Abstract
Blood–tumor barrier (BTB) constitutes an efficient organization of tight junctions that impairs the delivery of therapeutic drugs. However, the methods and molecular mechanisms underlying the BTB opening remain elusive. MicroRNAs (miRNAs) have recently emerged as key regulators of various biologic processes and therapeutic targets. In this study, we have identified microRNA-181a (miR-181a) as a critical miRNA in opening BTB. MicroRNA-181a expression was upregulated in glioma endothelial cells (GECs), which were obtained by coculturing endothelial cells (ECs) with glioma cells. Overexpression of miR-181a resulted in an impaired and permeability increased BTB, and meanwhile reduced the expression of zonula occluden (ZO)-1, occludin, and claudin-5. Kruppel-like factor 6 (KLF6), a transcription factor of the zinc-finger family, was downregulated in GECs. Mechanistic investigations defined it as a direct and functional downstream target of miR-181a, which was involved in the regulation of BTB permeability and the expression of ZO-1, occludin, and claudin-5. Furthermore, luciferase assays and chromatin immunoprecipitation assays showed that KLF6 upregulated the promoter activities and interacted with the promoters of ZO-1, occludin, and claudin-5 in GECs. Collectively, we showed the possibility that overexpression of miR-181a contributes to the increased permeability of BTB by targeting KLF6, thereby revealing potential therapeutic targets for the treatment of brain gliomas.
Keywords: blood–tumor barrier, brain endothelial cells, KLF6, MiR-181a, tight junction
Introduction
Glioblastoma multiforme is the most frequent and lethal type of malignant brain tumors. Despite the commonly available treatments including surgical resection, radiation, and chemotherapy,1 glioblastoma multiforme is difficult to treat because of the existence of blood–tumor barrier (BTB) that impairs the delivery of therapeutic drugs, causing the majority of drugs do not reach the brain at all or do so with negligible bioavailability.2,3 Thus, there is a critical need to develop successful methods to safely open the BTB and to spare adjacent nontumoral neural tissue to improve the chemotherapeutic treatment for malignant gliomas.
The blood–brain barrier (BBB) forms a protective and precisely regulated barrier that separates the central nervous system from peripheral blood circulation.4 Blood–tumor barrier, similar to BBB, is composed of microvascular endothelial cells (ECs), which are interconnected by tight junctions that in turn form a barrier restricting paracellular diffusion.5 The tight junctions involve the interactions of cytoskeletal molecular complexes including the zonula occludens (ZO) proteins, coupled to transmembrane proteins occludin and claudins.6
MicroRNAs (miRNAs) are short noncoding RNAs that negatively regulate gene expression in a sequence-specific manner, primarily via binding to the 3′-untranslated region (3′-UTR) of the target mRNAs.7 Mounting evidence implicates that miRNA is involved in various biologic processes and have become novel biomarkers, modulators, and therapeutic targets for diseases such as cancer, heart disease, and diabetes.8,9 Human microRNA-181a (miR-181a), a member of the miR-181 family, can function as either an oncogene or a tumor suppressor depending on the cancer type and cellular context. For example, elevated level of miR-181a was observed in ovarian cancer,10 but reduced level of miR-181a was observed in gliomas.11 Recent findings also suggested that miR-181a deregulation contributes to the metastasis of salivary adenoid cystic carcinoma and breast cancer.12,13 In addition, miR-181a is among the miRNAs primarily involved in EC aging and inflamm-aging.14 MicroRNA-181a has been reported to be expressed in vascular development and neo-lymphangiogenesis.15 However, the function of miR-181a in regulating BTB as well as the associated molecular mechanism have not been documented.
Kruppel-like factor 6 (KLF6) is a transcription factor of the zinc-finger family that regulates key cellular processes such as development, differentiation, cellular proliferation, and apoptosis.16 It has a more generalized role in tumorigenenesis as a tumor suppressor gene that is inactivated in a number of human cancers including glioma.17 In addition, KLF6 has been shown to have a role in EC motility.18 Immunohistochemical analysis showed that KLF6 protein was expressed in various regions of the adult forebrain and KLF6-positive cells manifested neuronal or endothelial phenotypes under physiologic conditions.19 Previous studies have revealed that KLF6 was also expressed in human corneal epithelial cells.20 However, the expression and function of KLF6 in human cerebral microvascular ECs forming the BTB have remained completely unknown.
Therefore, the aim of this project was to study the role of mir-181a in regulating BTB function and thereby to determine whether KLF6 is involved in this process and mediates the expression of tight junction-related proteins.
