Abstract
To assess reactive oxygen species (ROS) production by detecting the fluorescent oxidation product, hydroethidine has been used extensively. The present study was undertaken to evaluate the potential of the hydroethidine derivative as a radiotracer to measure in vivo brain ROS production. [3H]-labeled N-methyl-2,3-diamino-6-phenyl-dihydrophenanthridine ([3H]Hydromethidine) was synthesized, and evaluated using in vitro radical-induced oxidization and in vivo brain ROS production model. In vitro studies have indicated that [3H]Hydromethidine is converted to oxidized products by a superoxide radical (O2•−) and a hydroxyl radical (OH•−) but not hydrogen peroxide (H2O2). In vivo whole-body distribution study showed that [3H]Hydromethidine rapidly penetrated the brain and then was washed out in normal mice. Microinjection of sodium nitroprusside (SNP) into the brain was performed to produce ROS such as OH•− via Fenton reaction. A significant accumulation of radioactivity immediately after [3H]Hydromethidine injection was seen in the side of the brain treated with SNP (5 and 20 nmol) compared with that in the contralateral side. These results indicated that [3H]Hydromethidine freely penetrated into the brain where it was rapidly converted to oxidized forms, which were trapped there in response to the production of ROS. Thus, [3H]Hydromethidine should be useful as a radical trapping radiotracer in the brain.
Keywords: in vivo molecular imaging, radical trapping radiotracer, reactive oxygen species
Introduction
Reactive oxygen species (ROS) have a pivotal role as regulatory mediators in signal processes at moderate concentrations.1 An excessive amount of ROS causes the imbalance of redox state and damages cell function in living tissue and organs. A sustained increase in ROS production has been implicated in a wide variety of disease state such as inflammation, cancer, ischemia/reperfusion injury, neurodegenerative disorders, and oxidative stress.2, 3, 4, 5 The superoxide radical (O2•−) is usually the primary ROS produced and is subsequently converted into hydrogen peroxide (H2O2) through spontaneous or superoxide dismutase (SOD)-catalyzed dismutation. Reaction of O2•− and nitric oxide (NO) generates peroxynitrite (ONOO•−). Reaction of H2O2 and ONOO•− can generate the highly reactive hydroxyl radical (OH•−).6,7 O2•− is subsequently converted into OH•− and H2O2.8 To clarify the roles of different ROS in disease pathogenesis, highly sensitive and specific optical probes (fluorescent, luminescent, or chemiluminescent probes) for detecting ROS are being developed.9, 10, 11, 12
Hydroethidine, a fluorescent ROS probe, is extensively used in tissue experiments (in vitro and ex vivo) to detect O2•− production. The reaction between hydroethidine and O2•− is considered to generate an oxidative product with red fluorescence.13, 14, 15 Hydroethidine, an uncharged lipophilic compound is easily transported to a tissue or a cell, where it is converted to positively charged products (membrane-impermeable products), which become trapped there. Murakami et al.16 have reported that hydroethidine can detect the O2•− produced by occlusion of the middle cerebral artery using mutant mice with a heterozygous knock-out gene encoding mitochondrial manganese SOD. It has also been reported to detect O2•− production in the hippocampus of lithium-pilocarpine epilepsy rats,17 beta cells of type II diabetes mellitus rats,18 or the brain of multiple sclerosis mice.19
Recently, Hall et al.20 have reported the detection of O2•− production in real time in aging or ketamine-pretreated brain using an optical imaging technique with hydroethidine and fluorescence lifetime contrast-based unmixing. This optical imaging is useful as a relatively simple method to detect the production of O2•− but quantitative measurement is difficult with respect to tissue absorption of the optical signal. Generally, a radiolabeled probe such as 3H or 14C is used to acquire quantitative autoradiogram with high sensitivity. Probe-labeled positron emitters such as 11C or 18F enable noninvasive measurement of the whole body. Radiotracer of hydroethidine-related compounds is considered to be trapped in tissue based on the hypothesis of metabolic trapping as reaction between hydroethidine and ROS results in the generation of a cation-charged product, the oxidized form of hydroethidine.
In the present study, we synthesized 3H-labeled hydromethidine and evaluated its ability to detect ROS in vitro and in vivo. Hydromethidine was selected as a prototype of radiotracer since it is possible to synthesize 3H and 11C-labeled compounds by N-methylation. In vivo brain ROS generation was performed by striatal microinjection of sodium nitroprusside (SNP) generating ROS including OH•− by Fenton reaction.21 The OH•− is one of the most reactive ROS in neuronal tissue damage.
Materials and methods
Animals
All animal experiments in the present study were reviewed and approved by the Institutional Animal Care and Use Committee of Shionogi Research Laboratories (Osaka, Japan) and were consistent with the internal guidelines for animal experiments and in adherence to the ethics policy of Shionogi & Co., Ltd (Osaka, Japan). Male C57BL/6 J mice were purchased from CLEA Japan, Inc. (Tokyo, Japan) and were 8 weeks old at the time of experiments. They were allowed free access to chow and tap water and housed in a temperature-controlled room maintained on a 12-hour light/dark cycle with lights on at 0800 h.
