Abstract
Previous studies have suggested that schizophrenia patients have dysfunctional thermoregulation. The aim of this study was to examine whether brain temperature (BT) in schizophrenia patients differs from that in patients with bipolar disorder and healthy subjects by using magnetic resonance imaging. We also evaluated the possible relationship between BT and cerebral blood flow (CBF). We analyzed the temperature of lateral ventricles as the mean BT using diffusion-weighted imaging (DWI) thermometry, and evaluated the relationships between the BT and the CBF using pseudo-continuous arterial spin labeling (pCASL) among 3 diagnostic groups, 22 male patients with schizophrenia, 19 male patients with bipolar disorder, and 23 healthy male subjects. There were significant positive correlations between BT in the lateral ventricles and CBF in both the patients with bipolar disorder and healthy subjects. By contrast, there were significant negative correlations in patients with schizophrenia. We could not detect the significant difference in the surrogates of BT among three diagnostic groups. We showed that patients with schizophrenia, but not those with bipolar disorder, have dysfunctional thermoregulation in the brain. Brain temperature is highly dependent on cerebral metabolism and CBF, and thus uncoupling of cerebral metabolism and CBF may occur in schizophrenics.
Keywords: arterial spin labeling, bipolar disorder, brain temperature, diffusion-weighted imaging thermometry, schizophrenia
Introduction
Schizophrenia is a psychotic disorder characterized by altered perception, thought processes, and behaviors; and bipolar disorder is a mood disorder involving prolonged states of depression and mania.1 Historically, bipolar disorder and schizophrenia have been considered distinct nosological entities, with each disorder thought to have a different etiology and pathogenesis. However, the underling neural mechanisms of these disorders remain unclear. Functional brain imaging studies using positron emission tomography have most commonly reported that hyperdopaminergia are thought to be fundamental to the emergence of psychotic symptoms and to the mechanism of action of antipsychotics (for review, see Fusar-Poli and Meyer-Lindenberg2). Further, schizophrenia showed hypofrontality, a decrease in cerebral metabolic rate (CMR for glucose or oxygen) and a decrease in cerebral blood flow (CBF) in frontal regions revealed by positron emission tomography and single photon emission computed tomography (for review, see Hill et al3). However, increased prefronto-limbic CMR and CBF have been observed in some positron emission tomography and single photon emission computed tomography reports in bipolar disorder (for review, see Gonul et al4).
Cerebral metabolic rate and CBF have been found to be closely correlated in positron emission tomography studies in healthy human subjects.4 However, some developmental, degenerative, ischemic, and neoplastic processes have been associated with an uncoupling of CMR and CBF.5,6 Dunn et al7 also showed the uncoupling of CMR and CBF in depressed patients. As for bipolar disease, they showed that patients with bipolar disease showed a positive correlation between CMR and CBF globally like controls.7
In healthy humans, brain temperature (BT) is determined by the balance between heat produced by cerebral energy turnover and heat removal.8 Because heat removal is primarily dependent on CBF8 and the arterio-venous temperature difference across the brain,8,9 reduced cerebral perfusion relative to cerebral metabolism may indicate decreased central heat removal (i.e., higher BT).10 Previous studies have suggested that schizophrenia patients have dysfunctional thermoregulation,11, 12, 13, 14, 15, 16, 17 and these data suggest that patients with schizophrenia may exhibit abnormal BT.
Recently, human BT in lateral ventricle has been estimated noninvasively and accurately from the diffusion-weighted imaging (DWI).18,19 Cerebral blood flow can also be calculated noninvasively by pseudo-continuous arterial spin labeling (pCASL) imaging.20 The aim of the present study was to examine whether the temperature of lateral ventricle, regarded as the mean BT, in schizophrenia patients differs from that in patients with bipolar disorder and healthy subjects by using DWI. We also evaluated whether there were differences in the relationship between BT and CBF among the three diagnostic groups by using pCASL.
