Abstract
D-Serine is known to be essential for the activation of the N-methyl-D-aspartate (NMDA) receptor in the excitation of glutamatergic neurons, which have critical roles in long-term potentiation and memory formation. D-Serine is also thought to be involved in NMDA receptor-mediated neurotoxicity. The deletion of serine racemase (SRR), which synthesizes D-serine from L-serine, was recently reported to improve ischemic damage in mouse middle cerebral artery occlusion model. However, the cell type in which this phenomenon originates and the regulatory mechanism for D-/L-serine remain elusive. The D-/L-serine content in ischemic brain increased until 20 hours after recanalization and then leveled off gradually. The results of in vitro experiments using cultured cells suggested that D-serine is derived from neurons, while L-serine seems to be released from astroglia. Immunohistochemistry studies of brain tissue after cerebral ischemia showed that SRR is expressed in neurons, and 3-phosphoglycerate dehydrogenase (3-PGDH), which synthesizes L-serine from 3-phosphoglycerate, is located in astrocytes, supporting the results of the in vitro experiments. A western blot analysis showed that neither SRR nor 3-PGDH was upregulated after cerebral ischemia. Therefore, the increase in D-/L-serine was not related to an increase in SRR or 3-PGDH, but to an increase in the substrates of SRR and 3-PGDH.
Keywords: excitotoxicity, focal ischemia, glial cells, glutamate, neuronal–glial interaction
Introduction
Neurotoxicity mediated by the activation of N-methyl-D-aspartate (NMDA) receptors, a glutamate receptor subtype, has an important role in the pathogenesis of ischemic brain injury.1 The overstimulation of NMDA receptors by the massive release of glutamate during cerebral ischemia leads to an increase in Ca2+ influx into cells, the activation of catabolic enzymes, and the generation of reactive oxygen species, which have detrimental effects on the brain after transient ischemia.2,3 Although antagonists of the glutamate binding site of the NMDA receptor have been shown to have protective effects in a rodent model, they failed to induce a response in clinical trials.4,5 Therefore, the development of other stroke treatments to block excitotoxicity other than the use of glutamate antagonists is anticipated.
In addition to glutamate, the NMDA receptor requires a coagonist at a second site. Although glycine was initially thought to be a ligand for this site, subsequent evidence has revealed that D-serine binds to this site with a greater potency than glycine.6,7 D-Serine is needed to activate the NMDA receptor and is essential for the excitation of glutamatergic neurons, which have a critical role in long-term potentiation and are required for memory formation.8 However, D-serine is also involved in NMDA receptor-mediated neurotoxicity and has a pathophysiologic role in stroke and neurodegenerative diseases.9,10
In terms of cerebral ischemia, the deletion of serine racemase (SRR), which synthesizes D-serine from L-serine, has recently been reported to improve ischemic damage in a murine middle cerebral artery occlusion (MCAO) model.11 Therefore, the inhibition of D-serine production might have therapeutic potential for the treatment of ischemic stroke. However, the mechanism by which D-/L-serine is regulated during and after cerebral ischemia is not understood. The present study was conducted to determine the temporal profile of D-/L-serine and to clarify the cell types from which D-/L-serine is derived during cerebral ischemia. First, we investigated the D-/L-serine contents in the brain after ischemia using an in vivo ischemic model; we also examined the release of D-/L-serine from cultured cells after hypoxia. Second, we clarified the expressions of SRR and 3-phosphoglycerate dehydrogenase (3-PGDH), which synthesizes L-serine from 3-phosphoglycerate, in the brain after MCAO using immunohistochemistry and western blotting.
Materials and methods
Animals
All animal procedures were performed in accordance with The Animal Experimentation Guidelines of Keio University School of Medicine and were approved by the Laboratory Animal Care and Use Committee of Keio University. Experiments were performed in 6- to 8-week-old male SRR+/+ or SRR−/− mice (weight: 20 to 24 g). An SRR knockout (SRR−/−) mouse strain was produced as described previously.12 The mice were obtained from house colonies and were backcrossed onto the C57BL/6 background to generate homozygous animals free of background effects on phenotypes. Wild-type (WT) siblings were used as WT controls. Timed-pregnant Sprague-Dawley rats were purchased from Japan SLC, Inc. (Hamamatsu, Japan).
Chemicals
All chemicals and materials were obtained from the following sources: Dulbecco's modified Eagle's medium (DMEM) with or without glucose, penicillin, and streptomycin was obtained from Life Technologies (Grand Island, NY, USA); defined fetal bovine serum was obtained from HyClone Laboratories (Logan, UT, USA); Tissue-Tek O.C.T. Compound was obtained from Sakura Finetek Japan (Tokyo, Japan); an anti-SRR antibody was obtained from BD Biosciences (Franklin Lakes, NJ, USA); an antibody for Nissl was obtained from Millipore (Bedford, MA, USA); an antibody for glial fibrillary acidic protein (GFAP) was obtained from Dako (Glostrup, Denmark); secondary antibodies labeled with fluorescein or Texas-Red were obtained from Jackson Immunoresearch Laboratories (West Grove, PA, USA); and the derivatizing reagent 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F) was obtained from Tokyo Chemical Industry (Tokyo, Japan). Water was purified using a Milli-Q gradient A 10 system (Millipore); Alamar Blue was obtained from Invitrogen Life Technologies (Carlsbad, CA, USA); the LDH assay kit was obtained from Wako Chemicals (Osaka, Japan); all other chemicals were obtained from Sigma (St Louis, MO, USA).