Chemicals and Reagents
NaBH4, 3,8-diamino-6-phenanthridine, hypoxanthine, xanthine oxidase, and SOD were obtained from Sigma-Aldrich (St Louis, MO, USA). [methyl-3H]Methyl nosylate was obtained from Perkin-Elmer, Inc. (Waltham, MA, USA). Ethyl acetate (EtOAc), n-hexane, CH3COONH4, and dimethyl sulfoxide (DMSO) were obtained from Nacalai Tesque (Kyoto, Japan), and 4N-HCl/EtOAc from Watanabe Chemical Industries Ltd. (Hiroshima, Japan). PIC B8 low UV was obtained from Waters Corp. (Milford, MA, USA). Acetonitrile, NaHCO3, and FeSO4•7H2O were obtained from Kanto Chemical Co. Inc. (Tokyo, Japan). Di-tert-butyl dicarbonate ((Boc)2O), H2O2, and sodium pentacyanonitrosylferrate(III) dihydrate (SNP) were obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Isoflurane was from Abbott Japan Co., Ltd. (Tokyo, Japan), and NOC-18 from Dojindo Laboratories (Kumamoto, Japan).
Synthesis of [3H]Hydromethidine
The radiochemical reaction was monitored by high performance liquid chromatography (HPLC) system (LC-20A; Shimadzu, Kyoto, Japan) with a flow scintillation analyzer (Radiomatic 625TR; Perkin-Elmer, Inc.).
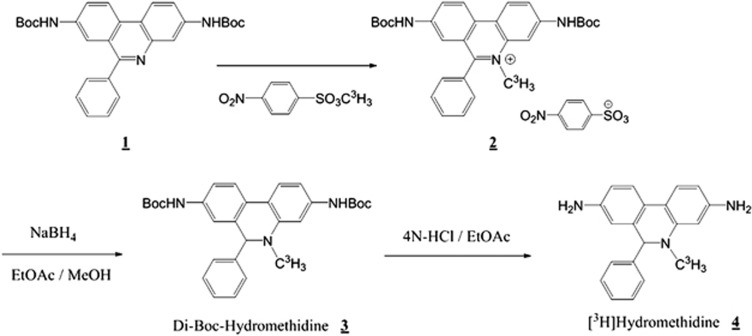
Labeling scheme for [3H]Hydromethidine is shown in Figure 1. Starting material (1) was prepared by treating 3,8-diamino-6-phenanthridine with (Boc)2O. [methyl-3H]Methyl nosylate (total activity: 370 MBq, specific activity: 740 GBq/mmol) and starting material (1) were dissolved in acetonitrile and stirred at 80°C for 5 hours. The reaction mixture was purified by preparative thin-layer chromatography (SiO2 60 F254; Merck, Darmstadt, Germany) using EtOAc as a developing solvent, and extracted with EtOAc/methanol (2:1) to give di-Boc-methidium 4-nitrobenzenesulfonate (2). Di-Boc methidium 4-nitirobenzenesulfonate was reduced by NaBH4 to give di-Boc-Hydromethidine (3), and deprotected with 4N HCl/EtOAc solution. The reaction mixture was concentrated, mixed with NaHCO3 aq. to adjust the pH to about 8, and extracted with EtOAc. The extract was purified by preparative thin-layer chromatography (Chromatorex DNH, Fuji Silysia Chemical Ltd., Aichi, Japan) using n-hexane/EtOAc (1:2) as developing solvents to give a solution of [3H]Hydromethidine (4). Specific activity was measured by liquid chromatography/mass spectrometry system (Quattro micro ESCi with Alliance 2690 and 2487 Dual Absorbance Detector; Waters Corp.). Radiochemical purity was measured by a radio-HPLC system using a Cadenza CD-C18 column (40°C, 3 μm, 3.0 × 75 mm; Imtakt Corp., Kyoto, Japan) eluted at 0.4 mL/min with a gradient of A (CH3COONH4 buffer containing 5 mmol/L PIC B8 low UV, pH 4.5) and B (acetonitrile), with B being increased linearly from 30% to 90% (v/v) over 15 minutes. Specific activity and radiochemical purity were 74 GBq/mmol and 98.8%, respectively. For the following experiments, [3H]Hydromethidine was diluted with distilled water containing 5% DMSO (v/v), giving 95.7% of radiochemical purity.
Figure 1.
Labeling scheme for [3H]Hydromethidine.
In vitro Reaction Between [3H]Hydromethidine and Reactive Oxygen Species
The hypoxanthine/xanthine oxidase system was used to generate O2•−. Hypoxanthine (0.42 mmol/L) containing [3H]Hydromethidine was incubated with xanthine oxidase (8.3 mU/mL) in phosphate-buffered saline (2.7 mmol/L KCl, 137 mmol/L NaCl, 10 mmol/L phosphate, pH 7.4) at 37°C for 20 minutes. Superoxide dismutase (250 U/mL) was used to scavenge the generated O2•−.
The Fenton reaction was used to generate OH•−. Fe2+ (1.7 mmol/L) containing [3H]Hydromethidine was incubated with H2O2 (74 mmol/L) in distilled water at room temperature for 20 minutes.
