Abstract
Stimulation of N-methyl-D-aspartate receptors (NMDAR) contributes to regenerative neuroplasticity following the initial excitotoxic insult during cerebral ischemia. Stimulation of NMDAR with the partial NMDAR agonist D-cycloserine (DCS) improves outcome and restores hippocampal synaptic plasticity in models of closed head injury. We thus hypothesized that DCS would improve outcome following restoration of spontaneous circulation (ROSC) from cardiac arrest (CA). DCS (10 mg/kg, IP) was administered to Sprague-Dawley rats (male, 250–330 g; 63–84 days old) 24 and 48 hours after 6 or 8 minutes of asphyxial CA. Heart rate and blood pressure declined similarly in all groups. Animals showed neurological deficits after 6 and 8 minutes CA (P<0.05, Tukey) and these deficits recovered more quickly after 6 minutes than after 8 minutes of CA. CA decreased the number of healthy neurons within CA1 with no difference between 6 and 8 minutes duration of CA (180.8±27.6 (naïve, n=5) versus 46.3±33.8 (all CA groups, n=27) neurons per mm CA1). DCS had no effect on neurological deficits or CA1 hippocampal cell counts (P>0.05, Tukey).
Keywords: glutamate, global ischemia, hippocampus, neuropediatrics, neuroregeneration, perinatal hypoxia
Introduction
Cardiac arrest (CA) is the third leading cause of death and disability in the USA.1 Recovery after CA is generally very poor and usually results in death or severe neurological impairment due to global cerebral ischemia. Over 300,000 people annually are affected by CA in the United States, whereby only 13,800 (4.6%) survive; 42,000/70,000 (60%) of patients that are resuscitated die from brain injury, and only 2,100–7,000/70,000 (3–10%) resume a somewhat normal lifestyle.2 Neurological impairments include short-term memory loss, speech impairments, and motor and cognitive dysfunction. Hyperactivation of N-methyl-D-aspartate receptors (NMDAR) play a major role in excitotoxic neuronal injury during cerebral ischemia. NMDAR also play an essential role in synaptic plasticity3 and may thus contribute to regeneration and restoration of cognitive function in days to weeks following the initial insult. Studies of closed head injury show that excitotoxic, hyperactivation of NMDAR occurs for a short period of time (<1 hour after injury), and is followed by a period of NMDAR downregulation. Stimulation of NMDAR with the partial agonist DCS during the period of downregulation improves neurological outcome.4 No study to our knowledge has addressed whether NMDAR stimulation from hours to days after global cerebral ischemia affects neurological outcome. Here we tested the hypothesis that activation of NMDAR with DCS would improve outcome following global cerebral ischemia caused by CA.
In this study, we induced global cerebral ischemia in rats via 6 or 8 minutes of CA. Animals resuscitated within 2 minutes after CA were administered DCS (10 mg/kg, intraperitoneally [IP]) at 24 and 48 hours after restoration of spontaneous circulation (ROSC). Neurological deficit scores (NDS) were taken 2 hours after ROSC and daily during a 7-day period. CA1 hippocampal cell counts were also measured to quantify neuronal cell loss resulting from ischemia/reperfusion. Although 6 and 8 minutes of CA produced significant neurological deficits and decreases in CA1 neuronal cell counts, results showed no effect of DCS on either measure.
Materials and methods
Animals
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, published by the National Institutes of Health (NIH) and were approved by the Institutional Animal Care and Use Committee of the University of Alaska Fairbanks. Male, Sprague-Dawley rats, purchased from Simonsen Laboratories (Gilroy, CA) were 250–330 g; 63–84 days old on the day of the experiment. Female rats were not used to avoid influence of the estrous cycle. Rats were group-housed initially for 1 week and transferred after quarantine to a procedure area where they were housed in pairs and allowed to acclimate for 2–3 weeks prior to use. Rats were housed at 21–23°C on a 12:12 hours light/dark cycle. Food and water was available ad libitum prior to experimentation and during recovery (1 week) after cardiac arrest, except that to normalize glycogen, rats were fasted 24 hours prior to surgery. Experiments consisted of four groups of animals whereby 6 or 8 minutes CA was followed by blinded, coded treatment with DCS or vehicle (0.9% saline). Assignment to 1 of 2 blinded and coded treatment groups alternated between experiments. Physiological parameters (age, body weight, Po2, Pco2, pH, acid base excess [ABE], hematocrit [HCT], plasma glucose, HCO3−, mean arterial blood pressure [MABP], and temporalis/rectal temperatures) were monitored before as well as after CA. A group of naïve rats of similar age and weight were included for a priori power analysis and subsequent comparison with treated groups.
Cardiac Arrest Procedure
The surgical procedure was performed as described previously and described in detail here.5, 6 Animals were anesthetized with 5% isoflurane and a 70:30 mixture of oxygen and nitrous oxide followed by endotracheal intubation. Anesthesia was maintained between 1–2% isoflurane. Head and rectal temperatures were maintained between 36.5°C–37.5°C using temperature controlled heating lamps and T-CSC32 temperature controllers (Omega, Stamford, CT) throughout the procedure and for 60 minutes post-ROSC. A three lead electrocardiogram (ECG) was attached to the fore and hind limbs to monitor heart rate. The femoral vein was cannulated using a single lumen (Tygon® flexible tubing; Saint-Gobain PPL Corp., 0.375-mm ID, 0.75-mm OD; length 0.029” & wall 0.014” Norton, Akron, OH) catheter and advanced∼2 cm towards the heart. The femoral artery was cannulated using the same type of tubing for continuous blood pressure monitoring and blood gas analysis. After cannulation vecuronium (0.3 mL, approximately 1 mg/kg) (Gensia Sicor pharmaceuticals, Irvine, CA) was injected IV followed by mechanical ventilation (60 breaths per min [bpm]) and isoflurane was lowered to 0.5%.