Preparation of Cultured Neurons and Astroglia
A primary culture of neurons was prepared from the cortex and striatum of fetal rats on embryonic day 16 as described previously.13 Mechanically dissociated cells were counted, and viable cells (1.5 × 106 cells/mL) without trypan blue were cultured in 12-well culture plates (0.8 mL/well, respectively) (Nalge Nunc., Rochester, NY, USA) coated with poly-L-lysine (5 μg/mL). The cells were cultured in a glucose-containing medium (final concentration, 12 mmol/L of D-glucose) comprises DMEM with 10% (v/v) fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in humidified air with 7% CO2. Cytosine arabinoside (10 μmol/L) was added 72 hours later to induce mitotic arrest of the astroglia. Assays were performed using 7- to 8-day-old cultures. The nutrient medium remained untouched until the experiments were performed. The astroglial cultures were prepared from the cerebral cortices of pups at 24 to 48 hours after birth.13 The brains were excised, the meninges and blood vessels were removed, and the front-parietal cortices were dissected out and mechanically disrupted. The dissociated cells (2.5 × 105 cells/mL) were plated (15 mL per flask) in uncoated 75 cm2-culture flasks (Sumitomo Bakelite, Tokyo, Japan) and cultured at 37°C in humidified air with 7% CO2. The culture medium was changed twice a week until the cultures reached confluence. The flask was then shaken overnight at 37°C to eliminate loosely adherent round cells, which were thought to be O2A progenitors and microglia. The adherent cells were treated with trypsin-EDTA solution for 1 to 2 minutes at 37°C, suspended in four volumes (60 mL) of fresh culture medium, and placed in uncoated 12-well culture plates (0.8 mL/well, respectively). The culture medium was changed twice a week, and the secondary astroglial cells were used once they reached confluence (day 21).
Experimental Protocol for D/L-Serine Release
To assess the effect of hypoxia on D/L-serine release, cells were placed in a hypoxic chamber (Multi-gas incubator APM-30DR; ASTEC, Fukuoka-ken, Japan) at 37°C in humidified air containing 1% O2 and 7% CO2 for 24 hours. After 24 hours of exposure, 0.3 mL of the culture medium supernatant was sampled and used for two-dimensional high performance liquid chromatography (2D-HPLC). The net production and release of D/L-serine in the medium were calculated by subtracting the D/L-serine content of the medium from that after 24 hours of exposure to hypoxia and were normalized according to the cellular protein content of each well as measured using the bicinchoninic acid method.14
Middle Cerebral Artery Occlusion
The procedures used for transient MCAO were identical to those previously described and are only summarized here.15, 16, 17 Mice were anesthetized with isoflurane (1.5% to 2%). A fiber optic probe was glued to the parietal bone and then connected to a laser Doppler flow meter (ALF 21R; Advance Co., Ltd., Tokyo, Japan) to monitor the cerebral blood flow (CBF). A heat-blunted 6.0 monofilament surgical suture was inserted into the external carotid artery, advanced into the internal carotid artery, and wedged into the circle of Willis to obstruct the origin of the middle cerebral artery. The filament was left in place for 40 minutes and then withdrawn. Only animals that exhibited an 85% reduction in CBF during MCAO and in which the CBF had recovered by 80% after 10 minutes of reperfusion were included in the present study.16,18,19 This protocol for MCAO led to reliable infarcts comparable in size and distribution (cortex and striatum) to those reported by others.20,21 In this study, one mouse for the stroke volume experiment was excluded by the blood flow criteria. In all the mice, the rectal temperature was maintained at 37.0±0.5 °C during surgery and during the recovery period until the animals regained consciousness.
Measurement of Infarct Volume
One day after ischemia, the mice were anesthetized and were perfused transcardially with 2% triphenyltetrazolium chloride. The brains were removed, frozen, and sectioned (thickness, 30 μm) using a cryostat. The brain sections were collected at 600-μm intervals, and the infarct volume was presented as a percentage relative to the entire right hemisphere volume.
Double-Label Immunohistochemistry
To identify cell types expressing SRR and 3-PGDH, mice were anesthetized with sodium pentobarbital (120 mg/kg) and perfused transcardially with 4% paraformaldehyde. The brains were then removed, cryoprotected in 20% sucrose phosphate buffer saline (PBS), frozen in Tissue-Tek O.C.T. Compound (Sakura Finetek Japan), sectioned through the parietal cortex, and stored at −80°C until use. Sections were rinsed in PBS (pH 7.4), permeabilized with 0.3% Triton X-100 for 10 minutes, and incubated at room temperature for 3 hours with a blocking solution containing appropriate normal serum (1:60; Vector Laboratories, Burlingame, CA, USA). Subsequently, the sections were incubated at 4°C for 12 to 72 hours in appropriate primary antibodies (anti-SRR antibody, 1:100, BD Biosciences; anti-3-PGDH, 1:300, a kind gift from Dr Shigeki Furuya; GFAP, 1:1,000, Dako) diluted in PBS containing blocking serum. Then, the sections were incubated at room temperature for 40 minutes in a mixture of the neuronal marker Nissl (1:100; Millipore) and/or species-specific secondary antibodies labeled with fluorescein or Texas-Red (Jackson Immunoresearch Laboratories). The sections were washed again and were mounted using ProLong Gold Antifade Reagent with DAPI (Invitrogen, Carlsbad, CA, USA). Images of double-labeled neocortex were sequentially acquired using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Two-Dimensional High Performance Liquid Chromatography
The amino-acid analysis was conducted using a previously described protocol12,22,23 with a small modification. Brain tissue was freeze-dried for 24 hours using a freeze dryer and was homogenized in a 19.5-fold volume (w/v) of methanol. For the cell cultures, cultured media were mixed with a 10-fold volume (v/v) of methanol. These homogenates were then centrifuged at 20,400 g for 10 minutes, and the supernatants were spin-dried using Savant SpeedVac (Thermo Scientific, Waltham, MA, USA). Then, the dried residues were resuspended in 200 mmol/L sodium borate. After the addition of 40 mmol/L NBD-F (Tokyo Chemical Industry), the reaction mixture was incubated at 60°C for 2 minutes. The reaction of fluorescent derivatization was stopped by adding 2% trifluoroacetate. The NBD-F-derivatives were subjected to 2D-HPLC (NANOSPACE SI-2 series; Shiseido), separated into each amino acid using a reversed-phase column, a monolithic-ODS column (750 mm × 0.53 mm ID, 454 prepared in a fused silica capillary, provided by Shiseido), and further separated into enantiomers using an enantioselective column (Sumichiral OA-2500S; Sumika Chemical Analysis Service, Kyoto, Japan).12,22 The fluorescence intensity was detected at 530 nm with excitation at 470 nm.