Materials and methods
Cell Lines and Cultures
The immortalized human brain endothelial cell line hCMEC/D3 (ECs) was kindly provided by Dr Couraud from the Institut Cochin, Paris, France. Cells were cultured on culture inserts (0.4 mm pore size; Corning, Lowell, MA, USA) coated with 150 μg/mL Cultrex Rat Collagen I (R&D Systems, Minneapolis, MN, USA). Cells were maintained in endothelial basal medium (EBM-2; Lonza, Walkersville, MD, USA), containing 5% fetal bovine serum ‘Gold' (FBS; PAA Laboratories GmbH, Pasching, Austria), 1% Penicillin-Streptomycin (Life Technologies Corporation, Paisley, UK), 1.4 μmol/L hydrocortisone (Sigma-Aldrich, St Louis, MO, USA), 1% chemically defined lipid concentrate (Life Technologies Corporation, Paisley, UK), 5 μg/mL ascorbic acid (Sigma-Aldrich), 10 mmol/L HEPES (PAA Laboratories GmbH) and 1 ng/mL human basic fibroblast growth factor (bFGF; Sigma-Aldrich). Endothelial cells were used between passages 30 and 40. Human glioblastoma U87 cell line and human embryonic kidney 293T cell line were purchased from the Shanghai Institutes for Biological Sciences Cell Resource Center and maintained in Dulbecco's modified Eagle's medium (DMEM) of high glucose with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin (Life Technologies Corporation, Paisley, UK). All cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Establishment of an In Vitro Blood–Tumor Barrier Model
The in vitro BTB model was established by coculturing ECs and U87 cells in a transwell system as described previously.21 Briefly, U87 cells were seeded at 2 × 104 per well in 6-well plates with suitable culture medium and cultured for 2 days before addition of the EC inserts. Endothelial cells were subsequently seeded at 2 × 105 per well on the upper side of inserts coated freshly with 150 μg/mL of Cultrex Rat Collagen I. The medium was then renewed every 2 days. After coculturing 4 days, the glioma endothelial cells (GECs) which were ECs cocultured with glioma cells were obtained.
Quantitative RT-PCR
Total RNA was extracted from cells with Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA). The RNA concentration and quality were determined for each sample by the 260/280 nm ratio using a Nanodrop Spectrophotometer (ND-100). For measuring miR-181a, RNA samples were reverse transcribed using Taqman MicroRNA Reverse Transcription Kit, and real-time PCR analysis was performed using Taqman Universal Master Mix ІІ with the TaqMan MicroRNA Assay of miR-181a and U6 (Applied Biosystems, Foster City, CA, USA). For quantification of the mRNA levels, reverse transcription and real-time PCR amplification were performed using the High Capacity cDNA Reverse Transcription Kits and TaqMan Universal Master Mix II with the gene expression assays (Applied Biosystems), respectively. The gene expression assays are the probe for KLF6 (Hs00810569_m1), ZO-1 (Hs01551861_m1), occludin (Hs00170162_m1), claudin-5 (Hs00533949_s1), and GAPDH (Hs03929097_g1). All quantitative RT-PCR analyses were conducted by means of a 7500 Fast Real-Time PCR System (Applied Biosystems). Relative expression values were calculated using the relative quantification (2−ΔΔCt) method.
Transfection and Generation of Stable Cell Lines
Oligonucleotides encoding miR-181a precursor and anti-miR-181a precursor were subcloned into the pGPH1/GFP/Neo (miR-181a (+)) and pGPU6/GFP/Neo (miR-181a (−)) plasmid vectors (GenePharma, Shanghai, China), respectively. Human full-length KLF6 gene with its 3′-UTR sequences and short hairpin RNA directed against human KLF6 gene were constructed in pEX-2 (KLF6 (+)) and pGPU6/GFP/Neo (KLF6 (−)) plasmid vectors (GenePharma), respectively. Plasmid carrying a nontargeting sequence was used as a negative control (NC) of miR-181a (+), miR-181a (−), or KLF6 (−). Empty pEX-2 vector was used as an NC of KLF6 (+). Plasmids of miR-181a (+)-NC, miR-181a (+), miR-181a (−)-NC, miR-181a (−), KLF6 (+)-NC, KLF6 (+), KLF6 (−)-NC and KLF6 (−) were used to conduct stable transfection, which was performed at about 80% confluency of ECs in 24-well plates using Lipofectamine LTX and Plus Reagents (Life Technologies Corporation, Carlsbad, CA, USA), according to the manufacturer's instructions. The stable transfected cells were selected by the culture medium containing 0.4 mg/mL G418 (Sigma-Aldrich). After approximately 4 weeks, G418-resistant cell clones were established. Three clones (#1, #2, and #3) of miR-181a (+), miR-181a (−), KLF6 (+), or KLF6 (−) stable transfected cells were used in the subsequent experiments. The sequences of the above constructs were provided in Supplementary Table 1.
Cell Transfection of MicroRNA
The miR-181a agomir, miR-181a antagomir, and their respective NC were synthesized (GenePharma). The sequences of these were provided in Supplementary Table 1. Cells were transfected using lipofectamine 2000 reagent (Life Technologies Corporation, Carlsbad, CA, USA). Transfection complexes were prepared according to the manufacturer's instructions. The transfected efficacy of miR-181a agomir and miR-181a antagomir was evaluated by quantitative RT-PCR, and the high transfection efficacy of these could sustain 7 days (Supplementary Figure 1). Stable transfected cells cotransfected with miR-181a agomir (or miR-181a antagomir) were divided into nine groups (Table 1).
