Abstract
In the current study, the presence of cerebral cortical microinfarcts (CMIs) was evaluated in a series of 21 patients with a symptomatic high-grade >50% stenosis of the carotid artery. A T2-weighted fluid-attenuated inversion recovery sequence and a T1-weighted turbo field echo sequence of the brain were obtained at 7.0 Tesla magnetic resonance imaging. Primary study endpoint was the number of CMIs and macroinfarcts. In total, 53 cerebral infarcts (35 macroinfarcts; 18 CMIs) were found ipsilateral to the symptomatic carotid artery, in 14 patients (67%). In four of these patients, both CMIs and macroinfarcts were visible. In the contralateral hemisphere, seven infarcts (five macroinfarcts and two CMIs) were found in five patients (24%). In the ipsilateral hemispheres, the number of CMIs and macroinfarcts were significantly correlated (P=0.02). Unpaired comparison of medians showed that the number of CMIs in the ipsilateral hemisphere was significantly higher than the number of CMIs in the contralateral hemisphere (P=0.04). No significant correlation was found between stenosis grade and the number of any infarct. The current study shows that in symptomatic patients with significant extracranial carotid artery stenosis, CMIs are part of the total cerebrovascular burden and these CMIs prevail with a similar pattern as observed macroinfarcts.
Keywords: atherosclerosis, brain ischemia, carotid artery, imaging, MRI
Introduction
Atherosclerosis of the carotid artery is an important risk for cerebral ischemia. In daily practice, patients are classified as high risk mainly based on the stenosis grade of the carotid artery.1,2 Recent literature confirms that plaque characteristics may contribute to specify subgroups with an increased risk of (recurrent) ischemic cerebral events.3, 4, 5 Moreover, the presence of infarcts in the brain parenchyma that downstream to these lesions may be important for accurate risk stratification. Patients with infarcts and cerebral symptoms were found to profit more from carotid endarterectomy (CEA), compared with patients with just ocular symptoms.6,7 Therefore, sensitive detection of brain lesions in patients with carotid artery disease may be fundamental for identifying patient (sub)groups with the highest risk of recurrent stroke who might benefit most from carotid surgery.
Magnetic resonance imaging (MRI) has a high distinctive capacity in the diagnosis of larger cortical and basal ganglia infarcts. However, from pathology studies it is known that smaller cerebral cortical microinfarcts (CMIs) can be too small for visualization with conventional in vivo imaging techniques.8 Although 3.0 Tesla (T) MRI enables to visualize larger CMIs, the smallest lesions remain undetected.9 However, recent publications have shown that sensitive detection of the total cerebrovascular burden, including very small CMIs of <3 mm in cortical length, is nowadays possible with stronger MRI field strengths, up to 7.0 T.8,10 Until now, the presence of these CMIs is mainly associated with vascular cognitive impairment.9,11
The aim of the present study was to evaluate the presence of CMIs in a series of patients with a symptomatic high-grade stenosis of the carotid artery on high-resolution 7.0 T MRI.
Materials and Methods
Subjects
In patients who are scheduled for CEA, 7.0 T MR images of the brain were obtained <36 hours before surgery. In all patients, a symptomatic carotid artery stenosis of ⩾50% was diagnosed, and the indication for revascularization was discussed within a multidisciplinary panel including neurologists, radiologists, and vascular surgeons. An exclusion criterion was inability to undergo 7.0 T MRI owing to the metallic implants not approved for ultra-high-field strength MR imaging. The medical ethics committee of the University Medical Center Utrecht gave approval for this prospective study, previously described,12 and all patients gave written informed consent. The current study was conducted according to the guidelines of the Declaration of Helsinki.