Evaluation of Cell Damage Using Alamar Blue Reduction Activity and Lactate Dehydrogenase Release
Cellular damage was evaluated quantitatively using the amount of lactate dehydrogenase (LDH) released into the culture media and was confirmed using a colorimetric assay based on Alamar Blue (Invitrogen Life Technologies) reduction.
The LDH release assay, a standard cell death/viability assay reflecting cellular membrane integrity, was applied. Three hundred microliters out of 0.5 mL of culture medium was collected from a 12-well culture plate and was used for the LDH activity assay after 24 hours of hypoxia (1% O2). The remaining cells were used for the Alamar Blue assay, as described below. The LDH activities in the sampled medium were measured using an LDH assay kit (Wako Chemicals) based on the conversion of lactate and NAD+ into pyruvate and NADH catalyzed by LDH. The produced NADH was measured (340 nm), and the LDH concentrations in the samples were calculated using the LDH standard solution. Six wells of appropriate sister culture cells were treated with 0.5 mL of 1% Triton-X 100 in PBS and the average LDH activity was considered as the maximal LDH release. The released LDH activity from each well exposed to hypoxia (n=6) was divided by the maximal LDH release and was considered as the percent of cell death in each well after 24 hours of exposure to hypoxia (1% O2).
Alamar Blue is a redox indicator that exhibits both fluorescence and colorimetric changes in response to metabolic activity and cellular viability.24,25 After the removal of nutrient medium from the 12-well culture plate, the cells were washed once with PBS, and 0.5 mL of DBSS containing 2 mmol/L glucose and Alamar Blue (10% final volume) was added. The cells were incubated for 60 minutes in a CO2 incubator (21% O2) for color development. The fluorescence level was measured at 0 and 60 minutes using a fluorescence microplate reader (Infinite F200 PRO; TECAN Japan, Kanagawa, Japan) with excitation at 535 nm and emission at 590 nm. As the fluorescent signals increased linearly for up to 60 minutes (data not shown), Alamar Blue reduction (cell viability) was expressed as the percent increase in the fluorescent signal at 60 minutes compared with that at 0 minute. Cell viability after 24 hours of exposure to hypoxia (1% O2) was evaluated using the percent of Alamar Blue reduction activity in the control cells (21% O2).
Western Blotting
The MCAO mice were anesthetized with diethylether and euthanized by the perfusion of PBS. Each brain was then dissected into the right/left parietal or temporal region. The central area of infarction was the right temporal region. Each tissue was homogenized in lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 15 mmol/L NaCl, 20 mmol/L EDTA, 1% Triton X-100, protease inhibitor cocktail) and centrifuged at 20,400 g for 10 minutes. The protein concentration of the supernatant was then analyzed using a BCA assay (Thermo Scientific), and 20 μg of protein was mixed with the sample buffer (320 mmol/L Tris-HCl (pH 6.8), 3.6% SDS, 18% glycerol, and 15% β-mercaptoethanol). The denatured samples were subjected to SDS-PAGE and transferred onto a PVDF membrane. Then, the membrane was immunoblotted with a mouse monoclonal antibody to SRR (BD Transduction Laboratories, Franklin Lake, NJ, USA), a rabbit monoclonal antibody to GAPDH (Cell Signaling Technology, Danvers, MA, USA), and a rabbit polyclonal antibody to 3-PGDH (a kind gift from Dr Shigeki Furuya).
In VivoD-Serine Production Assay
The ischemic core region of the brain was homogenized in 2 mL of in vivo assay buffer (20 mmol/L Tris-HCl, 4 mmol/L MgCl2, 0.5 mmol/L sodium benzoate, 0.1% Triton X-100) and the debris was removed by centrifugation at 20,400 g for 10 minutes. Then, the small molecules were removed from the homogenate using the Amicon Ultra-4 centrifugal filter device 3000 NMWL (Merck KGaA, Darmstadt, Germany), and the homogenate was concentrated. The protein concentration in the homogenate was measured using a BCA assay, and 600 μg of protein in 75 μL of the assay buffer was mixed with 5 μL of 10 mmol/L ATPγS (Wako, Tokyo, Japan). Then, 20 μL of 50 mmol/L L-serine was added and the mixture was incubated at 37°C for 2 hours. Finally, the reaction was stopped by the addition of a 10-fold volume (v/v) of methanol. After centrifugation at 20,400 g for 10 minutes, the D-serine in the supernatant of the assay mixture was analyzed using HPLC.