Materials and methods
Subjects
Subjects were consisted of 22 male individuals with schizophrenia, 19 male individuals with bipolar disorder, and 23 healthy male subjects. Diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria.21 All subjects were Japanese males and biologically unrelated. The symptoms of all schizophrenia subjects were assessed by using the Positive and Negative Syndrome Scale (PANSS),22 and the patients with bipolar disorder were rated with the 21-item Hamilton depression rating scale23 and Young mania rating scale.24 Daily doses of antipsychotics including depot antipsychotics and antidepressants were converted to chlorpromazine and imipramine equivalents using published guidelines, respectively.25 The characteristics of the participants are shown in Table 1.
Table 1. Demographic and clinical characteristics of the subjects.
|
Variable |
Healthy subjects |
Schizophrenia |
Bipolar disorder |
P |
|---|---|---|---|---|
| Male (n=23) | Male (n=22) | Male (n=19) | ||
| Mean±s.d. | Mean±s.d. | Mean±s.d. | ||
| Age (years) | 39.3±12.7 | 36.3±10.4 | 42.4±10.2 | 0.24 |
| Education (years) | 17.2±4.0 | 14.8±2.6 | 15.8±3.3 | 0.06 |
| Age at onset (years) | 22.7±6.1 | 32.7±10.5 | <0.001 | |
| Antipsychotic medicationa (mg/day) | 573.9±470.8 | 222.3±344.5 | 0.01 | |
| Antidepressant medicationb (mg/day) | 38.0±100.6 | 64.7±141.4 | 0.5 | |
| HAM-D | 12.2±9.0 | |||
| Young mania rating scale | 0.4±1.1 | |||
| PANSS positive | 14.8±4.4 | |||
| PANSS negative | 17.0±7.3 | |||
| PANSS general | 33.4±10.2 |
HAM-D, Hamilton's rating scale for depression; PANSS, positive and negative syndrome scale; s.d., standard deviation.
Chlorpromazine equivalent.
Imipramine equivalent.
Healthy subjects were recruited from the community through local magazine advertisements and our website announcement. These participants were interviewed for enrollment by a research psychiatrist using the Japanese version of the Mini-International Neuropsychiatric Interview.26 Participants were excluded if they had a prior medical history of central nervous system disease or severe head injury, or if they met the criteria for substance abuse or dependence. Those individuals who showed a history of psychiatric illness or contact with psychiatric services were excluded from the group of healthy subjects.
After the study was explained to each participant, written informed consent was obtained for participation. This study was approved by the ethics committee of the National Center of Neurology and Psychiatry, Japan.
Magnetic Resonance Imaging Data Acquisition and Processing
The magnetic resonance studies were performed on a 3-T MR system (Philips Medical Systems, Best, The Netherlands). The DWI was performed in the axial plane (repetition time/echo time, 5,760/62 ms; matrix, 80 × 80; field of view, 240 × 240 mm; 60 continuous transverse slices; slice thickness 3 mm with no interslice gap). To enhance the signal-to-noise ratio, acquisition was performed twice. Diffusion was measured along 15 noncollinear directions using a diffusion-weighted factor b in each direction of 1,000 s/mm2, and one image was acquired without using any diffusion gradient. The imaging parameters for all of the pCASL experiments were single-shot gradient-echo echo planar imaging in combination with parallel imaging (sensitivity encoding (SENSE) factor 2.0), repetition time=4,000 ms, echo time=12 ms, matrix=64 × 64, field of view=240 × 240, voxel size=3.75 × 3.75 mm, 20 slices acquired in ascending order, slice thickness=7 mm, 1-mm gap between slices, labeling duration=1,650 ms, postspin labeling delay=1520 ms, time interval between consecutive slice acquisitions=32.0 ms, radio frequency duration=0.5 ms, pause between radio frequency pulses =0.5 ms, labeling pulse flip angle=18°, bandwidth=3.3 kHz/pixel, echo train length=35. Thirty-two pairs of control/label images were acquired and averaged. The scan duration was 4 minutes and 24 seconds. For measurement of the magnetization of arterial blood and also for segmentation purposes, an echo planar imaging M0 image was obtained separately with the same geometry and the same imaging parameters as the pCASL without labeling. Details of the calculation of regional CBF (rCBF) are described elsewhere.19 The CBF maps were then normalized with the DARTEL (diffeomorphic anatomical registration using the exponentiated lie) registration method using a template made from the average CBF maps of healthy subjects previously recorded at our center.27 Each map was then spatially smoothed with a 4-mm full-width at half-maximum Gaussian kernel to decrease spatial noise and compensate for the inexactitude of normalization.