The radioactivity of an aliquot of each mixture after the incubation was measured with a liquid scintillation analyzer (Perkin-Elmer, Inc.). Another aliquot of the mixture was mixed with an equal volume of EtOAc before centrifugation (11 000 g, 4°C, 3 minutes), and the radioactivity of the organic layer was measured. The values are expressed as % remaining of [3H]Hydromethidine calculated by dividing the weight-corrected radioactivity of the organic layer by that of the incubated mixture.
Whole-Body Distribution in Mice
An aqueous solution (5% DMSO, v/v) of [3H]Hydromethidine (74 kBq) was injected intravenously into the tail vein of mice. The mice were killed by decaptation at postinjection time points of 1, 5, 10, 30, and 60 minutes under deep anesthesia with isoflurane, and the organs of interest were removed. The tissue weights were determined, and the radioactivity in the tissue was measured with a liquid scintillation analyzer (Perkin-Elmer, Inc.). The percentage of unmetabolized [3H]Hydromethidine in the radioactivity of blood was determined as follows. The radioactivity of an aliquot of blood samples was measured with a liquid scintillation analyzer. Another aliquot of blood samples was mixed with 2 mL of EtOAc, and the radioactivity of the organic layer was measured. The values are calculated by dividing the weight-corrected radioactivity of the organic layer by that of the blood sample. Remaining blood samples were centrifuged at 11 000 g for 3 minutes at 4°C to obtain plasma.
Intrastriatal Injection of Sodium Nitroprusside and NOC-18
Injection of SNP or NOC-18 into the striatum of mice was performed using a stereotactic apparatus under isoflurane anesthesia. Sodium nitroprusside (5 or 20 nmol/μL; dissolved in saline) or NOC-18 (20 nmol/μL; dissolved in phosphate-buffered saline) was injected into the right striatum using a 25-μL syringe with a 33-gauge needle controlled by an automated syringe pump at a rate of 0.25 μL/min for 4 minutes. The coordinates for the stereotactic injection were 0.74 mm anterior, 1.6 mm lateral, and 3.5 mm ventral from the bregma, according to the atlas of Paxinos and Franklin.22 After the injection, the needle was kept in the same position for an additional 3 minutes to allow diffusion of drugs and then retrieved slowly from the brain. At the same time, 1 μL of saline for SNP or phosphate-buffered saline for NOC-18 was injected into the left striatum by the same method.
Autoradiography
At 60 and 30 minutes after the intrastriatal injection of SNP and NOC-18, respectively, an aqueous solution (5% DMSO, v/v) of [3H]Hydromethidine (740 kBq) was injected intravenously into the tail vein of mice. The mice were killed by decapitation at postinjection time points of 1, 5, 20, or 60 minutes under deep anesthesia with isoflurane. The brains were rapidly removed and frozen, and sections (2 μm thick) were prepared using a cryostat. The sections were exposed to an imaging plate (BAS-TR; Fujifilm Corp., Tokyo, Japan) for 14 days. After exposure, the plates were read with a FLA-3000 (Fujifilm Corp.). Regions of interest were drawn on the images, and the photo-stimulated luminescence value for each region of interest (PSL/mm2) was determined using Multi Gauge version 2.3 (Fujifilm Corp.).
Results
In Vitro Oxidation Characteristic of [3H]Hydromethidine
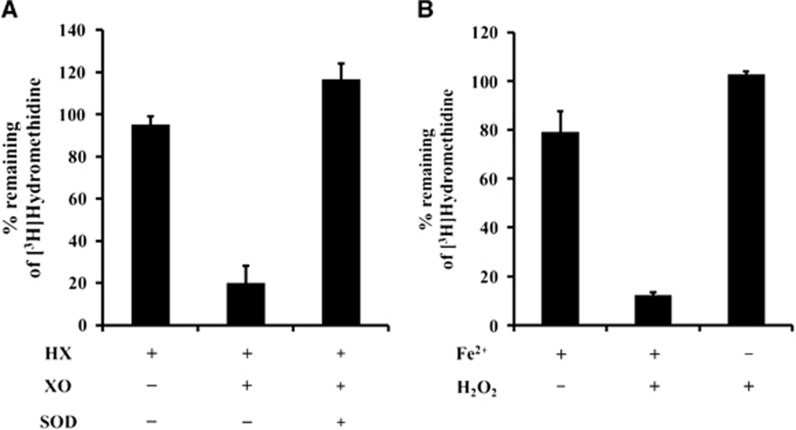
[3H]Hydromethidine was oxidized by O2•− produced with hypoxanthine and xanthine oxidase. Superoxide dismutase, which rapidly removes O2•−, inhibited the oxidation of [3H]Hydromethidine (Figure 2A). [3H]Hydromethidine showed oxidation in the presence of OH•− as shown in Figure 2B. However, its oxidation was not observed in the presence of H2O2.
Figure 2.
In vitro reactivity of [3H]Hydromethidine with reactive oxygen species (ROS) produced by hypoxanthine (HX)/xanthine oxidase (XO) system (A) or Fenton reaction (B). Data are expressed as % remaining of [3H]Hydromethidine after incubation (mean±s.d., n=3). Superoxide dismutase (SOD) was used to remove O2•−.