Table 1. The table of nine groups.
| Groups | Stable transfected cells | Cotransfection |
|---|---|---|
| Control | Untransfected | None |
| Pre-miR-181a-NC+ KLF6 (+)-NC group | KLF6 (+)-NC | miR-181a agomir NC |
| Pre-miR-181+ KLF6 (+) group | KLF6 (+) | miR-181a agomir |
| Pre-miR-181a-NC+ KLF6 (−)-NC group | KLF6 (−)-NC | miR-181a agomir NC |
| Pre-miR-181+ KLF6 (−) group | KLF6 (−) | miR-181a agomir |
| Anti-miR-181a-NC+ KLF6 (+)-NC group | KLF6 (+)-NC | miR-181a antagomir NC |
| Anti-miR-181a+ KLF6 (+) group | KLF6 (+) | miR-181a antagomir |
| Anti-miR-181a-NC+ KLF6 (−)-NC group | KLF6 (−)-NC | miR-181a antagomir NC |
| Anti-miR-181a+ KLF6 (−) group | KLF6 (−) | miR-181a antagomir |
KLF6, Kruppel-like factor 6; miR-181a, microRNA-181a; NC, negative control.
Transendothelial Electric Resistance Assays
Transendothelial electric resistance (TEER) assays were performed using a transwell system by coculturing ECs and U87 cells and measured using millicell-ERS instrument (Millipore, Billerica, MA, USA). U87 cells were seeded at 2 × 104 per well in 6-well plates with suitable culture medium and cultured for 2 days. After the cells were confluent, ECs were seeded at 2 × 105 per well in transwell permeable supports (pore size, 0.4 μm, surface area, 4.52 cm2) coated with 150 μg/mL of Cultrex Rat Collagen I. On the fourth day of coculture, TEER was measured after 30 minutes at room temperature directly after medium exchange to ensure temperature equilibration and same medium composition during the measurement. Background electric resistance was subtracted before final resistances were calculated. Expressed as Ω • cm2 using the surface area of the transwell insert.
Permeability Assays
In the lower chamber of transwell, culture medium was removed and replaced with the transport buffer composed of Hank's buffer saline solution with 10 mmol/L HEPES and 1 mmol/L sodium pyruvate (Life Technologies Corporation, Paisley, UK). In the apical side of transwell, the transport buffer was supplemented with 50 mmol/L Lucifer Yellow (LY, 457 Da), 50 mmol/L fluorescein isothiocyanate (FITC)-Dextran (4 kDa), or 20 mmol/L FITC-Dextran (70 kDa) (Sigma-Aldrich). Incubations were performed in a humidified incubator at 37°C and 5% CO2. Samples obtained were analyzed using a florescence multiwall plate reader (FlexStation 3; Molecular Devices, Sunnyvale, CA, USA). Expressed as permeability coefficients using the slopes of the curves, which were obtained through the volume cleared plotted against time.
Western Blot Assays
Cells were lysed and the supernatant extracts were quantified for protein using the BCA protein assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). Equal amounts of protein were separated by SDS-PAGE and transferred onto PVDF membrane. The membrane was blocked with 5% nonfat dry milk in TBST for 2 hours, and subsequently incubated with primary antibodies for KLF6 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), ZO-1 (1:500; Life Technologies Corporation, Frederick, MD, USA), occludin (1:250; Life Technologies Corporation, Frederick, MD, USA) and claudin-5 (1:250; Life Technologies Corporation, Frederick, MD, USA). The blots were washed three times and incubated with respective conjugated secondary antibodies at room temperature. After washing, immunoblots were visualized by enhanced chemiluminescence (ECL Kit; Santa Cruz Biotechnology) and scanned using the ChemImager 5500 V2.03 software (AlPha Innotech, San Leandro, CA, USA), and integrated light density values (IDVs) were calculated by the Fluorchem 2.0 software (Alpha Innotech, San Leandro, CA, USA) and normalized with that of GAPDH.
Immunofluorescence Assays
Immunofluorescence assay was performed to detect the expression and distribution of KLF6 and tight junction-related proteins. Cells were cultured on insert filters, fixed with 4% paraformaldehyde for 20 minutes and permeated for 10 minutes in PBS containing 0.2% Triton X-100 (KLF6), or fixed with ice-cold acetone for 10 minutes at −20°C and permeated with 0.2% Triton X-100 for 10 minutes at room temperature (ZO-1 and claudin-5), or fixed with methanol for 10 minutes at −20°C (occludin), followed by incubation in 5% BSA blocking buffer for 2 hours at room temperature. Subsequently, cells were then incubated with primary antibodies for KLF6 (1:50; Santa Cruz Biotechnology), ZO-1 (1:50; Life Technologies Corporation, Frederick, MD, USA), occludin (1:50; Life Technologies Corporation, Frederick, MD, USA), and claudin-5 (1:50; Life Technologies Corporation, Frederick, MD, USA), diluted in the blocking buffer overnight at 4°C. After three washes with PBS, cells were incubated with Alexa Fluor 555-labeled goat anti-mouse IgG or anti-rabbit IgG secondary antibody (1:500; Beyotime Institute of Biotechnology, Jiangsu, China) for 1 hour. The nuclei were counterstained with 0.5 μg/mL of DAPI. The staining was analyzed using immunofluorescence microscopy (Olympus, Tokyo, Japan) and merged by the Chemi Imager 5500 V2.03 software.