Imaging
Ultra-high-field strength MRI was performed on a 7.0 T MRI scanner (Philips Healthcare, Cleveland, OH, USA) with a 32-channel receive-coil and a volume transmit/receive-coil for transmission (Nova Medical, Wilmington, MA, USA). The MRI protocol consisted of a T2-weighted fluid-attenuated inversion recovery sequence13 and a T1-weighted turbo field echo sequence. The fluid-attenuated inversion recovery sequence was acquired in sagittal orientation with a field of view of 250 × 250 × 190 mm3, an acquired voxel size of 0.8 × 0.8 × 0.8 mm3, repetition time 8,000 ms, inversion time 2,250 ms, echo time 300 ms (equivalent echo time 154 ms), flip angle 100°, turbo spin echo factor 125, SENSE factor in anterior–posterior direction and right–left direction 2.5 × 2.5. Scanning time was 12 minutes 56 seconds. The T1 turbo field echo sequence was acquired in sagittal orientation with a field of view of 200 × 250 × 200 mm3, an acquired voxel size of 1.0 × 1.0 × 0.5 mm3 repetition time 4.8 ms, echo time 2.2 ms, flip angle 8° and scanning time was 1 minute 37 seconds.
Image analysis
Image analysis was performed with MeVisLab 2.4 (MeVis Medical Solutions AG, Bremen, Germany). The presence of CMIs and macroinfarcts on the high-resolution fluid-attenuated inversion recovery images and T1 turbo field echo images of the brain were described, relative to the side of the scheduled CEA. The brain was systematically divided in seven regions: frontal, parietal, occipital, temporal, caudate nucleus, lentiform nucleus, and thalamus. For each of these regions, presence or absence and number of CMIs and macroinfarcts in both hemispheres were determined. CMIs were defined as infarcts <3 mm in cortical length. Two observers (JH and AR), masked for each other's assessment, performed the image analysis; the intraclass correlation coefficient and 95% confidence interval of ipsilateral infarcts (macro and micro) has been evaluated for interobserver agreement. In a consensus meeting, final decision of all infarcts was made; the final infarcts were used for statistical analyses.
Statistics
First, the relation between number of infarcts and baseline characteristics (age, type of symptoms, and stenosis grade) was evaluated by a Kendall's tau test. Second, the relation between number of macroinfarcts and CMIs was evaluated by the Kendall's tau test and the median difference in number of CMIs and macroinfarcts in the ipsilateral and the contralateral hemisphere by a Mann–Whitney U-test.
Statistical analyses were performed in IBM SPSS Statistics version 20 (IBM Corporation, Armonk, NY, USA).
Results
Subjects
Twenty-one patients with a symptomatic stenosis in the carotid artery, scheduled for CEA between May 2011 and September 2013, were included. Carotid endarterectomy was planned on the left carotid artery in 12 patients and on the right side in the remaining 9 patients. Of these patients, seven were diagnosed with a stroke, nine with a transient ischemic attack and five with amaurosis fugax. Mean age of the patients (14 male) was 69±8 years. Baseline characteristics are presented in Table 1. Imaging was performed 1 day before surgery in 20 patients; for one patient, imaging and surgery were on the same day. The median interval between symptoms and imaging was 21 days (range 11–78 days). At increasing delays, the findings on MRI can less certainly be linked to the clinical symptoms. Nevertheless, the delay of 78 days was one single case. For the rest of the cohort, the range of delays between symptoms and imaging was 10–38 days. As the number of macro- and microinfarcts in this patient was not outlying, we decided not to exclude this patient from analysis.
Table 1. Baseline characteristics of all included patients (n=21).
|
Baseline characteristics | |
| Gender, male | 14 (67%) |
| Age, years | 69±8 |
| Cerebrovascular accident type | |
| Stroke | 7 (33%) |
| TIA | 9 (43%) |
| Amaurosis fugax | 5 (24%) |
| Interval event — MRI | 16 (10–78) |
| Interval event — surgery | 17 (11–79) |
| Stenosis side, left | 12 (57%) |
| Stenosis grade | |
| Symptomatic side | 70 (50–99) |
| Asymptomatic side | 25 (0–90) |
| Symptomatic history on asymptomatic side | 0 (0%) |
| No. patients with infarcts ipsilateral to symptomatic side | |
| Macroinfarcts | 14 (67%) |
| Microinfarcts | 6 (29%) |
| Any infarct | 14 (67%) |
| No. patients with infarcts contralateral to symptomatic side | |
| Macroinfarcts | 4 (19%) |
| Microinfarcts | 1 (4%) |
| Any infarct | 5 (24%) |
| No. infarcts ipsilateral to symptomatic side | 53 |
| Macroinfarcts | 1 (0–6) |
| Microinfarcts | 0 (0–7) |
| Any infarct | 1 (0–13) |
| No. infarcts contralateral to symptomatic side | 7 |
| Macroinfarcts | 0 (0–2) |
| Microinfarcts | 0 (0–2) |
| Any infarct | 0 (0–2) |
MRI, magnetic resonance imaging; TIA, transient ischemic attack.