Statistical Analysis
Data are presented as the mean±s.e.m. Comparisons between two groups were statistically evaluated using the Student's t-test. Multiple comparisons were evaluated using an analysis of variance (ANOVA) followed by the Newman-Keuls multiple comparison test. Differences were considered as significant at P<0.05.
Results
Serine Racemase−/− Mice have Smaller Infarcts after Middle Cerebral Artery Occlusion
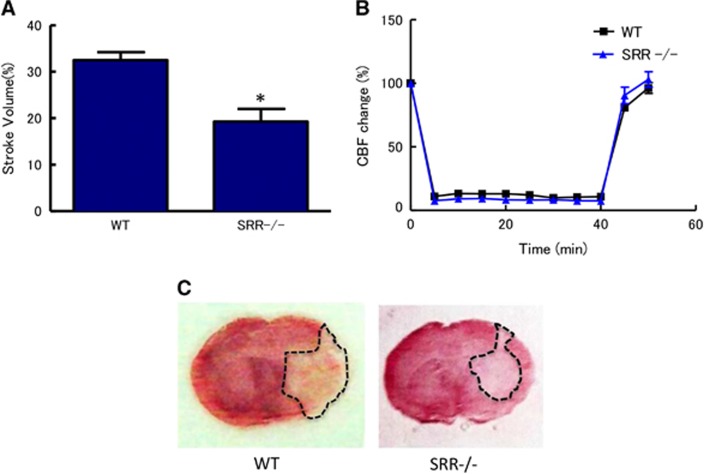
First, we examined the effects of SRR on brain injury at 24 hours after transient MCAO. The infarct volumes after 40 minutes of MCAO were 32.5% and 19.3% of the whole hemisphere in WT and SRR−/− mice, respectively (Figure 1A). The infarct volume in SRR−/− mice was significantly smaller than that in WT mice (Figures 1A and 1C). The reduction in the infarct volume could not be attributed to the differences in the degree of ischemia because the CBF reduction in the center of the ischemic territory was comparable in the WT and SRR−/− mice (Figure 1B).
Figure 1.
Reduced infarct volumes in serine racemase (SRR) −/− mice. (A) Infarct volume was determined 24 hours after middle cerebral artery occlusion (MCAO) (n=7 to 8/group); *P<0.05 vs. wild type (WT) (Student's t-test). (B) Core blood flow during and after MCAO (n=7 to 8/group); not significant between WT and SRR−/− (analysis of variance, ANOVA). (C) Representative triphenyltetrazolium chloride-stained brain sections derived from animals analyzed in (A). CBF, cerebral blood flow.
Temporal Profile of D-/L-Serine Contents after Middle Cerebral Artery Occlusion
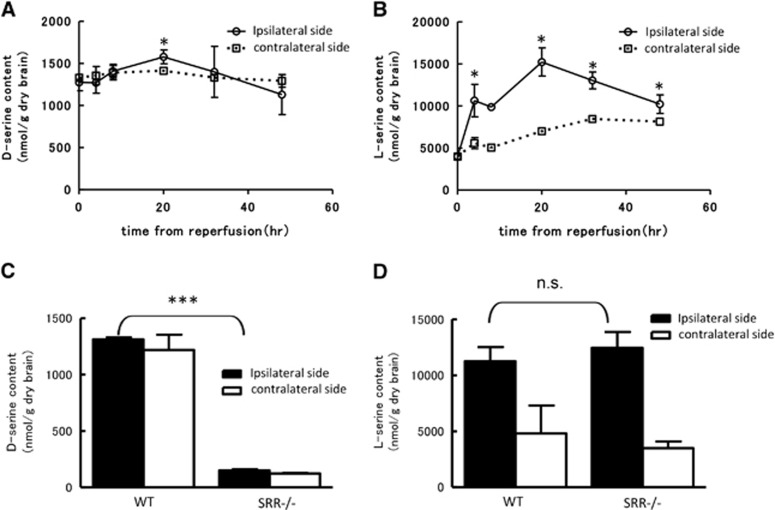
Next, we measured the contents of D-serine and L-serine in the cerebral cortex after MCAO using 2D-HPLC. Total serine was first separated from a methanol supernatant of the cortex using a reverse-phase column (Supplementary Figures A and C). Then, D-serine and L-serine were further separated using an enantio-selective column (Supplementary Figures B and D). The D-serine content in the ipsilateral cerebral cortex was gradually increased until 20 hours after reperfusion, but gradually decreased thereafter (Figure 2A). No increase in D-serine was observed in contralateral cortex. The L-serine content in the ipsilateral cortex prominently increased compared with 0 hour and peaked at 20 hours after reperfusion (Figure 2B). We also compared the difference in the D-serine and L-serine contents in the ipsilateral cortex between WT and SRR−/− mice at 20 hours after reperfusion, when both serine contents were at their maximum levels. The D-serine content in the SRR −/− mice was lower by about 90%, compared with that in the WT mice, whereas the L-serine contents were similar in the WT and SRR−/− mice (Figures 2C and 2D).
Figure 2.
Temporal profile of D-/L-serine content in the cortex after reperfusion. D-Serine (A) and L-serine (B) content in ipsilateral (ischemic) and contralateral hemisphere after middle cerebral artery occlusion (MCAO) in wild type (WT) mice (n=3−4/group). *P<0.05 vs. 0 hour (analysis of variance, ANOVA followed by Newman-Keuls test). D-Serine (C) and L-serine (D) content in WT and serine racemase (SRR) −/− mice at 20 hours after reperfusion (n=3 to 4/group). ***P<0.001, n.s., not significant vs. WT ipsilateral side (Student's t-test).
