coles JP, Chatfield DA, Bradley PG, Johnston AJ, et al. Physiological thresholds for irreversible tissue damage in contusional regions following traumatic brain injury. Brain. 2005;128:1931–1942. doi: 10.1093/brain/awh536. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32:1384–1390. doi: 10.1097/01.ccm.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- Stein SC, Graham DI, Chen XH, Smith DH.Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury Neurosurgery 200454687–691.discussion 691. [DOI] [PubMed] [Google Scholar]
- Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. doi: 10.1038/jcbfm.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard L, Sorensen AG, Chesler DA, Weisskoff RM, Koroshetz WJ, Wu O, et al. Combined diffusion-weighted and perfusion-weighted flow heterogeneity magnetic resonance imaging in acute stroke. Stroke. 2000;31:1097–1103. doi: 10.1161/01.str.31.5.1097. [DOI] [PubMed] [Google Scholar]
- Østergaard L. Principles of cerebral perfusion imaging by bolus tracking. J Magn Reson Imaging. 2005;22:710–717. doi: 10.1002/jmri.20460. [DOI] [PubMed] [Google Scholar]
- Bragin DE, Bush RC, Nemoto EM. Effect of cerebral perfusion pressure on cerebral cortical microvascular shunting at high intracranial pressure in rats. Stroke. 2013;44:177–181. doi: 10.1161/STROKEAHA.112.668293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R, Maxwell WL, Graham DI, Teasdale GM, Adams JH. Glial swelling following human cerebral contusion: an ultrastructural study. J Neurol Neurosurg Psychiatry. 1991;54:427–434. doi: 10.1136/jnnp.54.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Halley M. Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J Neurotrauma. 1994;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Irvine A, Adams JH, Graham DI, Gennarelli TA. Response of cerebral microvasculature to brain injury. J Pathol. 1988;155:327–335. doi: 10.1002/path.1711550408. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Wang S, Mehedi A, Katyshev V, Cleary K, Tapper A, et al. Pericyte-mediated vasoconstriction underlies TBI-induced hypoperfusion. Neurol Res. 2011;33:176–186. doi: 10.1179/016164111X12881719352372. [DOI] [PubMed] [Google Scholar]
- Kreipke CW, Schafer PC, Rossi NF, Rafols JA. Differential effects of endothelin receptor A and B antagonism on cerebral hypoperfusion following traumatic brain injury. Neurol Res. 2010;32:209–214. doi: 10.1179/174313209X414515. [DOI] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Eisenberg HM, Gary HE, Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–698. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Hlatky R, Valadka AB, Diaz P, Robertson CS.Comparison of cerebral blood flow in computed tomographic hypodense areas of the brain in head-injured patients Neurosurgery 200352340–345.discussion 345-6.. [DOI] [PubMed] [Google Scholar]
- Steiner LA, Coles JP, Johnston AJ, Czosnyka M, Fryer TD, Smielewski P, et al. Responses of posttraumatic pericontusional cerebral blood flow and blood volume to an increase in cerebral perfusion pressure. J Cereb Blood Flow Metab. 2003;23:1371–1377. doi: 10.1097/01.WCB.0000090861.67713.10. [DOI] [PubMed] [Google Scholar]
- Schroder ML, Muizelaar JP, Bullock MR, Salvant JB, Povlishock JT. Focal ischemia due to traumatic contusions documented by stable xenon-CT and ultrastructural studies. J Neurosurg. 1995;82:966–971. doi: 10.3171/jns.1995.82.6.0966. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Portella G, Barzo P, Signoretti S, Fatouros P, Beaumont A, et al. Distinguishing between cellular and vasogenic edema in head injured patients with focal lesions using magnetic resonance imaging. Acta Neurochir Suppl. 2000;76:349–351. doi: 10.1007/978-3-7091-6346-7_72. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Beit-Yannai E, Shohami E, Berman E, Cohen Y. Diffusion- and T2-weighted MRI of closed-head injury in rats: a time course study and correlation with histology. Magn Reson Imaging. 1997;15:77–85. doi: 10.1016/s0730-725x(96)00246-9. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Knoblach SM, Chew BG, O'Reilly MP, Faden AI, Pekar JJ. Diffusion and high resolution MRI of traumatic brain injury in rats: time course and correlation with histology. Exp Neurol. 2000;162:61–72. doi: 10.1006/exnr.2000.7256. [DOI] [PubMed] [Google Scholar]