Blood gases were continually sampled (with at least 10 minutes intervals between sampling) until physiological levels were normalized prior to CA induction. Arterial blood gases, including, Pco2, Po2, pH, HCO3− and ABE were analyzed (iSTAT analyzer with GC8+ cartridges; Abbott Laboratories, Abbott Park, IL) and maintained within normal limits by adjusting respiratory rate (60 ±10 rpm) and tidal volume (stroke volume 2.0 mL–3.2 mL/min). MABP and ECG were also continuously monitored using an AMP 6600 blood pressure amplifier and an AMP 6600 bioelectric amplifier (Gould Instrument Systems, Valley View, OH).
Cardiac arrest was induced by disconnecting the ventilator from the endotracheal tube for 6 or 8 minutes of asphyxiation. At 6 or 8 minutes, Positive End Expiratory Pressure (‘PEEP' 5 cm column of water) was implemented, ventilator was reconnected to deliver 100% oxygen at a rate of 80 rpm, epinephrine (10 μg/kg, IV) was administered and was followed immediately by manual chest compressions for up to 2 minutes or until MABP returned to a minimum of 50 mm Hg without compressions. Sodium bicarbonate (NaHCO3, 0.9cc, IV) was then administered to buffer acidosis. Blood gasses were sampled 10 minutes after ROSC. Immediately following blood gas sampling the ventilator rate was decreased from 80 to 60 rpm and oxygen was lowered from 100% to 30% in a mixture with N2O. Head and rectal temperatures were maintained between 36.5–37.5°C for 60 minutes and IPTT-300 temperature transponder tags (Biomedic Data Systems [BMDS] Inc., Seaford, DE) were implanted subcutaneously [SC] at the back of the neck for subsequent monitoring of Tb.
Post-Operative Care
Post-operative (post-op), rats were placed in a neonatal incubator set to 29°C overnight with water provided. Saline (0.9%, IP) was administered in a volume of 1 mL/100 g body weight at the end of surgery to prevent dehydration. After 8–16 hours animals were fed, weighed, cleaned, and assessed for NDS. This was repeated daily for 7 days (Note: NDS were also assessed 2 hours post-ROSC). Rats were fed a 50:50 mixture of rodent chow and sucrose mixed with water which was placed in a small petri dish to allow animals to self-feed. When animals were unable to feed themselves a gavage needle (approximately 11 cm long, 16ga x 1 ½”, blunt-ended, with bulb diameter of 4 mm) and a 3cc syringe were used to deliver the mixture into the animal's mouth. Animals were fed until they no longer swallowed in response to food delivery into the mouth. Most rats required assisted feeding and consumed the entire 3cc volume. Exclusion criteria: only animals resuscitated within 2 minutes post-CA were included in data analyses. In clinical medicine it is not standard protocol to administer analgesics after cardiac arrest.7 Post-operative analgesics were not provided because they were expected to interfere with the injury process and because analgesics are not given routinely following cardiac arrest in the clinic. Nonsteroidal anti-inflammatory analgesics and opioid analgesics affect inflammatory processes and acetaminophen can mitigate fever, a clinically relevant risk factor in CA.
Drug Administration
DCS (Sigma-Aldrich; St. Louis, MO) dissolved in saline was delivered IP at 10 mg/kg in a volume of 1.0 mL/kg. This DCS dose was shown previously to improve acquisition of spatial memory tasks without inducing concurrent side effects.8 Vehicle consisted of saline (0.9% NaCl, USP grade, 1.0 mL/kg, IP). DCS and vehicle were sterilized by filtering through a 0.2 μm filter (Pall Corporation, Ann Arbor, MI; PN 4192) into a sterile vial. Both solutions were prepared on the first day of injection (24 hours after ROSC) and given to rats 24 and 48 hours after CA. DCS or vehicle was administered as a coded solution and NDS assessed by an observer unaware of treatment. Adverse DCS symptoms were not observed nor reported in previous literature.9
Neurological Deficit Score Assessment
Neurological deficit scores were taken at 2 hours after ROSC and daily for 7 days after CA. Total NDS assessed five components: consciousness and respiration, cranial nerve function, motor function, sensory function and coordination.6 The NDS scale ranged between ‘0' (no neurological deficiency; normal function) to ‘100' (maximum neurological deficiency). Neurological deficit scores were performed by a single observer unaware of treatment. NDS were collected through day 7 until animals were euthanized and brain tissue collected for histopathology 8 days after ROSC.