Determination of D-/L-Serine Release from Cultured Cells during Experimental Ischemia
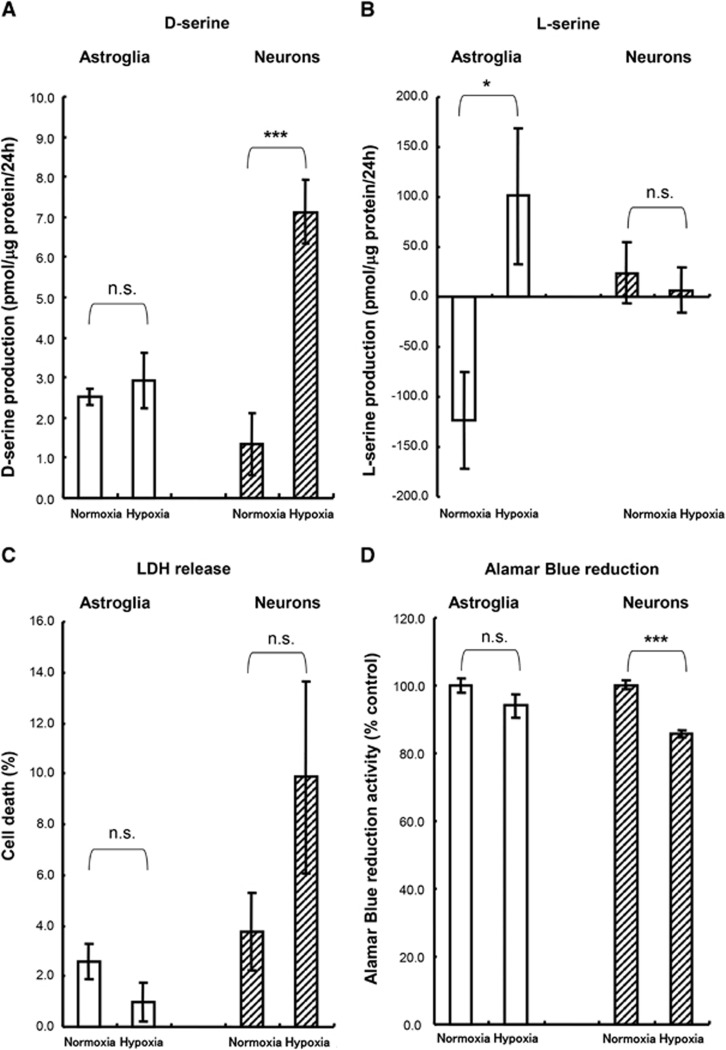
Next, we used cultured cells to determine which cell type can be ascribed to the increase in D-/L-serine after experimental ischemia. We measured the contents of D-/L-serine in cultured media containing neurons and astroglia after hypoxia. At 24 hours after hypoxia, the release of D-serine from the cultured neurons was significantly higher than that in a normoxia control (Figure 3A). In astroglial cultures, no change in the D-serine concentration was observed between the control and hypoxia conditions (Figure 3A). The L-serine value after 24 hours of normoxia incubation was a negative value because L-serine is taken up and consumed by astrocytes continuously for the maintenance of normal metabolism (Figure 3B). Although the L-serine concentration was lower than that at baseline in the normoxia control, the L-serine concentration was increased compared with the baseline after 24 hours of hypoxia (Figure 3B). However, the release of L-serine did not increase in the neuronal cultures (Figure 3B). These results suggested that neurons release D-serine, whereas L-serine is derived from astrocytes during cerebral ischemia. To examine whether cell death is induced after exposure to oxygen depletion, LDH released from damaged cultured cells was measured. After 20 hours of exposure to hypoxia, no significant LDH increase was observed in both cultured astroglia and neurons after oxygen deprivation (Figure 3C). These results showed that structural integrity was not affected during the 24 hours of hypoxia. Furthermore, the Alamar Blue reduction activities of those cells that had been exposed to hypoxia for 24 hours were evaluated. As shown in Figure 3D, the Alamar Blue reduction activities were not altered after hypoxia in astroglia, while the Alamar Blue reduction activity was decreased in neurons after hypoxia. These results suggest that the cellular viability of neurons was affected after hypoxia.
Figure 3.
D-/L-Serine release to culture medium from neurons and astroglia during hypoxia. D-Serine (A) and L-serine (B) contents in the medium after 24 hours hypoxia (n=6). The Y axis shows the quantity of D- or L-serine in the culture medium during hypoxia. A negative value indicates the consumption of D- or L-serine from the culture medium during hypoxia. (C) Cell death during hypoxia was calculated based on lactate dehydrogenase (LDH) release (n=6). (D) Alamar Blue reduction activity at 24 hours after hypoxia (n=6). n.s., not significant; *P<0.05; ***P<0.001 vs. normoxia (Student's t-test).