- Van Putten HP, Bouwhuis MG, Muizelaar JP, Lyeth BG, Berman RF. Diffusion-weighted imaging of edema following traumatic brain injury in rats: effects of secondary hypoxia. J Neurotrauma. 2005;22:857–872. doi: 10.1089/neu.2005.22.857. [DOI] [PubMed] [Google Scholar]
- Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59:467–477. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolizio G, Bissonnette B, Mason L, Ashwal S, Hartman R, Marcantonio S, et al. Effects of hemodilution after traumatic brain injury in juvenile rats. Paediatr Anaesth. 2011;21:1198–1208. doi: 10.1111/j.1460-9592.2011.03695.x. [DOI] [PubMed] [Google Scholar]
- Cernak I, Vink R, Zapple DN, Cruz MI, Ahmed F, Chang T, et al. The pathobiology of moderate diffuse traumatic brain injury as identified using a new experimental model of injury in rats. Neurobiol Dis. 2004;17:29–43. doi: 10.1016/j.nbd.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ebisu T, Naruse S, Horikawa Y, Ueda S, Tanaka C, Uto M, et al. Discrimination between different types of white matter edema with diffusion-weighted MR imaging. J Magn Reson Imaging. 1993;3:863–868. doi: 10.1002/jmri.1880030612. [DOI] [PubMed] [Google Scholar]
- Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- Vollmar B, Westermann S, Menger MD. Microvascular response to compartment syndrome-like external pressure elevation: an in vivo fluorescence microscopic study in the hamster striated muscle. J Trauma. 1999;46:91–96. doi: 10.1097/00005373-199901000-00015. [DOI] [PubMed] [Google Scholar]
- Bragin DE, Bush RC, Muller WS, Nemoto EM. High intracranial pressure effects on cerebral cortical microvascular flow in rats. J Neurotrauma. 2011;28:775–785. doi: 10.1089/neu.2010.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacol Ther. 2005;105:23–56. doi: 10.1016/j.pharmthera.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Rey FE, Li XC, Carretero OA, Garvin JL, Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91(phox) Circulation. 2002;106:2497–2502. doi: 10.1161/01.cir.0000038108.71560.70. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Wakai S, Asano T, Watanabe T, Kirino T, Sano K. The effect of a lipid hydroperoxide of arachidonic acid on the canine basilar artery. An experimental study on cerebral vasospasm. J Neurosurg. 1981;54:357–365. doi: 10.3171/jns.1981.54.3.0357. [DOI] [PubMed] [Google Scholar]
- Pyne-Geithman GJ, Nair SG, Stamper DN, Clark JF. Role of bilirubin oxidation products in the pathophysiology of DIND following SAH. Acta Neurochir Suppl. 2013;115:267–273. doi: 10.1007/978-3-7091-1192-5_47. [DOI] [PubMed] [Google Scholar]
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci. 1994;35:991–997. [PubMed] [Google Scholar]
- Haefliger IO, Anderson DR. Oxygen modulation of guanylate cyclase-mediated retinal pericyte relaxations with 3-morpholino-sydnonimine and atrial natriuretic peptide. Invest Ophthalmol Vis Sci. 1997;38:1563–1568. [PubMed] [Google Scholar]
- Mazzoni MC, Schmid-Schonbein GW. Mechanisms and consequences of cell activation in the microcirculation. Cardiovasc Res. 1996;32:709–719. [PubMed] [Google Scholar]
- Rovlias A, Kotsou S. Classification and regression tree for prediction of outcome after severe head injury using simple clinical and laboratory variables. J Neurotrauma. 2004;21:886–893. doi: 10.1089/0897715041526249. [DOI] [PubMed] [Google Scholar]
- Keskil S, Baykaner MK, Ceviker N, Aykol S. Head trauma and leucocytosis. Acta Neurochir (Wien) 1994;131:211–214. doi: 10.1007/BF01808615. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Kiening K, Schmiedek P, Lanksch W.Long-term observations of intracranial pressure after severe head injury. The phenomenon of secondary rise of intracranial pressure Neurosurgery 19933217–23.discussion 23-4. [DOI] [PubMed] [Google Scholar]
- Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- Schneider EB, Efron DT, MacKenzie EJ, Rivara FP, Nathens AB, Jurkovich GJ. Premorbid statin use is associated with improved survival and functional outcomes in older head-injured individuals. J Trauma. 2011;71:815–819. doi: 10.1097/TA.0b013e3182319de5. [DOI] [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol. 2001;88:1306–1307. doi: 10.1016/s0002-9149(01)02095-1. [DOI] [PubMed] [Google Scholar]
- Crone C. The permeability of capillaries in various organs as determined by use of the 'Indicator Diffusion' method. Acta Physiol Scand. 1963;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Renkin EM. B. W. Zweifach Award lecture. Regulation of the microcirculation. Microvasc Res. 1985;30:251–263. doi: 10.1016/0026-2862(85)90057-3. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Watabe H, Kudomi N, Kim KM, Enmi J, Hayashida K, et al. A theoretical model of oxygen delivery and metabolism for physiologic interpretation of quantitative cerebral blood flow and metabolic rate of oxygen. J Cereb Blood Flow Metab. 2003;23:1314–1323. doi: 10.1097/01.WCB.0000090506.76664.00. [DOI] [PubMed] [Google Scholar]
- Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9:1207–1219. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, et al. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab. 2008;28:961–972. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA. The luxury-perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet. 1966;2:1113–1115. doi: 10.1016/s0140-6736(66)92199-4. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Jespersen SN, Mouridsen K, Mikkelsen IK, Jonsdottir KY, Tietze A, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab. 2013;33:635–648. doi: 10.1038/jcbfm.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GN. Researches on the circulation time in organs and on the influences which affect it. Parts I.-III. J Physiol. 1893;15:1–89. doi: 10.1113/jphysiol.1893.sp000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard L, Jespersen SN, Mouridsen K, Mikkelsen IK, Jonsdottir KY, Tietze A, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab. 2013;33:635–648. doi: 10.1038/jcbfm.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa RS, Baron JC.Perfusion Thresholds in Cerebral IschemiaIn: Donnan GA, Baron JC, Davis SM, Sharp FR, (eds)Informa Healthcare USA, Inc.: New York; 200731–36. [Google Scholar]
- Enevoldsen EM, Jensen FT. Autoregulation and CO2 responses of cerebral blood flow in patients with acute severe head injury. J Neurosurg. 1978;48:689–703. doi: 10.3171/jns.1978.48.5.0689. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Kontos HA, Wei EP, Rosenblum WI, Becker DP. Changes in the cerebral vasculature after hypertension and trauma: a combined scanning and transmission electron microscopic analysis. Adv Exp Med Biol. 1980;131:227–241. doi: 10.1007/978-1-4684-3752-2_18. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP. Endothelium-dependent responses after experimental brain injury. J Neurotrauma. 1992;9:349–354. doi: 10.1089/neu.1992.9.349. [DOI] [PubMed] [Google Scholar]
- Wei EP, Dietrich WD, Povlishock JT, Navari RM, Kontos HA. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ Res. 1980;46:37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]
- Ellison MD, Erb DE, Kontos HA, Povlishock JT. Recovery of impaired endothelium-dependent relaxation after fluid-percussion brain injury in cats. Stroke. 1989;20:911–917. doi: 10.1161/01.str.20.7.911. [DOI] [PubMed] [Google Scholar]