Median (range) or mean±s.d. or number of (No.) patients (% from total).
None of the patients had a previous CEA of the ipsilateral carotid artery and none of the patients had a previous carotid revascularization or a history of symptoms on the contralateral carotid artery.
Cerebral burden
The determined interobserver agreement was strong for macroinfarcts and CMIs. The intraclass correlation coefficient was 0.78 (95% confidence interval=0.53 to 0.90) for macroinfarcts and 0.80 (95% confidence interval=0.55 to 0.92) for CMIs.
Both for the ipsilateral and contralateral hemisphere, no significant correlation was found between number of infarcts (macroinfarcts and CMIs) and stenosis grade. Both macroinfarcts and CMIs are also seen in patients with only ocular symptoms (amaurosis fugax). However, for both the ipsilateral and contralateral hemisphere, no significant correlation was found between number of infarcts (macroinfarcts and CMIs) and type of symptoms. In the ipsilateral hemisphere, no significant correlation was found between number of infarcts (macroinfarcts and CMIs) and age. In the contralateral hemisphere, a significant correlation only was found between macroinfarcts and age (P=0.04), not between CMIs and age (P=0.32). Results are shown in Table 2.
Table 2. Results of Kendalls tau correlation test, assessing the correlation between the stenosis grade and the number of infarcts and the correlation between CMIs and macroinfarcts.
|
Ipsilateral |
Contralateral |
|||
|---|---|---|---|---|
| τ | P | τ | P | |
| Age versus macroinfarcts | −0.07 | 0.71 | 0.39 | 0.04 |
| Age versus CMIs | 0.09 | 0.62 | −0.19 | 0.32 |
| CVA type versus macroinfarcts | −0.24 | 0.20 | −0.22 | 0.29 |
| CVA type versus CMIs | 0.02 | 0.94 | 0.04 | 0.48 |
| Stenosis grade versus macroinfarcts | −0.18 | 0.29 | −0.19 | 0.33 |
| Stenosis grade versus CMIs | −0.03 | 0.88 | 0.26 | 0.20 |
| CMIs versus macroinfarcts | 0.45 | 0.02 | −0.11 | 0.63 |
CMI, cerebral cortical microinfarct; CVA, cerebrovascular accident.
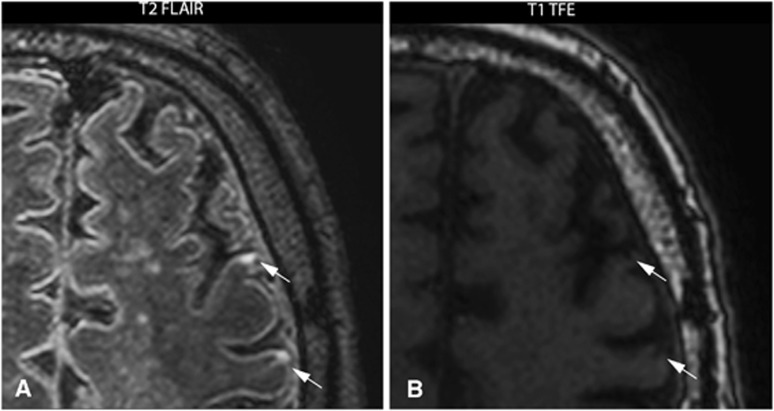
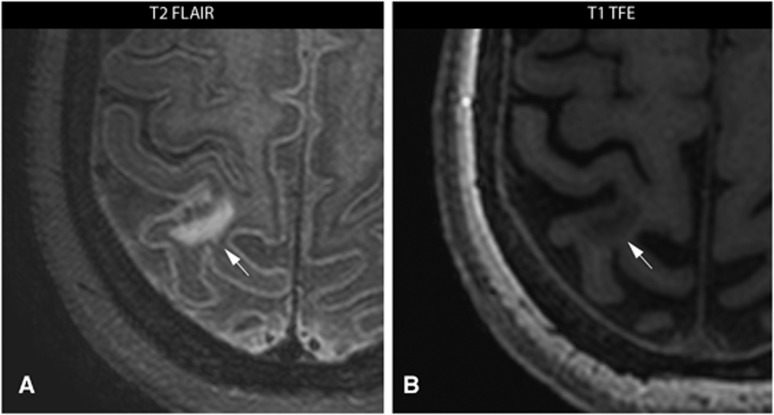
In 14 patients, 53 cerebral infarcts (18 macroinfarcts; 35 CMIs) were found ipsilateral to the carotid artery scheduled for CEA, compared with 7 infarcts in the contralateral hemisphere (5 macroinfarcts; 2 CMIs). An example of two CMIs in the ipsilateral hemisphere is given in Figure 1 and an example of one macroinfarct in the ipsilateral hemisphere is given in Figure 2.