Expression of Serine Racemase and 3-Phosphoglycerate Dehydrogenase and Distribution of D-/L-Serine in Tissues after Cerebral Ischemia
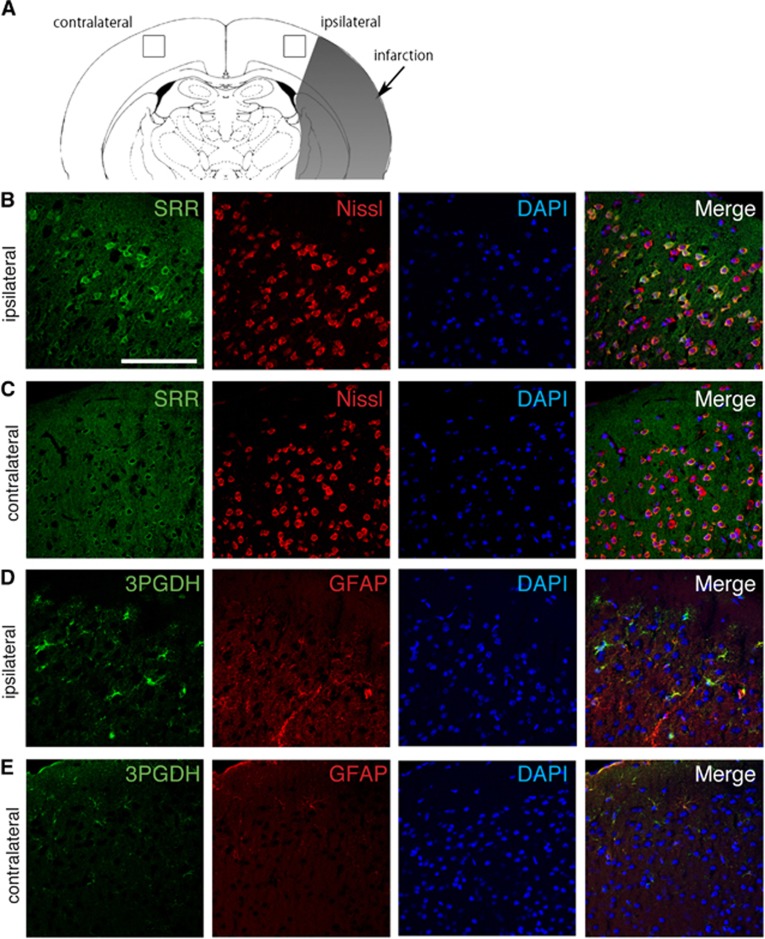
Next, we tried to investigate the cellular localization of key enzymes for D-/L-serine production after cerebral infarction. First, we performed immunohistochemistry for SRR in the ischemic brain. Expression of SRR was increased in the peri-infarct area on the ipsilateral side and was relatively low in the corresponding contralateral side (Figure 4A to 4C). The SRR signal was colocalized with the neuronal marker Nissl. Expression of 3-PGDH was also increased in the ipsilateral peri-infarct area, compared with the contralateral side, and was colocalized with the astroglial marker GFAP (Figures 4D and 4E). These findings suggested that D-serine was synthesized in neurons and L-serine was produced in astrocytes in the brain, supporting the results of the experiments with cultured cells.
Figure 4.
Cellular localization of serine racemase (SRR) and 3-phosphoglycerate dehydrogenase (3-PGDH) in the brain after middle cerebral artery occlusion (MCAO). The squares in the ipsilateral and contralateral cortex show the lesion where the pictures were taken (A). Immunohistochemistry of SRR, 3-PGDH, Nissl, and glial fibrillary acidic protein (GFAP) in the peri-infarct area of the ipsilateral hemisphere (B, D) and the corresponding area in the contralateral hemisphere (C, E) after 20 hours of reperfusion. Scale bar=100 μm.
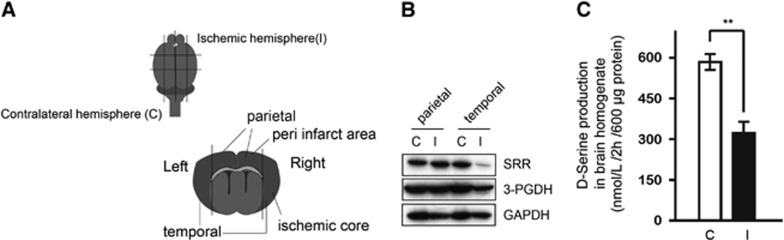
Western Blots for Serine Racemase and 3-Phosphoglycerate Dehydrogenase after Cerebral Ischemia and D-Serine Producing Activity in Brain Tissue after Ischemia
We also attempted to quantify the expressions of SRR and 3-PGDH after cerebral ischemia using a western blot analysis. Samples were separately collected from the temporal region (peri-infarct area) and the parietal region (ischemic core) at 20 hours after reperfusion (Figure 5A). In the peri-infarct lesion tissue, the expression of SRR was almost the same as that in the corresponding area in the contralateral hemisphere; however, the expression was markedly decreased in the ischemic core (Figure 5B). Although immunohistochemistry showed the upregulation of SRR in a small lesion of peri-infarct area, the expression of SRR was not increased overall. The 3-PGDH expression level was slightly decreased in the ischemic core; in the peri-ischemic area, the level was almost the same as that on the contralateral side (Figure 5B). Furthermore, the D-serine producing activity in brain homogenates was analyzed to evaluate the activity of SRR at 20 hours after reperfusion in WT mice. Brain homogenates were mixed with L-serine and incubated at 37°C for 2 hours. The amount of D-serine produced by the brain homogenate was subtracted from the amount for the same sample measured before incubation. The D-serine producing activity on the ipsilateral side of the MCAO was significantly reduced, compared with the contralateral side (Figure 5C).
Figure 5.
Expressions of serine racemase (SRR) and 3-phosphoglycerate dehydrogenase (3-PGDH) in the peri-infarct area and ischemic core. (A) Location where samples were obtained for the western blot. The parietal sample from the ischemic hemisphere contains tissue from the peri-infarct area, and the temporal sample contains tissue from the ischemic core. These tissues were compared with those from the corresponding area of the contralateral hemisphere. (B) Western blot for SRR and 3-PGDH in parietal (peri-infarct area) and temporal (ischemic core) samples at 20 hours after reperfusion in wild type (WT) mice. C: contralateral hemisphere, I: ischemic hemisphere. (C) D-Serine producing activity in brain homogenates at 20 hours after reperfusion in WT mice (n=4/group). The Y axis shows the amount of D-serine produced by 600 μg of protein for 2 hours. C, contralateral hemisphere; I, ischemic hemisphere. **P<0.01 vs ischemic hemisphere.