Histology
A group of naïve rats and treated rats on day 8 following ROSC were perfused with FAM solution (36% formaldehyde, 100% glacial acetic acid, and 100% methanol per 1 L solution mixed 1:1:8 by volume) for 19 minutes after a 1 minute initial perfusion with physiological saline. FAM was delivered at a constant rate of approximately 80 mL/min into the left ventricle with the descending aorta clamped. The head was immersed in FAM at 4°C for 24 hours. After an initial RO-H2O wash for 10–15 minutes, brains were carefully removed from the skull and stored in fresh FAM for another 24 hours at 4°C. Finally, brains were placed in 70% ethanol solution until trimmed and sectioned into coronal brain blocks for paraffin embedding. Coronal sections of 8μm were stained with hematoxylin and eosin. Rat brain sections examined contained the hippocampus at approximately −3.80 mm posterior to bregma.10 Normal (healthy, non-pyknotic) neuronal counts were made within a section of the CA1 region of hippocampus by an investigator blinded to the experimental conditions and the counts expressed as number of normal neurons present per mm of CA1 (at 40X magnification). Normal neurons were defined as having a well-defined cellular membrane and distinct cellular nucleus. MetaMorph 7.0 software was used to record cell counts.
Statistics
A priori power analysis (using G*Power software) was performed to estimate sample size needed to yield 80% power for detecting a therapeutically significant effect of treatment. We first determined the number of healthy neurons per mm of CA1 in naïve rats of similar age to proposed treatment groups to be 180.8±27.6 (mean±s.d., n=5). We then assumed that 8 minutes of CA would produce a 76% loss of healthy neurons based on results published previously using a protocol identical to that described in the present study.5 We defined a medically significant effect of treatment based on effects of therapeutic hypothermia, a treatment found to produce clinically significant effects. In this laboratory using this model of CA mild hypothermia produces a 3-fold increase in healthy neurons. We then calculated an effect size of 3.14 (mean1-mean2/s) where mean1, was calculated as a 76% loss from naïve values (43.2 healthy neurons per mm CA1); mean2, was calculated as 3 times mean1 (129.6 healthy neurons per mm CA1); and s, was defined as the s.d. of the mean of healthy neurons in naïve rats (27.6). This effect size was used in an a priori power analysis for a 2-tailed t-test to detect a difference between 2 independent means. Results indicated that a sample size of 3 rats for each treatment group (saline and DCS) with a total sample size of 6 would have an 82% power for detecting an effect size of 3.14. Subsequent sample size exceeded this estimate.
Data are expressed as mean±s.d. or s.e.m. as indicated with the exception of NDS data, which is expressed as median and first and third quartile values. Statistical evaluation was performed using MANOVA with repeated-measures over time followed by a Tukey multiple-comparison post-hoc test used to measure differences between group means (SAS v 9.1 software; SAS Institute Inc., Cary, NC, USA) or t-test where indicated when group interactions were significant; P<0.05 was considered significant. ANOVA single factor statistical test was used for CA1 hippocampal cell counts. A total of 37 rats were used for the DCS experiment all of which met inclusion criteria of maintaining a MABP of 50 mm Hg within 2 minutes of commencing chest compressions. All animals received treatments at 24 and 48 hours. Of these 37 rats, 1 rat treated with saline vehicle died 4 days after ROSC. Additionally, 4 rats were excluded from analysis due to poor brain tissue fixation (n=3), or because a tissue section at −3.80 mm from bregma was not available (n=1). These rats were excluded without knowledge of treatment.
Results
Physiology
Age, body weight, HCT, and plasma glucose (mg/dL) did not differ between experimental animal groups. pH, HCO3−, and ABE all significantly increased after CA while MABP decreased from baseline values after CA (Table 1; P<0.05, MANOVA, main effect of time for each variable). MABP values overall were lower in 8 minutes than in 6 minutes rats (Table 1; P=0.0016, MANOVA main effect of duration). Eight minutes CA rats had significantly lower Po2 than 6 minutes both before and after CA regardless of drug treatment (P=0.0294, MANOVA, main effect of duration). Tbrain and Trec values were overall slightly higher in 8 minutes rats than 6 minutes CA rats which had lower Tbrain values, but all within the 36.5–37.5°C range (Table 1; P=0.0220 [Tbrain], P=0.00033 [Trec] MANOVA, main effect of duration). Trec values in all groups were slightly higher ‘After CA', but were all within 36.5–37.5°C (Table 1; P=0.0141, main effect of time). There were only minor differences between physiological parameters prior to drug treatment.
Table 1. Physiological parameters measured before and after cardiac arrest in rats (mean±s.d.).