Calculation of the Brain Temperatures
Diffusion-weighted imaging analysis
We extracted the body of the lateral ventricle and calculated the mean temperature in the lateral ventricle using the automated temperature calculation method.19 In this method, the diffusion coefficient in the diffusion direction i was calculated by Equation [1] and then converted to a temperature.
 |
Here, Di is the diffusion coefficient (mm2/s) along the diffusion direction i, b is the applied diffusion weighting (s/mm2), and S0 and Si are the voxel signal intensities of the reference and diffusion-weighted images along diffusion direction i, respectively. The Di value was converted to the corresponding temperature using Equation [2].
Here, Ti is in the unit of degrees Celsius (°C). The temperature within the lateral ventricle was determined according to our previous paper.18
Statistical Analysis
The differences in age and years of education among patient groups and healthy subjects were evaluated using analysis of variance, and the differences in age at onset and dose of antipsychotics and antidepressants were evaluated using two-sample t test. The differences of BT in lateral ventricles were evaluated using analysis of covariance controlling for age. The post hoc test was done using Bonferroni's correction for multiple comparisons. The correlations between BT in lateral ventricles and the clinical symptoms of disorders were evaluated using partial correlation analysis controlling for age. Statistical analyses were performed using SPSS Statistics for Windows 17.0 software (SPSS Japan, Tokyo, Japan).
Correlations between the BT in the lateral ventricles and rCBF in patient groups and healthy subjects were assessed using the SPM8 (Welcome Department of Imaging Neuroscience, London, UK) software. We evaluated the correlation of each group by ‘full factorial' design in SPM8 with age as a covariate, and with the BT in the lateral ventricles as a covariate interacting with the factor ‘diagnosis'. Statistical analyses were performed only differences that met the following criteria were deemed significant. In this case, a height threshold of P<0.001 (uncorrected) and the extent threshold of P<0.05 (uncorrected) were adopted.
Results
The demographic and clinical characteristics of the participants are shown in Table 1. There were no significant differences in age and education years among the three groups in each sample.
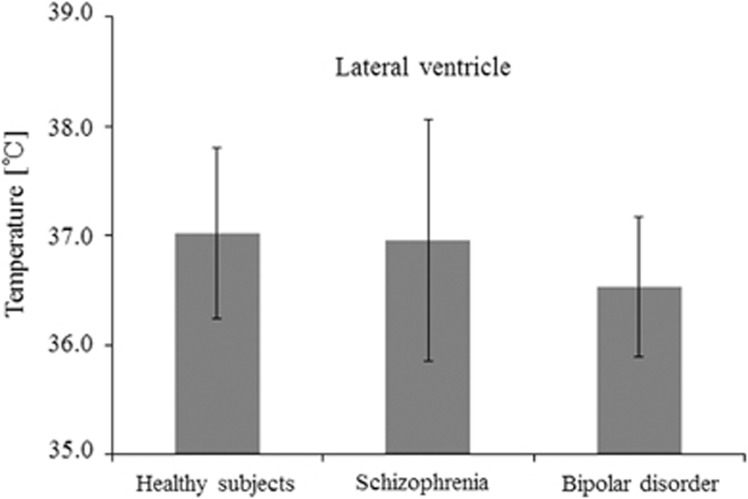
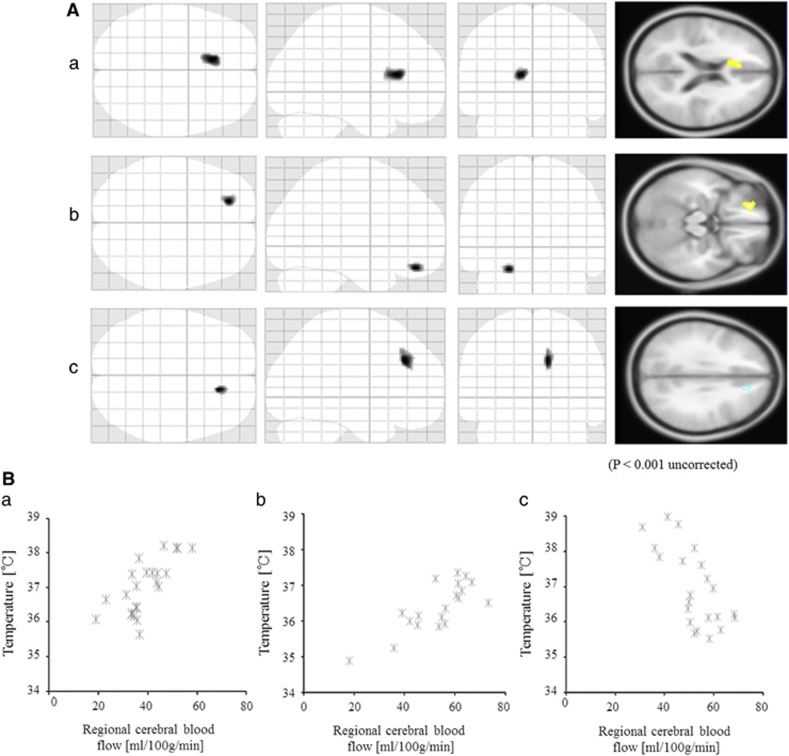