Reporter Vector Construction and Luciferase Reporter Assay
The sequences of KLF6 3′-UTR were amplified by PCR and cloned into a pmirGlO Dual-luciferase miRNA Target Expression Vector (Promega, Madison, WI, USA) to form 3′-UTR-luciferase reporter vector (KLF6-3′UTR-Wt) (GenePharma). To test the binding specificity, the sequences that interact with the miR-181a seed sequence were mutated as indicated (KLF6-3′UTR-Mut1, KLF6-3′UTR-Mut2, and KLF6-3′UTR-Mut3). The pmirGLO vector constructed with either 3′-UTR fragments or mutation of 3′-UTR fragments, and indicated miRNAs were cotransfected into human embryonic kidney 293T cells in 24-well plates using Lipofectamine 2000. Luciferase activity was analyzed 48 hours after transfection using the Dual-Luciferase Reporter System (Promega), and firefly luciferase activity was normalized by Renilla luciferase activity.
Human genomic DNA was used to amplify different promoter fragments, subcloned into pGL3-Basic-Luciferase vector (Promega) containing a firefly luciferase reporter gene and verified by DNA sequencing. Human full-length KLF6 was constructed in pEX3 (pGCMV/MCS/Neo) plasmid vector (GenePharma). Glioma endothelial cells were obtained through the establishment of BTB models. On transfection with pEX3-KLF6, exponential log-phase cells in 24-well plate were cotransfected with wild-type or deletion pGL3 vector, as well as renilla luciferase plasmid (pRL-TK, Promega; normalization of transfection efficiency), using FuGENE 6 (Roche Molecular Biochemicals, Indianapolis, IN, USA). Cells were harvested 48 hours after transfection and analyzed by luciferase assay using the Dual-Luciferase Reporter Assay System (Promega). The mean value of the experimental sample was subtracted readings of background luminescence from empty pGL3-basic vector-transfected cells. The relative luciferase activity was expressed as the ratio of firefly luciferase activity to renilla luciferase activity.
Chromatin Immunoprecipitation Assay
Simple ChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology, Danvers, Massachusetts, USA) was used for chromatin immunoprecipitation (ChIP) assays according to the manufacturer's protocol. Briefly, cells were crosslinked with 1% formaldehyde and collected in lysis buffer. Chromatin was then digested with Micrococcal Nuclease. Immunoprecipitation was incubated with 3 μg of anti-KLF6 antibody or normal rabbit IgG followed by immunoprecipitation with Protein G Agarose Beads during an overnight incubation at 4 °C with gentle shaking. As an input reference, 2% were removed before antibody supplemental and stored at −20°C. The ChIP DNA was reverse-crosslinked with 5 mol/L NaCl and Proteinase K and purified. Immunoprecipitated DNA was amplified by PCR using primers. The primers of each PCR set, the sizes of PCR products, and annealing temperatures were listed in Supplementary Table 2. In each PCR, the corresponding input was taken in parallel for PCR validation.
Statistical Analysis
Data were expressed as the mean±standard deviation (s.d.). Statistically significant differences between two groups were determined by Student's t-test. For three or more groups, statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Dunnett's posttest. P<0.05 was considered as statistically significant.
Results
MicroRNA-181a Expression Was Upregulated in Glioma Endothelial Cells
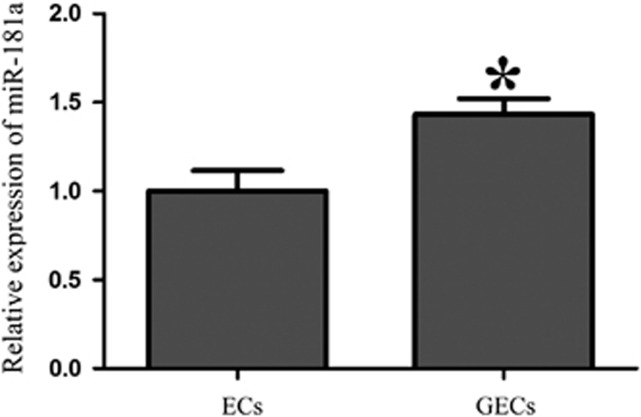
We determined the expression level of miR-181a in ECs and GECs. As shown in Figure 1, the miR-181a expression was higher in GECs than that in ECs, suggesting that miR-181a might be involved in the regulation of BTB function.
Figure 1.
Expression of microRNA-181a (miR-181a) in endothelial cells (ECs) and glioma endothelial cells (GECs). Relative expression levels of miR-181a in ECs and GECs. Data represent means±s.d. (n=5, each). *P<0.05 versus ECs group.