| Variable |
Cardiac Arrest (6 minutes) |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| D-cycloserine (n=10) | Vehicle (Saline, n=8) | |||
| Age (days) | 68.9±4.3 | 68.5±5.5 | ||
| Body Weight (g) | 284.7±10.7 | 283.7±17.6 | ||
| pH | 7.46±0.03 | 7.50±0.07 | 7.44±0.02 | 7.46±0.02 |
| PCO2 (mm Hg) | 38±2 | 38±2 | 39±2 | 41±3 |
| PO2 (mm Hg) | 119±7 | 135±44 | 122±7 | 128±14 |
| HCO3− (mmol/L) | 27.3±1.8 | 29.8±4.2 | 26.2±1.5 | 29.1±2.5 |
| Base Excess | 3.5±2.1 | 6.7±5.3 | 2.1±1.6 | 5.3±2.6 |
| HCT | 48.2±2.0 | 48.5±1.5 | ||
| MABP (mm Hg) | 120±16 | 96±14 | 116±10 | 93±17 |
| Tbrain (°C) | 36.6±0.2 | 36.7±0.2 | 36.7±0.2 | 36.6±0. |
| Trec (°C) | 36.8±0.2 | 37.0±0.3 | 36.8±0.2 | 36.8±0.5 |
| Plasma glucose (mg/dL) | 144±16 | 143±22 | ||
| Variable |
Cardiac Arrest (8 minutes) |
|||
| Before | After | Before | After | |
| |
D-cycloserine (n=7) |
Vehicle (Saline, n=6) |
||
| Age (days) | 74.0±6.4 | 68.8±6.1 | ||
| Body Weight (g) | 299.2±24.3 | 275.4±16.5 | ||
| pH | 7.45±0.01 | 7.48±0.04 | 7.44±0.03 | 7.50±0.02 |
| PCO2 (mm Hg) | 38±1 | 38±2 | 38±3 | 38±2 |
| PO2 (mm Hg)a | 112±10 | 116±34 | 119±10 | 102±2 |
| HCO3− (mmol/L) | 26.3±1.2 | 28.8±2.9 | 25.8±1.3 | 30.0±2.1 |
| Base Excess | 2.1±1.3 | 5.1±3.6 | 1.7±1.5 | 6.8±2.4 |
| HCT | 47.8±3.5 | 46.6±0.1 | ||
| MABP (mm Hg)a | 109±11 | 72±10 | 106±30 | 74±17 |
| Tbrain (°C)a | 36.9±0.3 | 36.9±0.4 | 36.8±0.3 | 36.8±0.2 |
| Trec (°C)a | 36.9±0.4 | 37.1±0.3 | 37.0±0.1 | 37.4±0.4 |
| Plasma glucose (mg/dL) | 144±24 | 124±16 | ||
P<0.05 vs 6 minutes Cardiac Arrest.
Asphyxial Cardiac Arrest
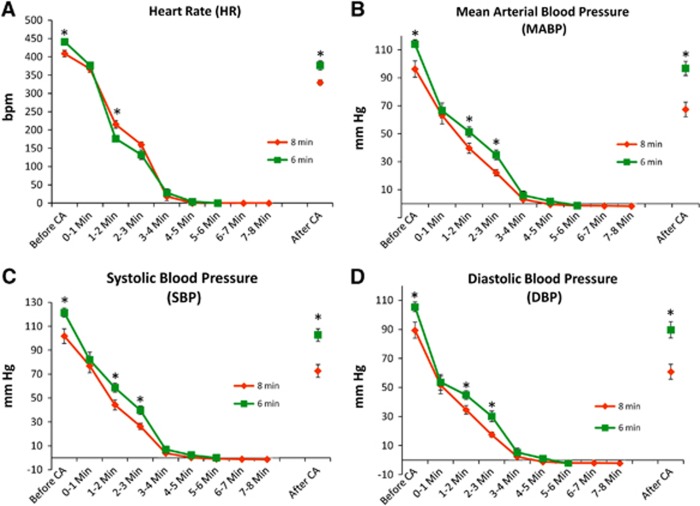
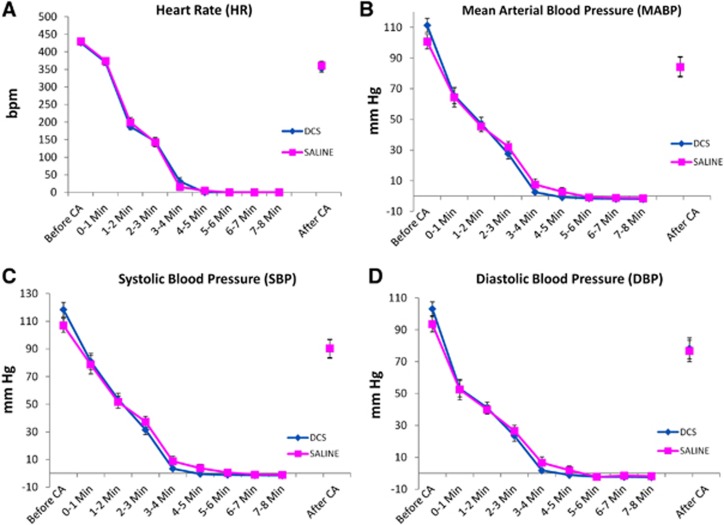
Heart rate (HR) and blood pressure (mean [MABP], systolic [SBP], and diastolic [DBP]) were compared before, during, and after CA between experimental groups. For the most part groups showed similar severity of CA with declines in heart rate and blood pressure over time (P<0.0001 for HR, P<0.0001 for MABP, P<0.0001 SBP; P<0.0001 DBP; MANOVA, main effects of time). However, for heart rate, MABP, SBP, and DBP there were some statistical differences between 6 and 8 minutes CA groups at some time points (Figure 1) (For HR, P<0.0001, MANOVA, time × duration of CA; for MABP P=0.0030, main effect of duration, P<0.001 time × duration of CA; for SBP, P=0.0035, main effect of duration; P=0.0016, time × duration; for DBP, P=0.0048, main effect of duration; P=0.0021, time × duration). No differences in heart rate or blood pressure were seen in groups subsequently treated with DCS or saline (Figure 2). Immediately after induction of asphyxiation, bradycardia (∼≥60% HR reduction) and hypotension (<50 mm Hg) was observed in all animals typically beginning within 2 minutes of CA. In both 6 minutes and 8 minutes CA groups, HR and MABP decreased slightly within 1 minute and decreased significantly within 2–4 minutes of CA with values near 0 mm Hg by 4 minutes of CA (see Figure 1). CA duration time intervals of ‘Before CA' (2 minutes prior to CA induction), ‘1–2 minutes', and ‘2–3 minutes' differed between both 6 minutes and 8 minutes CA rat groups (P<0.05, Tukey). DCS and vehicle saline groups had similar declines in HR and MABP and systolic and diastolic blood pressures during CA (Figure 2, P>0.05).