Whole-Body Distribution in Mice Intravenously Injected with [3H]Hydromethidine
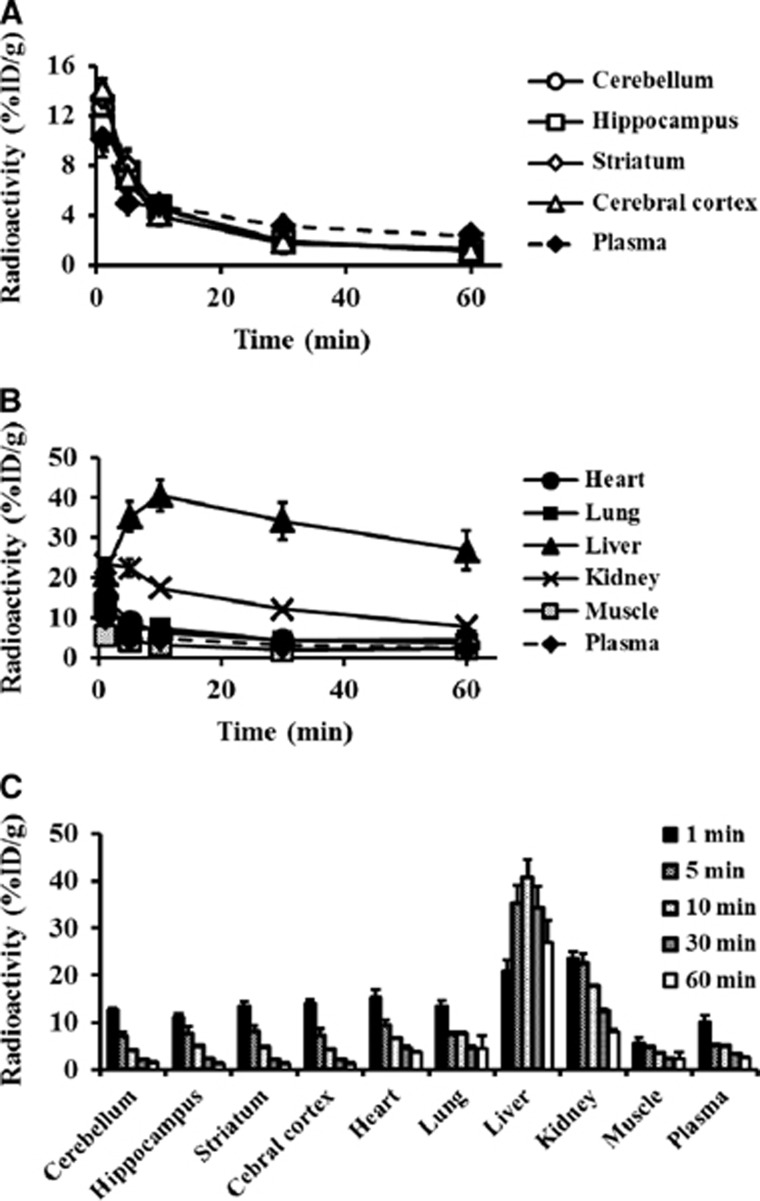
We examined the tissue distribution after intravenous injection of [3H]Hydromethidine into normal mice as shown in Figure 3. [3H]Hydromethidine rapidly penetrated the cell membranes including the blood–brain barrier, with high uptake by the brain, lungs, and heart immediately after the injection of the [3H]Hydromethidine. In the brain, almost homogeneous distribution as well as rapid clearance of radioactivity was observed in all regions. The radioactivity in the lungs, heart, and muscles also disappeared, while relatively high levels of radioactivity remained in the liver during the 60-minute observation period. The percentage of unmetabolized [3H]Hydromethidine in the radioactivity of blood was 95.1±27.7%, 73.1±13.4%, 59.9±6.8%, 55.8±4.6%, and 56.7±9.9% at 1, 5, 10, 30, and 60 minutes, respectively, postinjection of [3H]Hydromethidine.
Figure 3.
Distribution profiles of radioactivity in the brain (A) and other tissues (B and C) after intravenous injection of [3H]Hydromethidine to mice. Data are expressed as %ID/g (mean±s.d., n=3).
Brain Distribution after Intravenous Injection of [3H]Hydromethidine in Mice Given Intrastriatal Microinjection of Sodium Nitroprusside
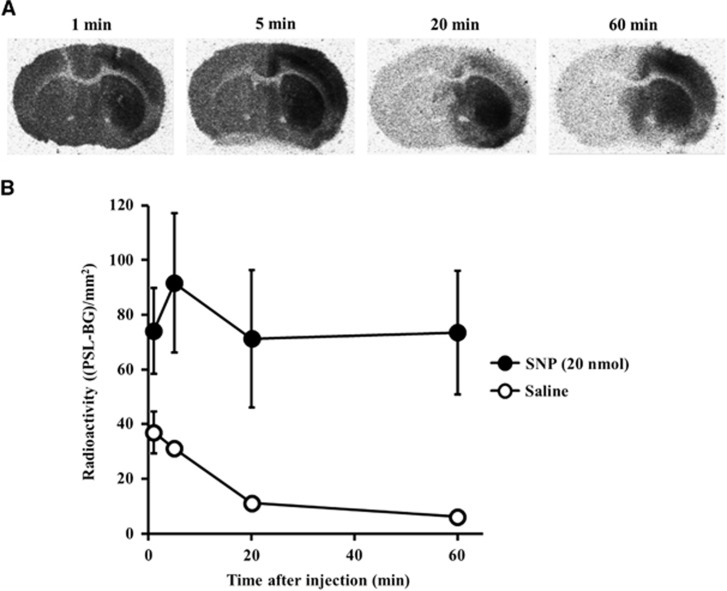
Typical autoradiograms obtained 1, 5, 20, and 60 minutes after [3H]Hydromethidine injection to mice treated with SNP (20 nmol) are shown in Figure 4. In the SNP-treated hemisphere, high accumulation of radioactivity was sustained during 60 minutes of period. In contrast, rapid decline in radioactivity accumulation in the contralateral hemisphere was observed. In the striatum, the ratios of radioactivity concentrations of the SNP-injected side to the contralateral side were 2.0 at 1 minute, 6.6 at 20 minutes, and 12.2 at 60 minutes after [3H]Hydromethidine injection.