Overexpression of MicroRNA-181a Impaired the Integrity, Increased the Permeability of Blood–Tumor Barrier, and Reduced the mRNA and Protein Expression of Tight Junction-Related Proteins in Glioma Endothelial Cells
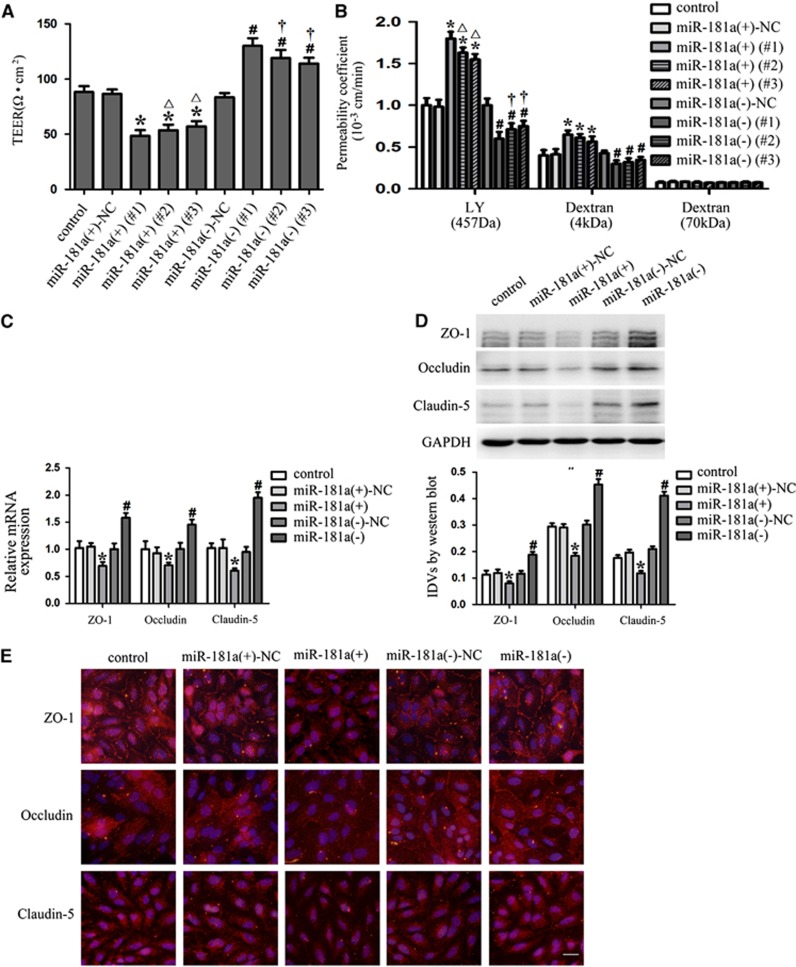
We next assessed the functional role of miR-181a in GECs by determining the effects of miRNA overexpression and inhibition on the integrity and permeability using TEER and permeability assays, respectively. Overexpression and inhibition of miR-181a were obtained by stable transfection and several clones were further generated. We detected three clones of these stable transfection for TEER and permeability assays. Detection of TEER was performed to evaluate the integrity of BTB. As shown in Figure 2A, the TEER values of GECs were decreased in the three clones of miR-181a (+) group compared with the miR-181a (+)-NC group, whereas the TEER values of GECs in the three clones of miR-181a (−) group were increased compared with the miR-181a (−)-NC group. The above results indicated that overexpression of miR-181a could impair the integrity of BTB. Permeability assays were further performed with molecules of different sizes to clarify the potential mechanisms relating the miR-181a-dependent regulation of BTB permeability. As shown in Figure 2B, the permeability coefficient values of LY and 4 kDa FITC-dextrans were increased in the three clones of miR-181a (+) group. In contrast, the three clones of miR-181a (−) group displayed the opposite effect on BTB permeability, which caused the decreased permeability coefficient values of LY and 4 kDa FITC-dextrans. No significant change in the permeability of 70 kDa FITC-dextran was detected, confirming that in vitro permeability of BTB is affected in a size-selective manner by the regulation of miR-181a.
Figure 2.
MicroRNA-181a regulated the integrity and permeability of blood–tumor barrier (BTB), and the expression of tight junction-related proteins in glioma endothelial cells (GECs). (A) The transendothelial electric resistance (TEER) values of BTB with the expression of miR-181a changed. (B) The permeability coefficient values of Lucifer Yellow (LY) (457 Da), 4 kDa fluorescein isothiocyanate (FITC)-dextrans, and 70 kDa FITC-dextran in BTB with the expression of miR-181a changed. (C) Quantitative RT-PCR analysis of tight junction-related proteins in GECs with the expression of miR-181a changed. (D) Western blot analysis of tight junction-related proteins in GECs with the expression of miR-181a changed, using GAPDH as an endogenous control. Representative protein expression and their integrated light density values (IDVs) are shown. Data represent means±s.d. (n=5, each). *P<0.05 versus miR-181a (+)-NC group, #P<0.05 versus miR-181a (−)-NC group, △P<0.05 versus miR-181a (+) (#1) group, †P<0.05 versus miR-181a (−) (#1) group. (E) Immunofluorescence staining of tight junction-related proteins in GECs with the expression of miR-181a changed. Nuclei were labeled with DAPI. Images are representative of independent experiments (n=4). Scale bars represent 20 μm. NC, negative control.