Figure 1.
Cardiovascular parameters monitored before, during, and after asphyxial cardiac arrest induced at 0 minute followed by measures to restore spontaneous circulation at 6 or 8 minutes; *P<0.05, 6 vs 8 minutes, Tukey; n=18 (6 minutes); n=13 (8 minutes). HR (A), MABP (B), SBP (C), and DBP (D).
Figure 2.
Cardiovascular parameters monitored before, during, and after induction of asphyxial cardiac arrest (6 or 8 minutes CA) in animals subsequently treated with saline vehicle or D-cycloserine (DCS), n=14 (saline); n=17 (DCS). The data points for the DCS group after CA are obscured by the data points for the saline group. HR (A), MABP (B), SBP (C), and DBP (D).
Neurological Deficit Scores
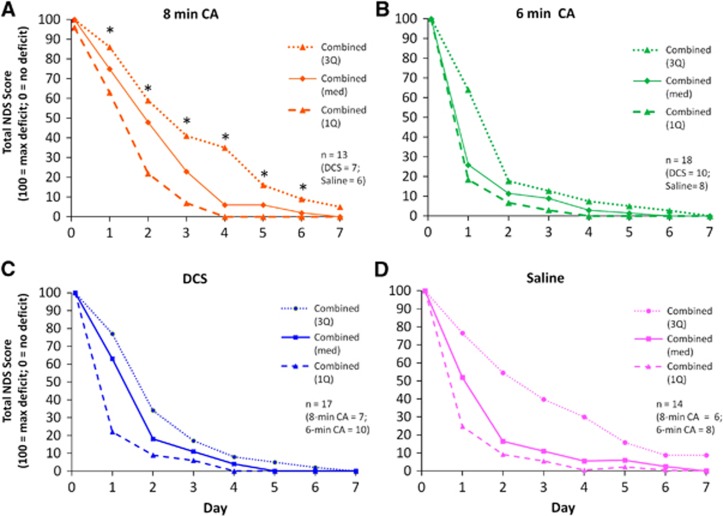
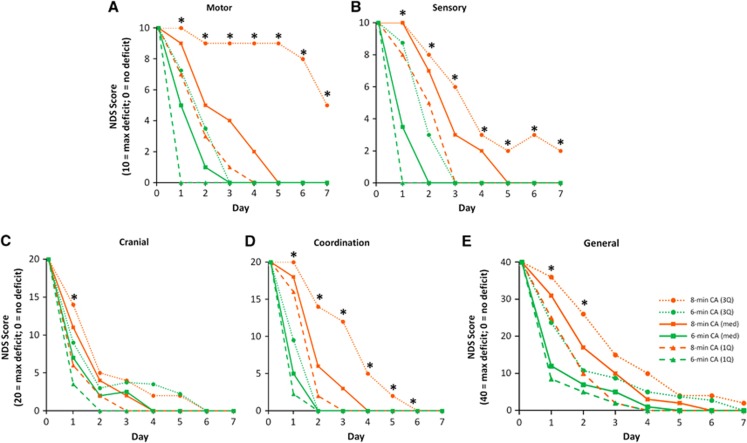
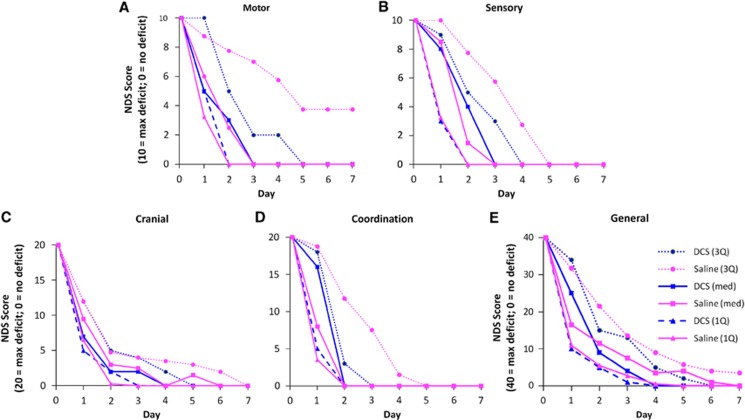
To assess the extent of injury caused by CA and the therapeutic effects of DCS we compared NDS between experimental groups. NDS scores were not normally distributed and transformations failed to improve normal distribution of residuals. Therefore, data were analyzed using MANOVA but are shown as median and the first and third quartiles. CA produced significant neurological deficit (indicated by higher NDS values). Total NDS spontaneously recovered over time (a majority of rats eventually had a NDS of ‘0' or ‘near 0' by the end of the assessment) (P<0.0001, MANOVA, main effect of time; Figure 3). Rats exposed to 8 minutes CA took more time to recover neurological function than rats exposed to 6 minutes (Figures 3A and 3B; P=0.0026, main effect of duration; P=0.0003, time × duration). DCS treatment had no effect on Total NDS (Figure 3C and 3D). Categorical NDS was also analyzed within the following categories: General Behavioral, Cranial Nerve Reflexes, Motor, Sensory, and Coordination. All categorical NDS showed significant differences between 6 and 8 minutes CA rats over time (NDS Days 1–3) (P<0.0001, main effect of time; Figure 4). Eight minutes CA also produced significant injury in rats for both Total and Categorical NDS (Motor NDS P=0.0039, main effect of duration; P=0.0058, time × duration; Figure 4A; Sensory NDS P=0.0008 main effect of duration; P=0.0004, time × duration; Figure 4B; and Coordination NDS P=0.0023, main effect of duration; P=0.0003, time × duration; Figure 4D; General P=0.0242, main effect of duration; P=0.0021, time × duration; Figure 4E; Cranial P=0.0167, time × duration; Figure 4C) which contributed to slower recovery. Overall, DCS did not produce a significant effect on NDS (Figure 5).