When the temperature in the lateral ventricles was compared by DWI thermometry, we could not detect a significant difference among the three diagnostic groups (Figure 1). Only the significant negative correlation was detected between the BT in lateral ventricles of schizophrenia and PANSS-general score (correlation coefficient=−0.54, P=0.012, d.f.=19). Second, we evaluated the relationships between the BT in the lateral ventricles and rCBF, and there were significant positive correlations in the left anterior cingulate in healthy subjects, and the left orbitofrontal region in patients with bipolar disorder (Figures 2A 2a, 2B, and 2b; Table 2). However, there were significant negative correlations in the right medial frontal region in patients with schizophrenia (Figure 2C and 2c; Table 2). The slope and R2 value of the linear approximate equation between the rCBF and the temperature in the lateral ventricles were slope=0.06, R2 value=0.53 in healthy subjects (Figure 2a); slope=0.04, R2 value=0.62 in the patients with bipolar disorder (Figure 2b); slope=−0.08, R2 value=0.49 in the patients with schizophrenia (Figure 2c).
Figure 1.
Clinical differences of brain temperature. There was no significant difference of temperature in the lateral ventricles among the three groups.
Figure 2.
The relationships between the brain temperature and regional cerebral blood flow. Three left images showed the statistically significant regions projected on a glass brain in the three orthogonal planes and one right image showed the regions on the standard T1-weighted image. (A) There were significant positive correlations in the left anterior cingulate in healthy subjects (shown in yellow). (a) The scatter plot of them. (B) There were significant positive correlations in the left orbitofrontal region in patients with bipolar disorder (shown in red). (b) The scatter plot of them. (C) There were significant negative correlations in the right medial frontal region in patients with schizophrenia (shown in blue). (c) The scatter plot of them.
Table 2. Regions of statistically significant correlations between regional cerebral blood flow and temperature of lateral ventricles using age as nuisance variable.
| Group | Cluster size | Z score | x | y | z | Brain region |
|---|---|---|---|---|---|---|
| Positive correlation | ||||||
| Healthy subjects | 184 | 4.52 | −10 | 26 | 16 | Left anterior cingulate gyrus |
| Patients with bipolar disorder | 82 | 4.33 | −22 | 44 | −20 | Left orbitofrontal gyrus |
| Negative correlation | ||||||
| Patients with schizophrenia | 99 | 3.94 | 14 | 34 | 34 | Right medial frontal gyrus |
Discussion
We found that there were different correlation patterns between BT in the lateral ventricles and CBF in the patients with schizophrenia compared with the patients with bipolar disorder and healthy subjects. To our knowledge, this is the first study focusing on the surrogate of BT and CBF simultaneously determined using magnetic resonance imaging.