Owing to the fact that the effects of miR-181a (+) (#1) and miR-181a (−) (#1) were most significant among other clones in the TEER and permeability assays, the clones of miR-181a (+) (#1) and miR-181a (−) (#1) were used in subsequent experiments and abbreviated as miR-181a (+) and miR-181a (−). To clarify the potential mechanisms in the changes of BTB permeability, the expression levels of ZO-1, occludin, and claudin-5 were measured by quantitative RT-PCR and western blot assays. The results showed that mRNA and protein expression levels of ZO-1, occludin, and claudin-5 were decreased in miR-181a (+) group, whereas those were increased in miR-181a (−) group (Figures 2C and 2D). Furthermore, immunofluorescence analysis of tight junction-related proteins showed that ZO-1 and occludin were localized on the cell–cell boundaries. However, claudin-5 was mainly expressed in cytoplasm. ZO-1 and occludin were continuously distributed in control, miR-181a (+)-NC, and miR-181a (−)-NC groups. MicroRNA-181a (−) group displayed the cobblestone morphology of confluent monolayers expressing abundant ZO-1 and occludin. However, discontinuous distribution of ZO-1 and occludin was observed in miR-181a (+) group. The expression of claudin-5 was also decreased in miR-181a (+) group, whereas that of miR-181a (−) group was increased (Figure 2E).
Kruppel-Like Factor 6 Expression Was Downregulated in Glioma Endothelial Cells
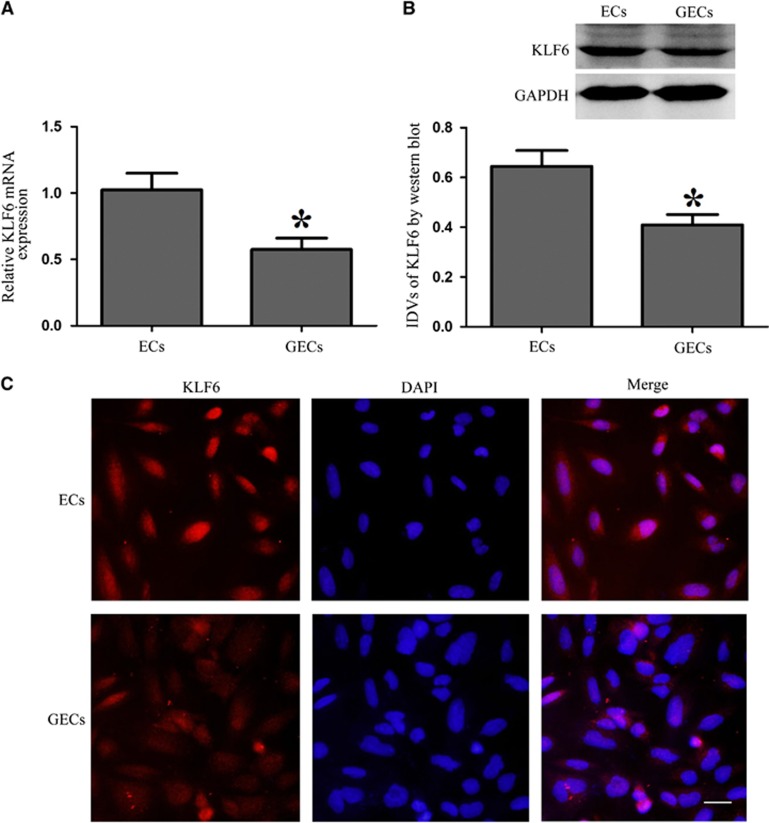
The mRNA and protein expression levels of KLF6 were analyzed by quantitative RT-PCR and western blotting. As shown in Figures 3A and 3B, KLF6 was expressed in both ECs and GECs, and the mRNA and protein expression levels of KLF6 were downregulated in GECs group compared with ECs group. Expression analysis indicated that both transcription and translation products of this factor were decreased in GECs. To further determine its distribution, immunofluorescence analysis was then performed. The results showed that KLF6 was mainly distributed in the nuclei of ECs and GECs, and showed a lower expression in GECs which was in accordance with the data from previous assays (Figure 3C).
Figure 3.
Expression of Kruppel-like factor 6 (KLF6) in endothelial cells (ECs) and glioma endothelial cells (GECs). (A) Quantitative RT-PCR analysis of KLF6 in ECs and GECs. (B) Western blot analysis of KLF6 in ECs and GECs, using GAPDH as an endogenous control. Representative protein expression and their integrated light density values (IDVs) are shown. Data represent means±s.d. (n=5, each). *P<0.05 versus ECs group. (C) Immunofluorescence staining of KLF6 in ECs and GECs. KLF6 (red) was labeled with secondary antibody against KLF6 antibody and nuclei (blue) were labeled with DAPI. Images are representative of independent experiments (n=4). Scale bars represent 20 μm.