Figure 4.
Accumulation of radioactivity in the brain after intravenous injection of [3H]Hydromethidine to mice treated with sodium nitroprusside (SNP) (20 nmol). SNP was microinjected to the right striatum. (A) Typical autoradiograms in brain obtained at 1, 5, 20, and 60 minutes after [3H]Hydromethidine injection. (B) Radioactivity profiles in the SNP-injected striatum (●) and saline-injected striatum (○), calculated from the autoradiograms. Data are expressed as (PSL-BG)/mm2 (mean±s.d., n=4).
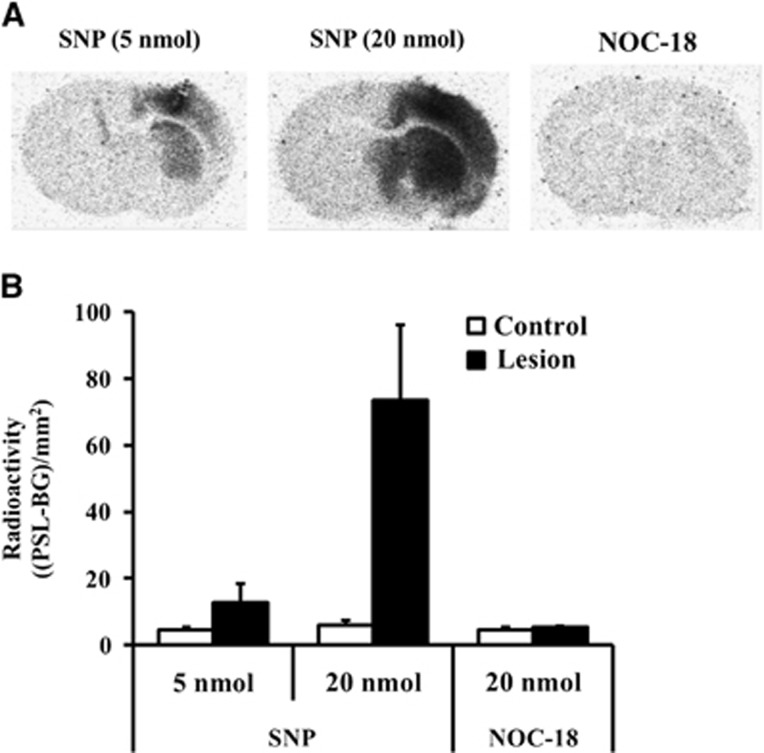
The degree of radioactivity accumulation at 60 minutes after injection of [3H]Hydromethidine was much dependent on the dose (5 or 20 nmol) of SNP. No significant difference of radioactivity concentrations was observed between the right and left hemispheres of mouse brain injected with NOC-18 (Figure 5).
Figure 5.
Accumulation of radioactivity in brain after intravenous injection of [3H]Hydromethidine to mice treated with sodium nitroprusside (SNP) (5 or 20 nmol) or NOC-18 (20 nmol). SNP or NOC-18 was microinjected to the right striatum. (A) Typical autoradiograms in brain obtained at 60 minutes after [3H]Hydromethidine injection. (B) Results of quantitative analysis of the autoradiograms. Radioactivity in the SNP or NOC-18-injected striatum (■) and control striatum (□) was calculated and expressed as (PSL-BG)/mm2 (mean±s.d., n=4).
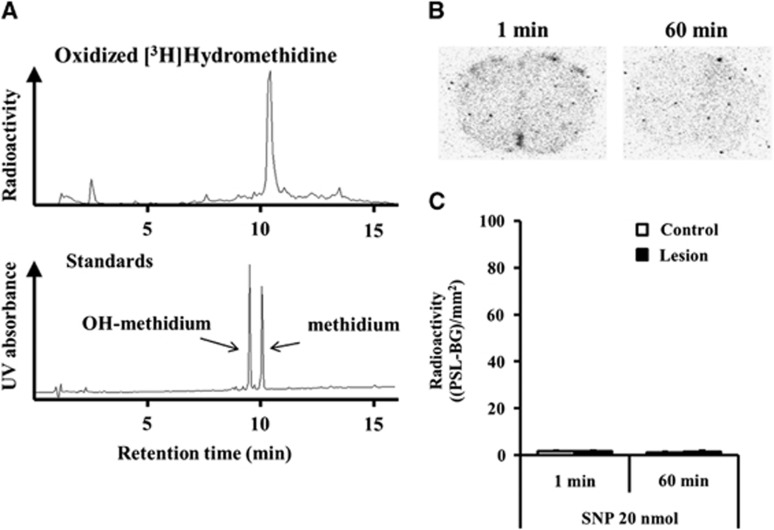
Figure 6 shows the brain distribution in SNP-microinjected mice when the oxidized product of [3H]Hydromethidine was intravenously injected. The oxidized product of [3H]Hydromethidine was acquired by generated O2•− using hypoxanthine/xanthine oxidase system in vitro and analyzed by radio-HPLC according to the method reported previously11 with slight modifications. The oxidized product of [3H]Hydromethidine was estimated to consist mainly of methidium cation in comparison with the retention of synthesized methidium cation or hydroxymethidium cation detected by UV.