Human studies examining acute stroke, brain tumors, and Moyamoya disease have shown a significant difference of BT compared with healthy subjects,10,28,29 and they indicated that the balance between heat production caused by CMR and heat removal by CBF helps to keep the temperature of the brain constant. A previous study focusing on psychiatric disease reported that all regional correlation coefficients for CMR and CBF were positive in the patients with bipolar disorder just as in the control subjects.7 Our results revealed that patients with bipolar disorder showed a positive correlation between the BT in the lateral ventricles and the CBF that was the same pattern in healthy subjects. These points may indicate that the thermoregulation system of the healthy subjects is the same as that of patients with bipolar disorder. However, the patients with schizophrenia showed a different correlation between the BT and the CBF. Typically, glucose is the principal energy substrate of the brain. However, it is reported that the brain of depressive patient uses an energy source other than glucose,30 and other study showed the dysregulation between CMR and CBF in the patients with major depressive disorder.7 Similarly, the patients with dementia of Alzheimer' type and with the alcohol abuse, who showed the decrement of brain glucose utilization and the increase in acetate uptake in the brain,31, 32, 33 presented with a dysregulation between CMR and CBF.34,35 The metabolic profiling study36 and postmortem study37 indicated the disordered energy metabolism in the brain of schizophrenia. Brain depends exclusively on glycolysis and oxidative phosphorylation to create ATP. Thus, any mitochondrial pathology will have the most pronounced effect on the brain. Recent postmortem studies in schizophrenia have revealed abnormalities in mitochondrial morphology, function, and gene expression.38 In vivo evidence for mitochondrial involvement in schizophrenia derives from magnetic resonance spectroscopy, an imaging technique that allows visualization of energy-related metabolite levels in the brain. N-acetyl-aspartate is an amino acid thought to be primarily synthesized in the mitochondria of neurons that can be measured by magnetic resonance spectroscopy. Some of magnetic resonance spectroscopy studies in schizophrenia have reported decreased N-acetyl-aspartate levels in several brain regions.39 Further, it is known that antipsychotics induce the dysfunction of mitochondria.40 The change in the brain energy metabolism in schizophrenia would alter the coupling of CBF and BT.
In this study, the significant positive or negative correlation between the BT of the lateral ventricles and CBF was observed in the medial frontal region and orbitofrontal region. The anterior cerebral artery runs through the neighborhood of the anterior part of the lateral ventricles, and the course of the artery is thought to influence the BT in the lateral ventricles.
Our data could not detect the significant difference of the BT in the lateral ventricle among three diagnostic groups. It is known that schizophrenia patients have dysfunctional thermoregulation (e.g., axillary, corneal, rectal, and oral esophageal),11, 12, 13, 14, 15, 16, 17 though there has been only one study that evaluated the BT of schizophrenic patients in vivo. In that report, there was posterior-dominant occipital-frontal temperature gradient in schizophrenics.17 We calculated the mean BT in the whole lateral ventricle, and did not evaluate in each segmented area. Further study with the measurement of temperature in small segmented lateral ventricles may show the temperature gradient of the schizophrenic brain. We detected the negative correlation between the BT and PANSS-general score, and this point was compatible with the previous one.17 In addition, it is known that antipsychotics may influence the temperature. Specifically, antipsychotics have been shown to have the capacity to lower core temperature,41,42 yet the schizophrenic patients in this study were prescribed larger volumes of antipsychotics, and showed higher BTs, compared with the patients with bipolar disorder. The schizophrenic participants in this study also showed relatively low PANSS scores, and PANSS scores have been shown to be negatively correlated with frontal CBF.43,44 Then, the little decline in CBF may have obscured the change in BT. A further study with controlled antipsychotics and severe schizophrenic patients may show the dysfunctional brain thermoregulation. Besides, this study contained relatively small sample size, then the degrees of freedoms for each of their correlations in the individual groups were low; 19 for schizophrenia patients, 16 for bipolar disorder, and 20 for healthy controls. The validity of the SPM is strongly dependent on the degrees of freedom, and experiments should be designed such that d.f. ⩾30.45 Future study with a larger number of subjects would be necessary to verify this study.
In conclusion, we showed that patients with schizophrenia, but not those with bipolar disorder, exhibited dysfunctional thermoregulation in the brain. Brain temperature is highly dependent on CMR and CBF, and thus uncoupling of CMR and CBF may occur in schizophrenics.