- Ding JY, Kreipke CW, Speirs SL, Schafer P, Schafer S, Rafols JA. Hypoxia-inducible factor-1alpha signaling in aquaporin upregulation after traumatic brain injury. Neurosci Lett. 2009;453:68–72. doi: 10.1016/j.neulet.2009.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226:2925–2933. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, et al. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One. 2012;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RA, Alam HB. Induced hypothermia for trauma: current research and practice. J Intensive Care Med. 2010;25:205–226. doi: 10.1177/0885066610366919. [DOI] [PubMed] [Google Scholar]
- Gao G, Oda Y, Wei EP, Povlishock JT. The adverse pial arteriolar and axonal consequences of traumatic brain injury complicated by hypoxia and their therapeutic modulation with hypothermia in rat. J Cereb Blood Flow Metab. 2010;30:628–637. doi: 10.1038/jcbfm.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, et al. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011;114:92–101. doi: 10.3171/2010.6.JNS10207. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Prado R, Dewanjee S, Dewanjee MK, et al. Widespread hemodynamic depression and focal platelet accumulation after fluid percussion brain injury: a double-label autoradiographic study in rats. J Cereb Blood Flow Metab. 1996;16:481–489. doi: 10.1097/00004647-199605000-00015. [DOI] [PubMed] [Google Scholar]
- Moran M, Moreno-Lastres D, Marin-Buera L, Arenas J, Martin MA, Ugalde C. Mitochondrial respiratory chain dysfunction: implications in neurodegeneration. Free Radic Biol Med. 2012;53:595–609. doi: 10.1016/j.freeradbiomed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Figaji AA, Zwane E, Thompson C, Fieggen AG, Argent AC, Le Roux PD, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 1: relationship with outcome. Childs Nerv Syst. 2009;25:1325–1333. doi: 10.1007/s00381-009-0822-x. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Prado R, Zhao W, Dewanjee MK, et al. Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats Neurosurgery 199843585–593.discussion 593-4. [DOI] [PubMed] [Google Scholar]
- Coles JP, Steiner LA, Johnston AJ, Fryer TD, Coleman MR, Smieleweski P, et al. Does induced hypertension reduce cerebral ischaemia within the traumatized human brain. Brain. 2004;127:2479–2490. doi: 10.1093/brain/awh268. [DOI] [PubMed] [Google Scholar]
- Budohoski KP, Zweifel C, Kasprowicz M, Sorrentino E, Diedler J, Brady KM, et al. What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. Br J Anaesth. 2012;108:89–99. doi: 10.1093/bja/aer324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen GM, Pettigrew KD, Paulson OB, Hertz MM, Patlak CS. Kinetic analysis of blood-brain barrier transport of D-glucose in man: quantitative evaluation in the presence of tracer backflux and capillary heterogeneity. Microvasc Res. 1990;39:28–49. doi: 10.1016/0026-2862(90)90057-x. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Tietze A, Nielsen T, Drasbek KR, Mouridsen K, Jespersen SN, et al. The relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res. 2013;73:5618–5624. doi: 10.1158/0008-5472.CAN-13-0964. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Zhao W, Alonso OF, Loor-Estades JY, Dietrich WD, Busto R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol. 1997;272:H2859–H2868. doi: 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate MG, Trivedi M, Fryer TD, Smielewski P, Chatfield DA, Williams GB, et al. Early derangements in oxygen and glucose metabolism following head injury: the ischemic penumbra and pathophysiological heterogeneity. Neurocrit Care. 2008;9:319–325. doi: 10.1007/s12028-008-9119-2. [DOI] [PubMed] [Google Scholar]
- Robertson CS, Grossman RG, Goodman JC, Narayan RK. The predictive value of cerebral anaerobic metabolism with cerebral infarction after head injury. J Neurosurg. 1987;67:361–368. doi: 10.3171/jns.1987.67.3.0361. [DOI] [PubMed] [Google Scholar]
- Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, et al. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J Cereb Blood Flow Metab. 1985;5:179–192. doi: 10.1038/jcbfm.1985.24. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Aamand R, Gutierrez-Jimenez E, Ho Y-L, Blicher JU, Madsen SM, et al. The capillary dysfunction hypothesis of Alzheimer's disease. Neurobiol Aging. 2013;34:1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- Zweifel C, Castellani G, Czosnyka M, Carrera E, Brady KM, Kirkpatrick PJ, et al. Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41:1963–1968. doi: 10.1161/STROKEAHA.109.577320. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD.Continuous assessment of the cerebral vasomotor reactivity in head injury Neurosurgery 19974111–17.discussion 17-9. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Aamand R, Karabegovic S, Tietze A, Blicher JU, Mikkelsen IK, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33:1825–1837. doi: 10.1038/jcbfm.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kate M, Hansen MB, Mouridsen K, Østergaard L, Choi V, Gould B, et al. Blood pressure reduction does not reduce perihematoma oxygenation. J Cereb Blood Flow Metab. 2013;34:81–86. doi: 10.1038/jcbfm.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro A. Observations On The Structure And Functions Of The Nervous System. Creech and Johnson: London; 1783. [Google Scholar]
- Kellie G. An account of the appearances observed in the dissection of two of the three individuals presumed to have perished in the storm of the 3rd, and whose bodie were discovered in the vicinity of Leith on the morning of the 4th November 1821 with some reflections on the pathology of the brain. Transact Med Chir Soc Edinburgh. 1824;1:84–169. [PMC free article] [PubMed] [Google Scholar]
- Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24 (Suppl 1:S59–S64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- Fog M. Cerebral circulation. The reaction of the pial arteries to a fall in blood pressure. Arch Neurol Psychiatry. 1937;37:351–364. [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Finnerty FA, Jr, Witkin L, Fazekas JF. Cerebral hemodynamics during cerebral ischemia induced by acute hypotension. J Clin Invest. 1954;33:1227–1232. doi: 10.1172/JCI102997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Jr, Raichle ME, Phelps ME, Ratcheson RA. Effects of increased intracranial pressure on cerebral blood volume, blood flow, and oxygen utilization in monkeys. J Neurosurg. 1975;43:385–398. doi: 10.3171/jns.1975.43.4.0385. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Feher G, Weigle CG, Knuese DE, Kampine JP. Video microscopy of cerebrocortical capillary flow: response to hypotension and intracranial hypertension. Am J Physiol. 1995;268:H2202–H2210. doi: 10.1152/ajpheart.1995.268.6.H2202. [DOI] [PubMed] [Google Scholar]
- Grande PO. The "Lund Concept" for the treatment of severe head trauma—physiological principles and clinical application. Intensive Care Med. 2006;32:1475–1484. doi: 10.1007/s00134-006-0294-3. [DOI] [PubMed] [Google Scholar]
- Muzevic D, Splavski B. The Lund concept for severe traumatic brain injury. Cochrane Database Syst Rev. 2013;12:CD010193. doi: 10.1002/14651858.CD010193.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 2010;25:239–249. doi: 10.1152/physiol.00011.2010. [DOI] [PubMed] [Google Scholar]
- Muizelaar JP, Marmarou A, Ward JD, Kontos HA, Choi SC, Becker DP, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Maas AI, Chieregato A, van der Plas AA. Hyperventilation in head injury: a review. Chest. 2005;127:1812–1827. doi: 10.1378/chest.127.5.1812. [DOI] [PubMed] [Google Scholar]
- Vogel J, Abounader R, Schrock H, Zeller K, Duelli R, Kuschinsky W. Parallel changes of blood flow and heterogeneity of capillary plasma perfusion in rat brains during hypocapnia. Am J Physiol. 1996;270:H1441–H1445. doi: 10.1152/ajpheart.1996.270.4.H1441. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Johannsen P, Cold GE, Østergaard L. Cerebral metabolic response to low blood flow: possible role of cytochrome oxidase inhibition. J Cereb Blood Flow Metab. 2005;25:1183–1196. doi: 10.1038/sj.jcbfm.9600113. [DOI] [PubMed] [Google Scholar]