Figure 6.
Accumulation of radioactivity in brain after intravenous injection of oxidized product of [3H]Hydromethidine to mice treated with sodium nitroprusside (SNP) (20 nmol). SNP was microinjected to the right striatum. (A) Radio-HPLC analysis of oxidized [3H]Hydromethidine solution for administration. Oxidized Hydromethidine standards were also analyzed by HPLC-UV. (B) Typical autoradiograms in brain obtained at 1 and 60 minutes after oxidized product of [3H]Hydromethidine injection. (C) Results of quantitative analysis of the autoradiograms. Radioactivity in the SNP-injected striatum (■) and control striatum (□) was calculated and expressed as (PSL-BG)/mm2 (mean±s.d., n=3). HPLC, high performance liquid chromatography.
When the oxidized product was intravenously injected into mice, no significant uptake of radioactivity was observed in the brain, which indicates that the oxidation metabolite of [3H]Hydromethidine did not penetrate the blood–brain barrier due to its cationic charge.
Discussion
We found that [3H]Hydromethidine had the desired characteristic of a radical trapping probe based on the results of whole body kinetics as well as autoradiography of brain injected with SNP after injection of [3H]Hydromethidine tracer. [3H]Hydromethidine was rapidly distributed to organs such as brain, heart, and lung after tracer injection. This was followed by its rapid clearance from the organs for normal mouse tissue. In the brain, a significantly high accumulation of radioactivity in the SNP-injected side (striatum and cerebral cortex) was seen even at 1 minute after injection of the tracer and the radioactivity concentrations were kept almost the same level during 60 minutes of period. In contrast, the radioactivity in the contralateral normal side (saline-injected side) of the brain was rapidly disappeared. In addition, the extent of radioactivity accumulation in the SNP-injected side was much dependent on the injected dose of SNP. It has been reported that SNP caused cell death dose-dependently when microinjected into the striatum.23 In addition, cell death induced by intrastriatal microinjection of SNP was caused by iron-related ROS such as OH•− via the Fenton reaction but not by NO or CN. Rauhala et al.21 also showed that SNP generated OH•− by the Fenton reaction in vitro in the presence of ascorbate. We have also reported that intrastriatal microinjection of SNP (50 nmol) caused neuronal cell death; however, neither NOC-18 (50 nmol), another type of NO donor, nor sodium cyanide (50 nmol) caused cell death.24 The accumulation of radioactivity observed in the SNP-injected side suggests that [3H]Hydromethidine was oxidized by ROS such as OH•− and then the oxidized form was trapped in the tissue. It is possible that [3H]Hydromethidine was also oxidized by other ROS since SNP generates not only OH•− but also O2•−, NO, or ONOO•−.25 In addition, lipid peroxidation of cell membrane induced by ROS is considered to oxidize [3H]Hydromethidine in intact tissues. As for the involvement of NO radical, we found that no accumulation of radioactivity was seen in the NOC-18-injected side. In this experiment, 20 nmol of SNP is considered to release 20 nmol of NO under the optimum conditions, whereas 40 nmol of NO might be released by 20 nmol of NOC-18. The NO-generating ability of NOC-18 has been reported to be greater than that of SNP.26 However, NOC-18 is a relatively slow NO donor that has a prolonged half-life of 20 hours. Therefore, the optimal dose and time for the treatment of NO donor is important for controlling NO level under the in vivo conditions. Further studies on the reactivity of [3H]Hydromethidine with reactive nitrogen species such as NO and ONOO•− both in vitro and in vivo will be needed. This result of NOC-18 suggests that oxidation of [3H]Hydromethidine may not be mediated by NO under at least the present experimental conditions. Thus, the accumulation of radioactivity induced by SNP is considered to be mainly due to oxidative conversion of [3H]Hydromethidine by ROS such as OH•−.