Discussion
The present study showed that SRR−/− mice have a smaller infarct volume than WT mice. In addition, the temporal profiles of D-/L-serine after cerebral ischemia were investigated in a reperfusion model for the first time. The D-/L-serine content increased until 20 hours after recanalization and then leveled off gradually. The results of in vitro experiments using cultured neurons and astroglia suggested that D-serine is derived from neurons, while L-serine was thought to be released from astrocytes. Immunohistochemistry of brain tissue after cerebral ischemia showed that SRR is expressed in neurons and that 3-PGDH is located in astrocytes, supporting the results of the in vitro experiments. The amounts of SRR and 3-PGDH were not upregulated when examined using western blotting. Therefore, the increase in D-/L-serine does not result from the increase in SRR or 3-PGDH.
Serine racemase−/− mice were previously reported to have a smaller infarct than WT mice after MCAO.11 Neuroprotection in SRR−/− mice is expected to be due to a reduction in D-serine, which exacerbates the excitotoxicity after cerebral ischemia. We first report the D-serine content in SRR−/− mice after transient MCAO. The D-serine content in naïve SRR−/− mice has been reported to be almost 10% of that in WT mice.12 If so, our data showed that the D-serine content in the SRR−/− brain remained at about the same level even at 20 hours after reperfusion, compared with the baseline. These findings also mean that a D-serine peak after reperfusion was not seen in SRR−/− mice. However, the 20-hour peak is unlikely to affect ischemic brain damage, since the therapeutic time window for NMDA is reported to be up to several hours.26,27 Therefore, the difference in the stroke volume was likely caused by the decrease in the D-serine concentration in the SRR−/− mice at baseline.
The reduction in D-serine producing activity was, at least in part, due to the decrease in SRR protein as shown by western blot. Although the D-serine producing activity was reduced in MCAO-brain homogenate, the D-serine content increased after MCAO. One possible explanation is that the reduced expression of SRR affected the degradation of D-serine, because SRR itself also catalyzes the degradation of cellular D-serine in the forebrain region.28 So, D-serine once transformed from L-serine would retain its level if SRR expression was decreased. Also, D-serine located in the extracellular space would be important because it would be capable of stimulating NMDA receptor and exacerbating excitotoxicity during cerebral ischemia. Although the overall increase in the total content of D-serine after MCAO was very subtle, the transfer from intracellular to extracellular might be facilitated after cerebral ischemia, as suggested by the results of our in vitro experiments. The D-serine level in the extracellular space should be investigated using microdialysis in the near future.
Our findings are consistent with a recent report showing that SRR exists in neurons and that 3-PGDH is located in astrocytes.29 On the basis of these findings, it could be hypothesized that L-serine is synthesized and released from astrocytes to be taken up by neurons for further conversion to D-serine. Then, D-serine is released from neurons and is taken up again by astrocytes to be degraded by diamine oxidase.22 The L-serine that is produced and released by the astrocytes is thought to be shuttled to neurons to support neurotransmission. This shuttle system is quite similar to the ANLSH, the hypothesis describing lactate shuttling between neurons and astrocytes.30, 31, 32
Cultured neuronal cells that had been exposed to hypoxia (1% O2) for 24 hours were somewhat unhealthy but were not damaged severely, since their axons and dendrites were thinner than those of the control cells but were not torn or cleaved. In fact, LDH release, which reflects membrane lysis and integrity, was not significantly elevated after 24 hours of hypoxic insult. Therefore, an elevation in D-serine release from neurons after hypoxia does not necessarily indicate passive release as a result of membrane lysis. In contrast, the Alamar Blue reduction activity, which is dependent on normal mitochondrial function in neurons,24,25 decreased significantly after 24 hours of hypoxia. Neuronal depolarization reportedly elicits the release of endogenous D-serine from primary neuronal cultures.33 Reduced ATP production as a result of impaired mitochondrial function might have induced D-serine release from neurons. In contrast to neurons, neither LDH release nor Alamar Blue reduction was affected in astroglia after 24 hours of hypoxia.
The 3-PGDH expression level was not increased overall in the ischemic hemisphere in our western blot analysis. The increase in the L-serine content during ischemia might arise from an increase in 3-phoshoglycerate, a substrate of 3-PGDH, through the upregulation of glycolysis during ischemia. The glucose utilization rate is increased in ischemic brain,34 and downstream products of 3-phoshoglycerate are also likely to be increased. In terms of the increase in D-serine after reperfusion, the upregulation of L-serine might also serve as a possible explanation. L-Serine is a substrate of SRR and drastically increases by up to 200% after ischemia.
The function of L-serine released during cerebral ischemia has not yet been clarified. The intravenous administration of L-serine is reported to be neuroprotective as a result of vascular dilatation through its activity as a Ca2+ channel blocker.35 However, L-serine was reported to be a trophic factor released from astrocytes, which promote the survival, dendritogenesis, and electrophysiologic development of cultured neurons.36 Therefore, it is likely that L-serine from astrocytes also directly affects neurons during cerebral ischemia. Studies of conditional knockout mice for 3-PGDH in astrocytes would likely help to solve this problem.