MicroRNA-181a Inhibited the Expression of Kruppel-Like Factor 6 by Targeting Its 3′-Untranslated Region
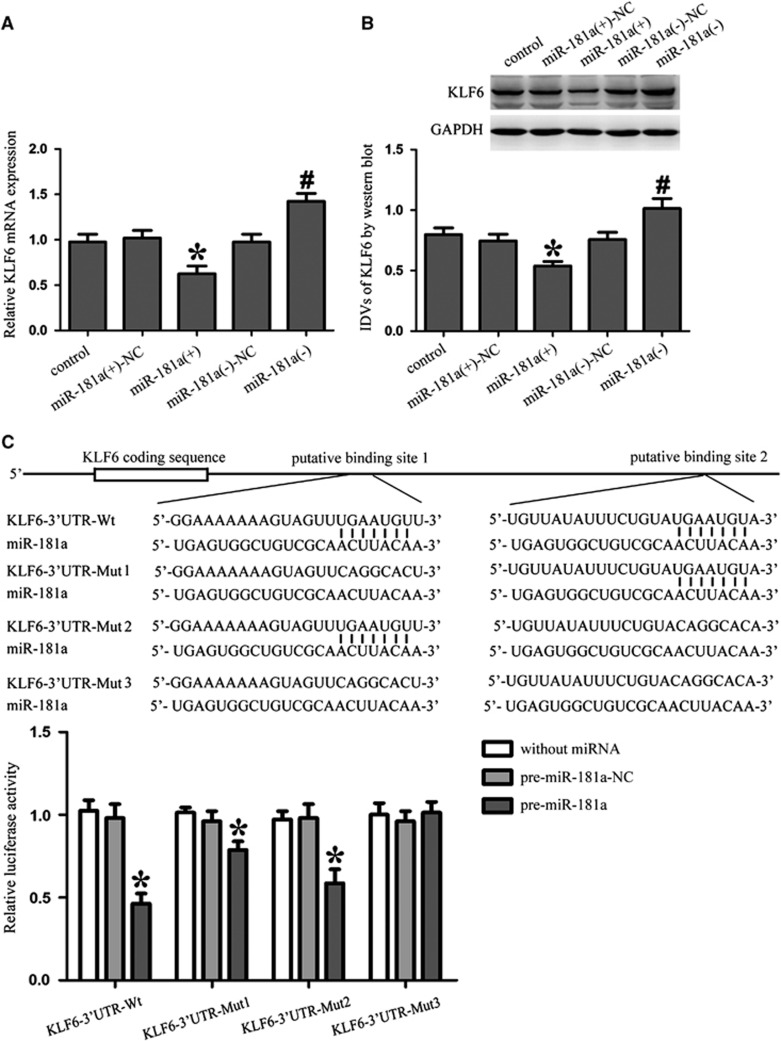
To uncover the mRNA targets of miR-181a in GECs, we used bioinformatics databases (Targetscan, Pictar, and RNAhybrid) to identify potential targets. To experimentally verify these potential targets, cells were transfected with miR-181a and mRNA and protein target expression levels were detected by quantitative RT-PCR and western blotting, respectively. It was confirmed that KLF6 was one of the candidates. Results showed that the mRNA and protein expression levels of KLF6 were decreased in miR-181a (+) group compared with miR-181a (+)-NC group whereas that of miR-181a (−) group was increased compared with miR-181a (−)-NC group. These results indicated that miR-181a could inhibit the expression of KLF6 in GECs (Figures 4A and 4B).
Figure 4.
MicroRNA-181a (MiR-181a) inhibits the expression of Kruppel-like factor 6 (KLF6) by targeting its 3′-untranslated region (3′-UTR). (A) Quantitative RT-PCR analysis of KLF6 in glioma endothelial cells (GECs) with the expression of miR-181a changed. (B) Western blot analysis of KLF6 with the expression of miR-181a changed. Representative protein expression and their integrated light density values (IDVs) are shown. Data represent means±s.d. (n=5, each). *P<0.05 versus miR-181a (+)-NC group, #P<0.05 versus miR-181a (−)-NC group. (C) Schematic representation of the predicted binding sites for miR-181a in the 3′-UTR of KLF6 (KLF6-3′UTR-Wt) and the site mutagenesis design for the reporter assay (KLF6-3′UTR-Mut1, KLF6-3′UTR-Mut2, and KLF6-3′UTR-Mut3). Luciferase reporter assay of human embryonic kidney (HEK) 293T cells cotransfected with the reporter plasmid (or the corresponding mutant reporter) and the indicated miRNAs. Renilla/firefly luciferase ratios were calculated and further normalized to the without miRNA group which was set as 1. Data represent means±s.d. (n=5, each). *P<0.05 versus pre-miR-181a-NC group. NC, negative control.
To elucidate the molecular mechanisms responsible for the miR-181a-induced inhibition of KLF6 expression, luciferase reporter assay was conducted. Using TargetScan 6.2, KLF6 was predicted to harbor two putative miR-181a binding sites in the 3′-UTR, which are conserved among species. The seeds for miR-181a to KLF6 3′-UTR were indicated. To verify that KLF6 was a functional target of miR-181a, we cloned a reporter plasmid containing the wild-type 3′-UTR of KLF6 (KLF6-3′UTR-Wt). Cotransfection of pre-miR-181a and KLF6-3′UTR-Wt strongly decreased the luciferase activity, while cotransfection of pre-miR-181a-NC and KLF6-3′UTR-Wt did not change the luciferase activity. This indicated that miR-181a targeted the 3′-UTR of KLF6. In parallel, we constructed reporter plasmids that two conserved seed sequences of miR-181a were mutated individually (KLF6-3′UTR-Mut1 and KLF6-3′UTR-Mut2) or were mutated in combination (KLF6-3′UTR-Mut3) to determine the regions responsible for miR-181a. Luciferase activity was markedly diminished by 18% in cells transfected with pre-miR-181a and KLF6-3′UTR-Mut1, compared with cells transfected with pre-miR-181a-NC and KLF6-3′UTR-Mut1. Furthermore, luciferase activity was markedly diminished by 40% in cells transfected with pre-miR-181a and KLF6-3′UTR-Mut2, compared with cells transfected with pre-miR-181a-NC and KLF6-3′UTR-Mut2. However, there was no significant difference in KLF6-3′UTR-Mut3 groups (Figure 4C). Thus, the putative binding site 1 was determined to be a mainly functional target site for miR-181a. These results showed that miR-181a functioned as a regulator of KLF6 by binding to the two sites (putative binding sites 1 and 2) in KLF6 3′-UTR.