Figure 1.
7.0 Tesla T2-weighted fluid-attenuated inversion recovery (FLAIR) image (A) and a T1-weighted turbo field echo (TFE) image (B) of a 74-year-old male with a symptomatic high-grade stenosis of the left carotid artery scheduled for carotid endarterectomy. Two cortical microinfarcts (arrows) ipsilateral to the symptomatic carotid artery are visualized in transversal plane.
Figure 2.
7.0 Tesla T2-weighted fluid-attenuated inversion recovery (FLAIR) image (A) and a T1-weighted turbo field echo (TFE) image (B) of a 58-year-old female with a symptomatic high-grade stenosis of the left carotid artery scheduled for carotid endarterectomy. One macroinfarct (arrows) ipsilateral to the symptomatic carotid artery is visualized in transversal plane.
In all 14 patients with visible infarcts in the ipsilateral hemisphere, macroinfarcts were visible and CMIs were present in 4 of these patients. The presence of CMIs in the ipsilateral hemisphere was significantly correlated with the presence of macroinfarcts (P=0.02) (Table 2). Furthermore, both the number of CMIs and the number of macroinfarcts were significantly higher in the ipsilateral hemisphere compared with the contralateral hemisphere (P=0.04 and P=0.001, respectively) (Table 3).
Table 3. Results of Mann–Whitney U-test, assessing the difference in the number of infarcts ipsilateral versus contralateral.
| Median number of infarcts (range) | Ipsilateral | Contralateral | P |
|---|---|---|---|
| CMIs | 0 (0–7) | 0 (0–2) | 0.04** |
| Macroinfarcts | 1 (0–6) | 0 (0–2) | 0.001*** |
CMIs, cerebral cortical microinfarcts.
Discussion
The current study is the first study that presents in vivo visualization of CMIs in patients with extensive atherosclerosis of the extracranial vessels. The results show that CMIs prevail with the same pattern as macroinfarcts. Although the number of CMIs is less than the number of macroinfarcts, a siginificant correlation between the presence of CMIs and macroinfarcts was observed and CMIs were significant more prevalent in the hemisphere ipsilateral to the symptomatic carotid artery stenosis compared with the contralateral hemisphere.
CMIs are thus far mainly investigated in relation to dementia.8,11 However, recently, autopsy studies showed that CMIs are not limited to patients with dementia; in 6% to 43% of patients without dementia CMIs were found.11 Besides, also a strong correlation was found between intracranial atherosclerosis and CMIs.14 A prevalence of 43% in non-demented patients was found in a postmortem study in a general population cohort of people with an age of 75 years and older.15 As the slice thickness in their specimens was already 10 micrometer, the size of microinfarcts visualized in this study were assumed to be much smaller than feasible for high-resolution 7.0 T MRI. In the current study, the prevalence of CMIs was 29% in the ipsilateral hemisphere and 5% in the contralateral hemisphere in a series of patients with a symptomatic high-grade atherosclerotic stenosis of the carotid artery. Because the CMIs in our study were found both in the ipsilateral and contralateral hemisphere and even in patients with only ocular symptoms, these infarcts may be the result of more generalized atherosclerosis. The significant higher prevalence of CMIs in the ipsilateral hemisphere in combination with the significant correlation between CMIs and macroinfarcts in the ipsilateral hemisphere is in agreement with the presence of a high-grade and possible vulnerable carotid plaque and patient symptoms.