The authors declare no conflict of interest.
Footnotes
This study was supported by a Health and Labor Sciences Research Grant (for Comprehensive Research on Disability, Health, and Welfare) (MO and HK), an Intramural Research Grant (24-11) for Neurological and Psychiatric Disorders of NCNP (MO and HK), and a grant for ‘Understanding of molecular and environmental bases for brain health' carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (HK).
References
- Goodwin FK, Jamison KKR.Manic-depressive illness: bipolar disorders and recurrent depression2nd edn.Oxford University Press: New York; 200754–59. [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull. 2013;39:33–42. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatry Scand. 2004;110:243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Coburn K, Kula M. Cerebral blood flow, metabolic, receptor, and transporter changes in bipolar disorder: the role of PET and SPECT studies. Int Rev Psychiatry. 2009;21:323–335. doi: 10.1080/09540260902962131. [DOI] [PubMed] [Google Scholar]
- Fink GR, Pawlik G, Stefan H, Pietrzyk U, Wienhard K, Heiss WD. Temporal lobe epilepsy: evidence for interictal uncoupling of blood flow and glucose metabolism in temporomesial structures. J Neurol Sci. 1996;137:28–34. doi: 10.1016/0022-510x(95)00323-t. [DOI] [PubMed] [Google Scholar]
- Mirza M, Tutus A, Erdogan F, Kula M, Tomar A, Silov G, et al. Interictal SPECT with tc-99m HMPAO studies in migraine patients. Acta Neurol Belg. 1998;98:190–194. [PubMed] [Google Scholar]
- Dunn RT, Willis MW, Benson BE, Repella JD, Kimbrell TA, Ketter TA, et al. Preliminary findings of uncoupling of flow and metabolism in unipolar compared with bipolar affective illness and normal controls. Psychiatry Res. 2005;140:181–198. doi: 10.1016/j.pscychresns.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonskiy DA, Ackerman JJ, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci USA. 2000;97:7603–7608. doi: 10.1073/pnas.97.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol. 2006;60:438–446. doi: 10.1002/ana.20957. [DOI] [PubMed] [Google Scholar]
- Spivak B, Weizman A, Wolovick L, Hermesh H, Tyano S, Munitz H. Neuroleptic malignant syndrome during abrupt reduction of neuroleptic treatment. Acta Psychiatry Scand. 1990;81:168–169. doi: 10.1111/j.1600-0447.1990.tb06473.x. [DOI] [PubMed] [Google Scholar]
- Cape G. Neuroleptic malignant syndrome—a cautionary tale and a surprising outcome. Br J Psychiatry. 1994;164:120–122. doi: 10.1192/bjp.164.1.120. [DOI] [PubMed] [Google Scholar]
- Hermesh H, Shiloh R, Epstein Y, Manaim H, Weizman A, Munitz H. Heat intolerance in patients with chronic schizophrenia maintained with antipsychotic drugs. Am J Psychiatry. 2000;157:1327–1329. doi: 10.1176/appi.ajp.157.8.1327. [DOI] [PubMed] [Google Scholar]
- Shiloh R, Weizman A, Epstein Y, Rosenberg S, Valevski A, Dorfman-Etrog P, et al. Abnormal thermoregulation in drug-free male schizophrenia patients. Eur Neuropsychopharmacol. 2001;11:285–288. doi: 10.1016/s0924-977x(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Shiloh R, Portuguese S, Bodinger L, Katz N, Sigler M, Hermesh H, et al. Increased corneal temperature in drug-free male schizophrenia patients. Eur Neuropsychopharmacol. 2003;13:49–52. doi: 10.1016/s0924-977x(02)00080-9. [DOI] [PubMed] [Google Scholar]
- Chong TW, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69:149–157. doi: 10.1016/s0920-9964(03)00222-6. [DOI] [PubMed] [Google Scholar]
- Shiloh R, Kushnir T, Gilat Y, Gross-Isseroff R, Hermesh H, Munitz H, et al. In vivo occipital-frontal temperature-gradient in schizophrenia patients and its possible association with psychopathology: a magnetic resonance spectroscopy study. Eur Neuropsychopharmacol. 2008;18:557–564. doi: 10.1016/j.euroneuro.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Sakai K, Yamada K, Sugimoto N. Calculation methods for ventricular DWI thermometry: phantom and volunteer studies. NMR Biomed. 2012;25:340–346. doi: 10.1002/nbm.1755. [DOI] [PubMed] [Google Scholar]