Figure 3.
Total Neurological Deficit Scores (NDS) for rats subjected to 8 minutes (A) or 6 minutes (B) of asphyxial cardiac arrest (CA), and for rats subjected to 6 or 8 minutes CA and subsequently treated with D-cycloserine (DCS) (C), or Vehicle (Saline) (D). There were no significant differences between NDS scores for DCS and saline treated rats; however, neurological deficits persisted longer after 8 minutes CA than after 6 minutes CA. *P<0.05, Tukey, 8 versus 6 minutes, n=18 (6 minutes); n=13 (8 minutes). Data are shown as medians bracketed by first and third quartiles.
Figure 4.
Categorical Neurological Deficit Scores (NDS) consisting of (A) motor, (B) sensory, (C) cranial, (D) coordination, and (E) general behavioral components for groups subjected to 6 or 8 minutes asphyxial cardiac arrest (CA). *P<0.05, 8 versus 6 minutes, n=18 (6 minutes); n=13 (8 minutes); DCS and saline treatments are combined. Data are shown as medians bracketed by first and third quartiles.
Figure 5.
Categorical Neurological Deficit Scores (NDS) consisting of (A) motor, (B) sensory, (C) cranial, (D) coordination, and (E) general behavioral components are shown for groups of rats treated with saline vehicle or D-cycloserine (DCS), following 6 minutes or 8 minutes of asphyxial cardiac arrest; n=14 (saline); n=17 (DCS). Data are shown as medians bracketed by first and third quartiles.
Histopathology
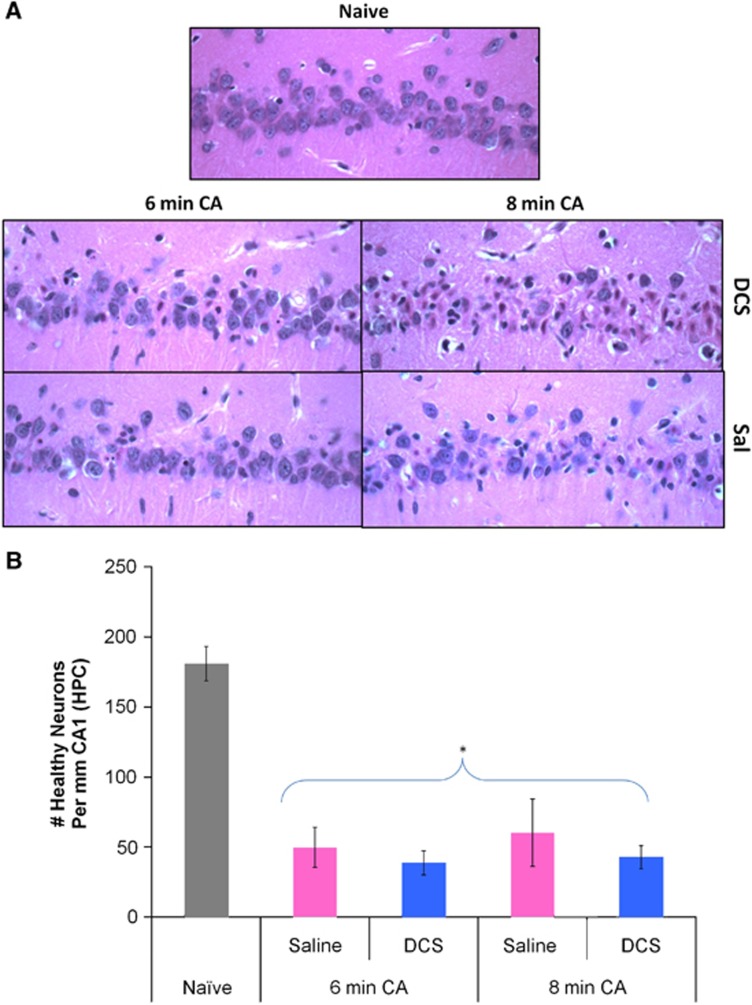
Ischemic neurons (shrunken pyknotic cells with darkened triangular-shaped nuclei, and eosinophilic nucleolus and cytoplasm) were present in the CA1 region of all CA treated rats (Figure 6A). Counts of healthy CA1 neurons showed a significant decrease when compared with naïve rats. Mean±s.d. for CA and naïve rats were as follows: 180.8±27.6 naïve (n=5) versus 46.3±33.8 healthy neurons per mm CA1 [all CA groups, n=27] (P=7.12 × 10–5, two-sample unequal variances t-test). There were no significant differences seen in CA1 neuron counts for 6 and 8 minutes CA rats and DCS had no significant effect on cell counts (Figure 6B).