In addition to the detection of CMIs, the current study is the second study thus far that evaluate cerebral (macro)infarcts at an ultra-high-field strength of 7.0 T. A previous study has shown that 7.0 T MRI enable to depict normal brain anatomy and ischemic lesions in patients with subacute and chronic stroke with a higher spatial resolution and more anatomic details.16 In the current study, this high-spatial resolution was used to depict both macroinfarcts and CMIs.
In vivo visualization of CMIs was described first on ultra-high-field strength 7.0 T MRI.10,11 Nevertheless, recent literature has shown that 3.0 T MRI is also able to visualize these ultra-small lesion.9 However, in this study, only cognitive impaired patients were analyzed and the smallest CMIs were still not visible. In literature, a certain range of definitions of CMIs is used, deferring from invisible with the naked eye to <5 mm.8,17 Although, the feasibility of imaging CMIs at 3.0 T MRI would make it more applicable in clinical settings, the clinical value of these microscopic lesions must be clarified first, which also implies that it must be clarified which size is clinically relevant.
As it is not guaranteed to find visible cerebrovascular damage in patients with a transient ischemic attack or amaurosis fugax, the finding that stroke patients have more often visible cerebral damage can be expected. However, both the number of macroinfarcts and CMIs are significantly higher in the symptomatic hemisphere, also in patients with only ocular symptoms. These findings suggest that CMIs prevail with the same pattern as macroinfarcts, and might also be an effect of the symptomatic vulnerable carotid plaque. The correlation between number of macroinfarcts and CMIs suggests that CMIs comprise a relevant part of the total cerebrovascular burden. Consequently, the ability to visualize CMIs in vivo enables to assess the cerebrovascular burden more accurate, which makes visualization of more subtle differences between patients possible. Furthermore, as CMIs are mainly associated with cognitive impairment, this might suggest that the presence of CMIs gives additional information about the cognitive state of patients with a symptomatic carotid artery stenosis. More research is needed to evaluate this hypothesis and to clarify whether surgical or medical treatment of these patients prevents increasing prevalence of CMIs and thus further development of cognitive impairment.
Although this is the first study that describes in vivo visualization of CMIs in a group of patients with extensive extracranial atherosclerosis at 7.0 T, the current study has also limitations. First of all, it is a series of highly selected patients. As performing 7.0 T MRI with metallic implants is still restricted, the included patients might have been the more vital patients within this series of patients. Nevertheless, for future studies, this might be less of an issue, as recent literature confirms that not all metallic implants should be considered as a major contraindication for 7.0 T MRI.18 Second, the current study is performed in a non-consecutive small group of patients with a symptomatic, high-grade stenosis. To validate the clinical benefit of visualizing CMIs more extensive research is warranted with asymptomatic patients, patients with a lower stenosis grade and healthy volunteers. Besides a head-to-head comparison with 3.0 T MRI is needed to explore the optimal clinical applicability.
Previous studies have shown that surgical treatment in the form of CEA is preferred over conservative treatment in patients with a symptomatic carotid artery stenosis of >70% and moderately beneficial for patients with a symptomatic carotid artery stenosis of >50%.19, 20, 21, 22 Outcome of these studies was a clinical cerebrovascular event, whether visible on computer tomography (CT) or not. As CMIs are too small to visualize on CT, they were not taken into account in this setting. Thereby, in previous studies, CMIs are mainly related to global cognitive impairment and not to acute symptoms.11 In the current study, no correlation was found between the stenosis grade and the visible CMIs (Table 2). This could be explained by the small range of stenosis grades in the current study because only patients with a high-grade symptomatic carotid artery stenosis are included. Although CMIs are seen with a similar pattern as macroifarcts, the clinical value of CMIs for risk stratification of patients with carotid artery atherosclerosis remains unknown.