Acknowledgments
The authors thank Shiseido Co., Ltd., for essential technical support in performing the 2D-HPLC experiments. This work was supported by JSPS KAKENHI Grant Number 26461279.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Takaoka R, Ohira M, Abe T, Tanahashi N, Suzuki N. Reactive oxygen species generated by mitochondrial injury in human brain microvessel endothelial cells. Clin Hemorheol Microcirc. 2006;34:163–168. [PubMed] [Google Scholar]
- Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Grotta J, Clark W, Coull B, Pettigrew LC, Mackay B, Goldstein LB, et al. Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke. Results of a phase IIa randomized trial. Stroke. 1995;26:602–605. doi: 10.1161/01.str.26.4.602. [DOI] [PubMed] [Google Scholar]
- Davis SM, Albers GW, Diener HC, Lees KR, Norris J. Termination of acute stroke studies involving selfotel treatment. ASSIST steering committed. Lancet. 1997;349:32. doi: 10.1016/s0140-6736(05)62166-6. [DOI] [PubMed] [Google Scholar]
- Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW. Induction of serine racemase expression and D-Serine release from microglia by amyloid beta-peptide. J Neuroinflammation. 2004;1:2. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, et al. D-Serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Hashimoto K, Harai T, Mori H. NMDA- and beta-amyloid1-42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci. 2008;28:14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shleper M, Kartvelishvily E, Wolosker H. D-Serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25:9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Ahmad AS, Zeynalov E, Gazi SK, Sikka G, Ehmsen JT, et al. Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J Neurosci. 2010;30:1413–1416. doi: 10.1523/JNEUROSCI.4297-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y, Konno R, Sasabe J, Ueno K, Tojo Y, Mita M, et al. Alteration of intrinsic amounts of D-Serine in the mice lacking serine racemase and D-amino acid oxidase. Amino Acids. 2012;43:1919–1931. doi: 10.1007/s00726-012-1398-4. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Izawa Y, Suzuki N.Astroglial pentose phosphate pathway rates in response to high-glucose environments ASN Neuro 20124art:e00078doi: 10.1042/AN20120002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Abe T, Zhou P, Jackman K, Capone C, Casolla B, Hochrainer K, et al. Lipoprotein receptor-related protein-6 protects the brain from ischemic injury. Stroke. 2013;44:2284–2291. doi: 10.1161/STROKEAHA.113.001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz A, Abe T, Hochrainer K, Shimamura M, Anrather J, Racchumi G, et al. Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J Neurosci. 2008;28:1649–1658. doi: 10.1523/JNEUROSCI.5205-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman K, Kahles T, Lane D, Garcia-Bonilla L, Abe T, Capone C, et al. Progranulin deficiency promotes post-ischemic blood-brain barrier disruption. J Neurosci. 2013;33:19579–19589. doi: 10.1523/JNEUROSCI.4318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, et al. Prostaglandin E(2) EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Abe T, Kunz A, Shimamura M, Zhou P, Anrather J, Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29:66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, et al. BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe J, Miyoshi Y, Suzuki M, Mita M, Konno R, Matsuoka M, et al. D-amino acid oxidase controls motoneuron degeneration through D-Serine. Proc Natl Acad Sci USA. 2012;109:627–632. doi: 10.1073/pnas.1114639109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Sasabe J, Furuya S, Mita M, Hamase K, Aiso S. Type 1 diabetes mellitus in mice increases hippocampal D-Serine in the acute phase after streptozotocin injection. Brain Res. 2012;1466:167–176. doi: 10.1016/j.brainres.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Abe T, Takahashi S, Fukuuchi Y. Reduction of Alamar Blue, a novel redox indicator, is dependent on both the glycolytic and oxidative metabolism of glucose in rat cultured neurons. Neurosci Lett. 2002;326:179–182. doi: 10.1016/s0304-3940(02)00347-6. [DOI] [PubMed] [Google Scholar]
- Page B, Page M, Noel C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int J Oncol. 1993;3:473–476. [PubMed] [Google Scholar]
- Meldrum B. Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovasc Brain Metab Rev. 1990;2:27–57. [PubMed] [Google Scholar]
- Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, et al. Serine racemase modulates intracellular D-Serine levels through an alpha,beta-elimination activity. J Biol Chem. 2005;280:1754–1763. doi: 10.1074/jbc.M405726200. [DOI] [PubMed] [Google Scholar]
- Ehmsen JT, Ma TM, Sason H, Rosenberg D, Ogo T, Furuya S, et al. D-Serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci. 2013;33:12464–12469. doi: 10.1523/JNEUROSCI.4914-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Kartvelishvily E, Shleper M, Klinker CM, Bowser MT, Wolosker H. Neuronal release of D-Serine: a physiological pathway controlling extracellular D-Serine concentration. FASEB J. 2010;24:2951–2961. doi: 10.1096/fj.09-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC, Brucklacher RM, Vannucci SJ. Glycolysis and perinatal hypoxic-ischemic brain damage. Dev Neurosci. 2005;27:185–190. doi: 10.1159/000085991. [DOI] [PubMed] [Google Scholar]
- Ren TJ, Qiang R, Jiang ZL, Wang GH, Sun L, Jiang R, et al. Improvement in regional CBF by L-serine contributes to its neuroprotective effect in rats after focal cerebral ischemia. PLoS ONE. 2013;8:e67044. doi: 10.1371/journal.pone.0067044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Tabata T, Mitoma J, Yamada K, Yamasaki M, Makino A, et al. L-serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc Natl Acad Sci USA. 2000;97:11528–11533. doi: 10.1073/pnas.200364497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.