We also investigated whether the oxidative products of [3H]Hydromethidine, which had been oxidized in the presence of xanthine oxidase and hypoxanthine, were able to penetrate into the brain. We found that the oxidative product produced by generation of O2•− was mostly the [3H]Hydromethidium cation. The in vitro ROS reactivity study suggests that the reaction between [3H]Hydromethidine and OH•− or O2•− produces its oxidative forms. The results with SNP-treated mice clearly showed that [3H]Hydromethidine was easily transported to a tissue or a cell and converted to a membrane-impermeable oxidization product, which became trapped there. A significant accumulation of radioactivity in the SNP-injected brain strongly suggests that the oxidized form of [3H]Hydromethidine produced by OH•− or O2•− was trapped in the brain. Hydroethidine has been reported to be rapidly distributed into the tissues including the brain16,27 and the oxidation products are retained in cells in the brain. These findings suggest that hydroethidine-related compounds can serve as radical trapping radiotracer. In addition, the autoradiography study indicated that entered [3H]Hydromethidine in the brain was rapidly oxidized to cationic form and trapped in the brain. As the input function (unoxidized [3H]Hydromethidine in the brain) rapidly declined with time, the conversion of [3H]Hydromethidine in the brain seemed to be almost completed within few minutes after the bolus injection. The amount of trapped oxidized form was mainly dependent on three factors, the delivery process from plasma (regional blood flow), the oxidation rate in the brain, and the washout rate of unoxidized [3H]Hydromethidine from the brain. As previously reported that no significant effects on cerebral blood flow were observed in rat brain injected with SNP (50 nmol/μL),28 the delivery process from the plasma seemed to be unaffected by SNP injection. The washout rate of unoxidized [3H]Hydromethidine from the brain was rapid. Therefore, the amount of trapped oxidized form in the brain seemed to be dependent on the oxidation rate in the brain.
Brain ROS have been implicated in a variety of pathophysiologic conditions such as Alzheimer's disease, Parkinsonism, multiple sclerosis, ischemia-reperfusion injury, and brain trauma.29,30 As mentioned above, [3H]Hydromethidine is considered to be a useful probe for assessing the role of ROS in the pathologic brain state. We have been able to confirm the accumulation of [3H]Hydromethidine suggesting production of ROS in the rodent ischemia-reperfusion model (in preparation). Although we focused on the brain ROS in this study, the use of [3H]Hydromethidine is not restricted to the central nervous system. The rapid clearance of [3H]Hydromethidine in several tissues of control mice suggests that it might be possible to detect the ROS in peripheral tissues such as the lungs, heart, and kidney. Further studies will be needed to detect ROS in peripheral tissues considering the relatively high distribution in the liver. A high level of ROS production has also been reported in cancer tissue. Jung et al.31 have reported a positive relationship between the uptake of 18F-FDG and the level of ROS in tumor cells. In tumor cells, upregulated anaerobic metabolism, known as the Warburg effect,32 was observed. There may be a close relation between this change in metabolism and ROS production. These reports suggest the usefulness of [3H]Hydromethidine for assessing the role of ROS in tumor tissues and cells. [3H]Hydromethidine was also thought to be useful for assessing ROS in tumor animal models because of its low uptake by mouse skeletal muscle.
Superoxide radical (O2•−), primary ROS species, is subsequently converted into H2O2 through spontaneous or SOD-catalyzed dismutation. The reaction of O2•− and NO generates the powerful oxidant ONOO•−. Reaction of H2O2 and ONOO•− can give rise to highly reactive OH•−.6,7 Therefore, it is also important to know the selectivity and intensity of the radical trapping probe for the type of ROS. We found that [3H]Hydromethidine reacted with O2•− and OH•− but not with H2O2 from the results of in vitro study. Hydroethidine is widely used as an ROS probe specifically for O2•−. As described above, dynamic optical imaging showed that brain O2•− was increased in SOD-deficient mice or ketamine-treated mice. In addition, the product of hydroethidine oxidation produced by O2•− in vivo was ethidium, not 2-hydroxyethidium from analysis of the fluorescence life time when hydroethidine was intravenously injected into mice at a dose of 50 mg/kg. Recent in vitro studies have suggested that the specific oxidation product of hydroethidine by O2•− is not ethidium, but 2-OH ethidium.11 In the present study, the oxidative product of [3H]Hydromethidine in the presence of hypoxanthine and xanthine oxidase was found to be mostly the [3H]Hydromethidium cation. Several ROS such as OH•− or O2•− might be produced by SNP treatment because of complex conditions of in vivo systems. The sensitivity of [3H]Hydromethidine also seemed to be high since the injected dose was 0.15 mg/kg if calculated based on the specific activity. Further studies will be needed on the effects of the differences of the specific activity for detecting ROS.
Generally, radiolabeled probes are highly sensitive and quantitative. A metabolic trapping tracer using oxidative conversion would allow the amount of ROS produced in a given tissue or cell to be quantified by measurement of the oxidized products produced over a defined period. As shown in Figure 3, the radioactivity level at 60 minutes after tracer injection is considered to be an indicator of ROS level since unmetabolite of [3H]Hydromethidine was mostly eliminated from the brain. In addition, Patlak plot analysis of the brain radioactivity concentrations enables calculation of the relative ROS amount when the kinetics of the unmetabolite of [3H]Hydromethidine in plasma is used as the input function.
Noninvasive measurement using [3H]-labeled compounds is not possible because of the low energy of beta rays. Time-dependent change in ROS could be detected in the same animals using positron-labeled hydroethidine-related compounds because Hydromethidine can be labeled using [11C]methylation instead of [3H] labeling. Therefore, small animal positron emission tomography (PET) studies using [11C]Hydromethidine should be very useful for studying the pathophysiologic roles of ROS in diseases.
The present study showed that radiolabeled hydromethidine could enable assessment of ROS levels for treatments of inflammation and ischemia in animals. To the best of our knowledge, this is the first report showing the usefulness of radical trapping radiotracers for detecting brain ROS in vivo.