In conclusion, the current study shows that in symptomatic patients with significant extracranial carotid artery stenosis, CMIs also impress as a relevant part of the total cerebrovascular burden and these CMIs prevail with a similar pattern as observed macroinfarcts. Assessment of this technique is needed in asymptomatic patients, patients with a lower stenosis grade, and healthy volunteers to explore and to validate the clinical benefit of visualizing cortical microinfarcts in these patients.
Acknowledgments
We greatly acknowledge the use of MeVisLab (MeVis Medical Solutions AG, Bremen, Germany).
The authors declare no conflict of interest.
References
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke. 2000;31:615–621. doi: 10.1161/01.str.31.3.615. [DOI] [PubMed] [Google Scholar]
- Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Hosseini AA, Kandiyil N, Macsweeney ST, Altaf N, Auer DP. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol. 2013;73:774–784. doi: 10.1002/ana.23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44:3071–3077. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- Howard DP, van Lammeren GW, Redgrave JN, Moll FL, de Vries JP, de Kleijn DP, et al. Histological features of carotid plaque in patients with ocular ischemia versus cerebral events. Stroke. 2013;44:734–739. doi: 10.1161/STROKEAHA.112.678672. [DOI] [PubMed] [Google Scholar]
- Verhoeven B, Hellings WE, Moll FL, de Vries JP, de Kleijn DP, de Bruin P, et al. Carotid atherosclerotic plaques in patients with transient ischemic attacks and stroke have unstable characteristics compared with plaques in asymptomatic and amaurosis fugax patients. J Vasc Surg. 2005;42:1075–1081. doi: 10.1016/j.jvs.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii Y, Maeda M, Kida H, Matsuo K, Shindo A, Taniguchi A, et al. In vivo detection of cortical microinfarcts on ultrahigh-field MRI. J Neuroimaging. 2013;23:28–32. doi: 10.1111/j.1552-6569.2012.00722.x. [DOI] [PubMed] [Google Scholar]
- van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, Spliet WG, Hendrikse J, Luijten PR, et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab. 2013;33:322–329. doi: 10.1038/jcbfm.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog AG, Bovens SM, Koning W, Hendrikse J, Pasterkamp G, Moll FL, et al. PLACD-7T study: atherosclerotic carotid plaque components correlated with cerebral damage at 7 Tesla magnetic resonance imaging. Curr Cardiol Rev. 2011;7:28–34. doi: 10.2174/157340311795677743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser F, Zwanenburg JJ, Hoogduin JM, Luijten PR. High-resolution magnetization-prepared 3D-FLAIR imaging at 7.0 Tesla. Magn Reson Med. 2010;64:194–202. doi: 10.1002/mrm.22397. [DOI] [PubMed] [Google Scholar]
- Zheng L, Vinters HV, Mack WJ, Zarow C, Ellis WG, Chui HC. Cerebral atherosclerosis is associated with cystic infarcts and microinfarcts but not Alzheimer pathologic changes. Stroke. 2013;44:2835–2841. doi: 10.1161/STROKEAHA.113.001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18:645–658. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]
- Madai VI, von Samson-Himmelstjerna FC, Bauer M, Stengl KL, Mutke MA, Tovar-Martinez E, et al. Ultrahigh-field MRI in human ischemic stroke—a 7 tesla study. PLoS One. 2012;7:e37631. doi: 10.1371/journal.pone.0037631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund M, Passant U, Sjobeck M, Ghebremedhin E, Englund E. Cerebral amyloid angiopathy and cortical microinfarcts as putative substrates of vascular dementia. Int J Geriatr Psychiatry. 2006;21:681–687. doi: 10.1002/gps.1550. [DOI] [PubMed] [Google Scholar]
- Wezel J, Kooij BJ, Webb AG.Assessing the MR compatibility of dental retainer wires at 7 Tesla Magn Reson Medadvance online publication, 11 November 2013; doi: 10.1002/mrm.25019 [DOI] [PubMed]
- European Carotid Surgery Trialists' Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Gutnikov SA, Warlow CP. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003;34:514–523. doi: 10.1161/01.str.0000054671.71777.c7. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. doi: 10.1016/s0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]