Figure 6.
(A) Representative images (at 40X magnification) showing pyknotic (ischemic) and healthy neurons in the CA1 hippocampal region of drug (D-cycloserine; DCS) or Vehicle (Sal) treated rats 8 days after 6 minutes or 8 minutes of asphyxial cardiac arrest (CA). (B) Number of healthy neurons per mm of hippocampal (HPC) CA1 in naïve rats and rats subjected to 6 minutes or 8 minutes of CA and treated with saline or DCS. No significant differences in CA1 neuronal cell counts were noted between experimental groups; however, neuronal counts in all CA groups differed from naïve rats. *P<0.05 CA, n=27 versus naïve, n=5, t-test; mean±s.e.m.
Discussion
The aim of this study was to determine whether activation of NMDAR by DCS could improve neuronal outcome by promoting neuroplasticity and regeneration subsequent to asphyxial CA. Our results showed that DCS had no significant effect on neurological improvement after 6 minutes or 8 minutes CA as assessed by NDS and histopathology. These results fail to support our initial hypothesis that DCS would improve neurological outcome after CA. The present study demonstrates that DCS has no effect given the reported experimental conditions although the study does not determine why DCS fails to improve outcome.
It was somewhat unexpected for DCS to not have any effect on brain injury following CA. Previous studies have shown that DCS, a partial and selective agonist of NMDAR at the glycine site, improves recovery after closed head injury,4 enhances hippocampal LTP in the previously damaged CA1 brain region9 and facilitates changes in hippocampus-and-amygdala dependent learning in the absence of brain injury.11, 12, 13, 14 Negative results may be due to a number of factors. DCS treatment may not have been optimal if NMDARs were not downregulated at the time of treatment. NMDAR downregulation occurs following closed head injury4 and although a similar phenomenon is expected following CA, NMDAR downregulation after CA has not been studied. Nonetheless, with or without NMDAR downregulation, neuroplasticity is emerging as an essential component to overall outcome after stroke and cardiac arrest,15 where drugs that promote neurogenesis and neuroplasticity promote neurorecovery.16 DCS increases NMDAR channel opening, which results in higher spatial learning performances17 and improves memory or accelerates learning acquisition in adult and aged mice without brain injury.8, 18, 19 In light of evidence that DCS promotes neuroplasticity NMDAR downregulation should not be necessary for DCS neurorestorative efficacy.
Shorter duration of CA may be necessary to see improvement in CA1 histopathology. This possibility is supported by the observation that 6 minutes and 8 minutes of CA produced the same decrease in the number of healthy neurons in the CA1 region suggesting that a maximal degree of CA1 injury was sustained by 6 minutes of CA. DCS drug dosage could have been too low or too high to show any therapeutic effect. D-cydoserine dosage was the same as that shown to improve outcome following closed head injury in mice;4 however, the dose-response relationship may depend on the timing of administration as well as the species and severity of brain injury. Measures of hippocampal dependent cognitive dysfunction (e.g. Morris water maze, radial arm maze, contextual fear conditioning, or fear-extinction conditioning behavioral tests) may be more sensitive measures to assess the time course of CA1 hippocampal deficiency and DCS efficacy. Poor recovery from CA is understood to be attributed to progressive neuronal death over a period of days to months after injury so assessment of the time course of hippocampal function is warranted. In addition, the discrepancy in findings between the restorative effects of DCS in the closed head injury model and CA may be due to differences in the species studied as well as to different neurological insults under study. Differences may also be due to different metabolites/adducts affecting the various neurological models in unpredictable ways.
Physiological parameters were consistent among DCS and saline treated groups so it is unlikely that a difference in the severity of CA confounded the effects of DCS. pH, HCO3−, and ABE all increased significantly after CA probably in response to the administration of HCO3− immediately after ROSC in order to counteract acidosis. Longer CA duration caused significantly lower Po2 and MABP. These differences are consistent with what is expected from longer duration of CA and with the worse outcome observed after the longer duration of CA. Cardiac arrest generally results in lower pH in tissues, arterial hypotension, and hypercapnia clinically in prenatal, pediatric, and some adult populations.20 The asphyxial CA rat model produced neuronal injury that is consistent with previous CA literature by which HR and BP decreased within 3–4 minutes of CA onset.5, 6 Due to random variation, the decline in HR and blood pressure differed in groups subsequently resuscitated after 6 or 8 minutes. Blood pressure declined slightly faster in rats subsequently exposed to 8 minutes of asphyxia. Importantly, however, no differences were seen between DCS and saline (both declined at similar rates). Another important factor to note is the elevated Tbrain in 8 minutes CA rats. Slower recovery time in 8 minutes CA rats could have been attributed to overall higher Tbrain, or to slightly more severe decreases in BP during 1–3 minutes of asphyxia.