The authors declare no conflict of interest.
References
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Hall ED, Andrus PK, Althaus JS, Vonvoigtlander PF. Hydroxyl radical production and lipid peroxidation parallels selective post-ischemic vulnerability in gerbil brain. J Neurosci Res. 1993;34:107–112. doi: 10.1002/jnr.490340111. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mzur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Hogg N, Darley-Usmar M, Wilson MT, Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992;281:419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, et al. Mitochondrial oxidative stress in heart failure. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- Asano M, Doi M, Baba K, Taniguchi M, Shibano M, Tanaka S, et al. Bio-imaging of hydroxyl radicals in plant cells using the fluorescent molecular probe rohdamine B hydrazide, without any pretreatment. J Biosci Bioeng. 2014;118:98–100. doi: 10.1016/j.jbiosc.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Michalski R, Zielonka J, Hardy M, Joseph J, Kalyanaraman B. Hydropropidine: A novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic Biol Med. 2013;54:135–147. doi: 10.1016/j.freeradbiomed.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski R, Michalowski B, Sikora A, Zielonka J, Kalyanaraman B. On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and ex vivo tissues: a reassessment. Free Radic Biol Med. 2014;67:278–284. doi: 10.1016/j.freeradbiomed.2013.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Barbacanne MA, Souchard JP, Darblade B, Iliou JP, Nepveu F, Pipy B, et al. Detection of superoxide anion released extracellularly by endothelial cells using cytochrome c reduction, ESR, fluorescence and lucigenin-enhanced chemiluminescence techniques. Free Radic Biol Med. 2000;29:388–396. doi: 10.1016/s0891-5849(00)00336-1. [DOI] [PubMed] [Google Scholar]
- Fernandes DC, Wosniak J, Jr, Pescatore LA, Bertoline MA, Liberman M, Laurindo FR, et al. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol. 2007;292:C413–C422. doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganeses superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestana RRF, Kijo ER, Hernandes MS. Britto LRG. Reactive oxygen species generated by NADPH oxidase are involved in neurodegeneration in the pilocarpine model of the temporal lobe epilepsy. Neurosci Lett. 2010;484:187–191. doi: 10.1016/j.neulet.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem. 2003;278:9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of ketogenic diet in murine model of multiple sclerosis. PLoS ONE. 2012;7:e35476. doi: 10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DJ, Han SH, Chepetan A, Inui EG, Rogers M, Dugan LL. Dynamic optical imaging of metabolic and NADPH oxidase-derived superoxide in live mouse brain using fluorescence lifetime unmixing. J Cereb Blood Flow Metab. 2012;32:23–32. doi: 10.1038/jcbfm.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhala P, Khaldi A, Mohanakumar KP, Chiueh CC. Apparent role of hydroxyl radicals in oxidative brain injury induced by sodium nitroprusside. Free Radic Biol Med. 1998;24:1065–1073. doi: 10.1016/s0891-5849(97)00386-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ.The mouse brain in stereotaxic coordinates; 2nd edn. Academic Press: San Diego [Google Scholar]
- Nazari OA, Mizumo K, Kume T, Takada-Takatori Y, Izumi Y, Akaike Y. In vivo brain oxidative stress model induced by microinjection of sodium nitroprusside in mice. J Pharmacol Sci. 2012;120:105–111. doi: 10.1254/jphs.12143fp. [DOI] [PubMed] [Google Scholar]
- Yanamoto K, Hosoi R, Uesaka Y, Abe K, Tsukada H, Inoue O. Intrastriatal microinjection of sodium nitroprusside induces cell death and reduces binding of dopaminergic receptors. Synapse. 2003;50:137–143. doi: 10.1002/syn.10256. [DOI] [PubMed] [Google Scholar]
- Aleryani S, Milo E, Kostka P. Formation of peroxynitrite during thiol-mediated reduction of sodium nitroprusside. Biochim Biophys Acta. 1999;1472:181–190. doi: 10.1016/s0304-4165(99)00119-1. [DOI] [PubMed] [Google Scholar]
- Tanno M, Sueyoshi S, Miyata N, Nagkagawa S. Nitric oxide generation from aromatic N-nitrosoureas at ambient temperature. Chem Pharm Bull. 1996;44:1849–1852. [Google Scholar]
- Quick KL, Dugan LL. Superoxide stress identifies neurons at-risk in a model of ataxia-telangiectasia. Ann Neurol. 2001;49:627–635. [PubMed] [Google Scholar]
- Inoue O, Taguchi H, Watanabe T, Hosoi R, Kobayashi K, Nishimura T, et al. Uncoupling of flow and metabolism induced by sodium nitroprusside in rat cerebral cortex. Neuroreport. 2004;15:141–145. doi: 10.1097/00001756-200401190-00027. [DOI] [PubMed] [Google Scholar]
- Choi BY, Jang BG, Kim JH, Lee BE, Sohn M, Song HK, et al. Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 2012;1481:49–58. doi: 10.1016/j.brainres.2012.08.032. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53:S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jung KH, Lee JH, Quach CHT, Paik JY, Oh H, Park JW, et al. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1α activation. J Nucl Med. 2013;54:2161–2167. doi: 10.2967/jnumed.112.115436. [DOI] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]