- Schumann P, Touzani O, Young AR, Verard L, Morello R, MacKenzie ET. Effects of indomethacin on cerebral blood flow and oxygen metabolism: a positron emission tomographic investigation in the anaesthetized baboon. Neurosci Lett. 1996;220:137–141. doi: 10.1016/s0304-3940(96)13210-9. [DOI] [PubMed] [Google Scholar]
- Jensen K, Ohrstrom J, Cold GE, Astrup J. The effects of indomethacin on intracranial pressure, cerebral blood flow and cerebral metabolism in patients with severe head injury and intracranial hypertension. Acta Neurochir (Wien) 1991;108:116–121. doi: 10.1007/BF01418518. [DOI] [PubMed] [Google Scholar]
- Dahl B, Bergholt B, Cold GE, Astrup J, Mosdal B, Jensen K, et al. CO(2) and indomethacin vasoreactivity in patients with head injury. Acta Neurochir (Wien) 1996;138:265–273. doi: 10.1007/BF01411736. [DOI] [PubMed] [Google Scholar]
- Biestro AA, Alberti RA, Soca AE, Cancela M, Puppo CB, Borovich B. Use of indomethacin in brain-injured patients with cerebral perfusion pressure impairment: preliminary report. J Neurosurg. 1995;83:627–630. doi: 10.3171/jns.1995.83.4.0627. [DOI] [PubMed] [Google Scholar]
- Roberts RG, Redman JW. Indomethacin—a review of its role in the management of traumatic brain injury. Crit Care Resusc. 2002;4:271–280. [PubMed] [Google Scholar]
- Rasmussen M. Treatment of elevated intracranial pressure with indomethacin: friend or foe. Acta Anaesthesiol Scand. 2005;49:341–350. doi: 10.1111/j.1399-6576.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- Imberti R, Fuardo M, Bellinzona G, Pagani M, Langer M. The use of indomethacin in the treatment of plateau waves: effects on cerebral perfusion and oxygenation. J Neurosurg. 2005;102:455–459. doi: 10.3171/jns.2005.102.3.0455. [DOI] [PubMed] [Google Scholar]
- Puppo C, Lopez L, Farina G, Caragna E, Moraes L, Iturralde A, et al. Indomethacin and cerebral autoregulation in severe head injured patients: a transcranial Doppler study Acta Neurochir (Wien) 2007149139–149.discussion 149. [DOI] [PubMed] [Google Scholar]
- Muizelaar JP, Wei EP, Kontos HA, Becker DP. Mannitol causes compensatory cerebral vasoconstriction and vasodilation in response to blood viscosity changes. J Neurosurg. 1983;59:822–828. doi: 10.3171/jns.1983.59.5.0822. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. 2009;26:301–312. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandestig A, Romner B, Grande PO. Therapeutic hypothermia in children and adults with severe traumatic brain injury. Ther Hypothermia Temp Manag. 2014;4:10–20. doi: 10.1089/ther.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley S, Reid J, McLatchie R, Hayton J, Clark C, Macdougall M, et al. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care. 2014;18:R75. doi: 10.1186/cc13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreckinger M, Marion DW. Contemporary management of traumatic intracranial hypertension: is there a role for therapeutic hypothermia. Neurocrit Care. 2009;11:427–436. doi: 10.1007/s12028-009-9256-2. [DOI] [PubMed] [Google Scholar]
- Sakoh M, Gjedde A. Neuroprotection in hypothermia linked to redistribution of oxygen in brain. Am J Physiol Heart Circ Physiol. 2003;285:H17–H25. doi: 10.1152/ajpheart.01112.2002. [DOI] [PubMed] [Google Scholar]
- Mouridsen K, Hansen MB, Østergaard L, Jespersen SN.Reliable estimation of capillary transit time distributions using DSC-MRI J Cereb Blood Flow Metab 2014. doi: 10.1038/jcbfm.2014.111 [DOI] [PMC free article] [PubMed]
- Sette G, Baron JC, Mazoyer B, Levasseur M, Pappata S, Crouzel C. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease. Positron emission tomography. Brain. 1989;112 (Pt 4:931–951. doi: 10.1093/brain/112.4.931. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Aamand R, Gutierrez-Jimenez E, Ho YC, Blicher JU, Madsen SM, et al. The capillary dysfunction hypothesis of Alzheimer's disease. Neurobiol Aging. 2013;34:1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]