Although DCS offers the advantage of partial agonist efficacy, other nonspecific effects such as inhibition of pyridoxal phosphate dependent enzymes and the potential to interfere with energy metabolism/intermediary metabolism may make it a less than ideal drug to be used in neurological studies.21, 22
In conclusion, DCS had no significant effect on neurological improvement following asphyxial CA. Asphyxial CA duration (6 and 8 minutes) played a significantly larger role in affecting neurological outcome as expressed in NDS and CA1 neuron counts than DCS treatment. While further studies to explain why DCS failed to improve outcome may be warranted, the purpose of this negative report is to avoid publication bias, which might otherwise contribute to the failure for basic science to translate to the clinic.23
The authors declare no conflict of interest.
Footnotes
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers NS41069–06, R15NS070779, and Alaska INBRE P20GM103395, UAF Center for Research Services, and Flint-Hills Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank K Dave, I Saul, J Sun, J Moore, TJ Jinka, KE Iceman and ZA Carlson for technical assistance and J McIntyre and Z Barati for assistance with statistical analysis.
References
- Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz M, Gobert D, Schohl A, Poquerusse J, Podgorski K, Spratt P, et al. Rapid Hebbian axonal remodeling mediated by visual stimulation. Science. 2014;344:904–909. doi: 10.1126/science.1251593. [DOI] [PubMed] [Google Scholar]
- Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci USA. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA. The Arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke. 2006;37:1261–1265. doi: 10.1161/01.STR.0000217409.60731.38. [DOI] [PubMed] [Google Scholar]
- Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15:1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122 (18 Suppl 3:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- Lelong V, Dauphin F, Boulouard M. RS 67333 and D-cycloserine accelerate learning acquisition in the rat. Neuropharmacology. 2001;41:517–522. doi: 10.1016/s0028-3908(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Yaka R, Biegon A, Grigoriadis N, Simeonidou C, Grigoriadis S, Alexandrovich AG, et al. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. Faseb J. 2007;21:2033–2041. doi: 10.1096/fj.06-7856com. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.The Rat Brain in Stereotaxic Coordinates4th edn1998 [DOI] [PubMed]
- Flood JF, Morley JE, Lanthorn TH. Effect on memory processing by D-cycloserine, an agonist of the NMDA/glycine receptor. Eur J Pharmacol. 1992;221:249–254. doi: 10.1016/0014-2999(92)90709-d. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- Monahan JB, Handelmann GE, Hood WF, Cordi AA. D-cycloserine, a positive modulator of the N-methyl-D-aspartate receptor, enhances performance of learning tasks in rats. Pharmacol Biochem Behav. 1989;34:649–653. doi: 10.1016/0091-3057(89)90571-6. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Honma M, Koyama S, Kim Y. D-cycloserine facilitates procedural learning but not declarative learning in healthy humans: a randomized controlled trial of the effect of D-cycloserine and valproic acid on overnight properties in the performance of non-emotional memory tasks. Neurobiol Learn Mem. 2011;95:505–509. doi: 10.1016/j.nlm.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Sarkamo T, Ripolles P, Vepsalainen H, Autti T, Silvennoinen HM, Salli E, et al. Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: a voxel-based morphometry study. Front Hum Neurosci. 2014;8:245. doi: 10.3389/fnhum.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HF, Pan XL, Wang JW, Kong HM, Fu YM. Protein-drug interactome analysis of SSRI-mediated neurorecovery following stroke. Biosystems. 2014;120:1–9. doi: 10.1016/j.biosystems.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Pitkanen M, Sirvio J, MacDonald E, Niemi S, Ekonsalo T, Riekkinen P., Sr. The effects of D-cycloserine and MK-801 on the performance of rats in two spatial learning and memory tasks. Eur Neuropsychopharmacol. 1995;5:457–463. [PubMed] [Google Scholar]
- Baxter MG, Lanthorn TH, Frick KM, Golski S, Wan RQ, Olton DS. D-cycloserine, a novel cognitive enhancer, improves spatial memory in aged rats. Neurobiol Aging. 1994;15:207–213. doi: 10.1016/0197-4580(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, Maretti L, et al. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology (Berl) 2011;218:461–470. doi: 10.1007/s00213-011-2330-4. [DOI] [PubMed] [Google Scholar]
- Katz LM, Wang Y, Rockoff S, Bouldin TW. Low-dose Carbicarb improves cerebral outcome after asphyxial cardiac arrest in rats. Ann Emerg Med. 2002;39:359–365. doi: 10.1067/mem.2002.121522. [DOI] [PubMed] [Google Scholar]
- Beeler T, Churchich JE. Reactivity of the phosphopyridoxal groups of cystathionase. J Biol Chem. 1976;251:5267–5271. [PubMed] [Google Scholar]
- Perez-Sala D, Ayuso MS, Rico M, Parrilla R, Rando RR. The interaction of cycloserine with pyruvate and other biologically relevant alpha-ketoacids. Biochem Pharmacol. 1989;38:1037–1044. doi: 10.1016/0006-2952(89)90246-3. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]