- Sakai K, Yamada K, Sugimoto N. Automated temperature calculation method for DWI-thermometry: volunteer study. Proc SPIE. 2013;8669:866922. doi: 10.1109/EMBC.2013.6609546. [DOI] [PubMed] [Google Scholar]
- Ota M, Sato N, Nakata Y, Ito K, Kamiya K, Maikusa N, et al. Abnormalities of cerebral blood flow in multiple sclerosis: a pseudo-continuous arterial spin labeling MRI study. Magn Reson Imaging. 2013;31:990–995. doi: 10.1016/j.mri.2013.03.016. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association DSM-IV: Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Press: Washington, DC; 1994 [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale of depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Inagaki A, Inada T, Fujii Y, Yagi G. Equivalent dose of psychotropics. Seiwa Shoten: Tokyo; 1999. [Google Scholar]
- Otsubo T, Tanaka K, Koda R, Shinoda J, Sano N, Tanaka S. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59:517–526. doi: 10.1111/j.1440-1819.2005.01408.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Jayasundar R, Singh VP. In vivo temperature measurements in brain tumors using proton MR spectroscopy. Neurol India. 2002;50:436–439. [PubMed] [Google Scholar]
- Yamada K, Sakai K, Akazawa K, Yuen S, Sugimoto N, Sasajima H, et al. Moyamoya patients exhibit higher brain temperatures than normal controls. Neuroreport. 2010;21:851–855. doi: 10.1097/WNR.0b013e32833d6b7a. [DOI] [PubMed] [Google Scholar]
- Lambert G, Johansson M, Agren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry. 2000;57:787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Gulanski BI, De Feyter HM, Weinzimer SA, Pittman B, Guidone E, et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest. 2013;123:1605–1614. doi: 10.1172/JCI65153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara Y, Ichiya Y, Ichimiya A, Sasaki M, Akashi Y, Yoshida T, et al. The relationship between the cerebral blood flow, oxygen consumption and glucose metabolism in primary degenerative dementia. Kaku Igaku. 1995;32:253–262. [PubMed] [Google Scholar]
- Zheng G, Zhang LJ, Zhong J, Wang Z, Qi R, Shi D, et al. Cerebral blood flow measured by arterial-spin labeling MRI: a useful biomarker for characterization of minimal hepatic encephalopathy in patients with cirrhosis. Eur J Radiol. 2013;82:1981–1988. doi: 10.1016/j.ejrad.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen T, Sun L, Zhao Z, Qi X, Zhou K, et al. Potential metabolite markers of schizophrenia. Mol Psychiatry. 2013;18:67–78. doi: 10.1038/mp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29:311–324. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, et al. Neurometabolites in schizophrenia and bipolar disorder—a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Shannon V, Heart DL, Paredes RM, Navaira E, Catano G, Maffi SK, et al. Clozapine-induced mitochondria alterations and inflammation in brain and insulin-responsive cells. PLoS ONE. 2013;8:e59012. doi: 10.1371/journal.pone.0059012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh R, Bodinger L, Katz N, Sigler M, Stryjer R, Hermesh H, et al. Lower corneal temperature in neuroleptic-treated vs. drug-free schizophrenia patients. Neuropsychobiology. 2003;48:1–4. doi: 10.1159/000071820. [DOI] [PubMed] [Google Scholar]
- Shiloh R, Hermesh H, Weizer N, Dorfman-Etrog P, Weizman A, Munitz H. Acute antipsychotic drug administration lowers body temperature in drug-free male schizophrenic patients. Eur Neuropsychopharmacol. 2000;10:443–445. doi: 10.1016/s0924-977x(00)00106-1. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 2011;194:64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping. 1995;2:189–210. [Google Scholar]