Overexpression of MicroRNA-181a Impaired the Integrity and Increased the Permeability of Blood–Tumor Barrier, and Reduced the mRNA and Protein Expression Levels of Tight Junction-Related Proteins in Glioma Endothelial Cells by Downregulating Kruppel-Like Factor 6
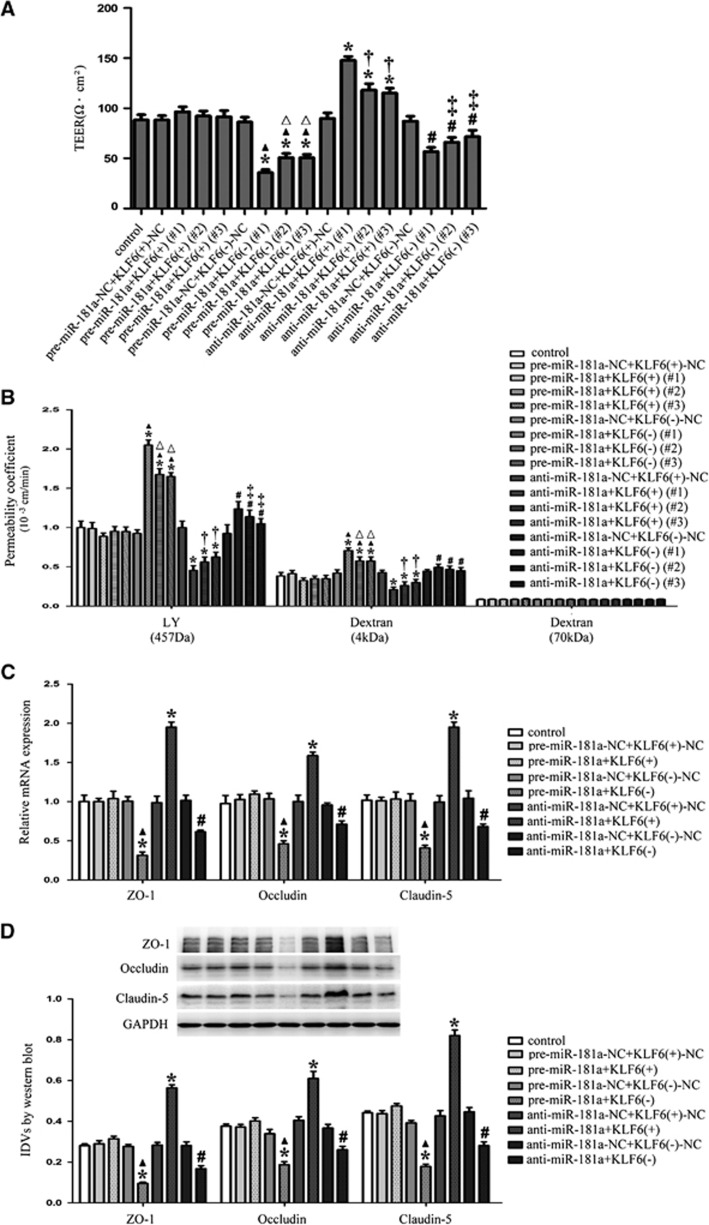
To clarify whether KLF6 was involved in the miR-181a-mediated regulation of the BTB function, the combinations of transfection were conducted and the integrity and permeability of BTB as well as the expression of ZO-1, occludin, and claudin-5 were further investigated. KLF6 (+) and KLF6 (−) were obtained by stable transfection and several clones were further generated. We detected three clones of these stable transfection for the TEER and permeability assays. As shown in Figure 5A, the TEER values of three clones in the pre-miR-181a+KLF6 (−) group were decreased whereas that of the three clones of anti-miR-181a+KLF6 (+) group were increased compared with the control group. Furthermore, the TEER values of GECs were decreased in the three clones of pre-miR-181a+KLF6 (−) group and anti-miR-181a+KLF6 (−) group compared with the three clones of pre-miR-181a+KLF6 (+) group and anti-miR-181a+KLF6 (+) group, respectively. As shown in Figure 5B, the permeability coefficient values of LY and 4 kDa FITC-dextrans were increased in the three clones of pre-miR-181a+KLF6 (−) group. In contrast, the permeability coefficient values of LY and 4 kDa FITC-dextrans were decreased in the three clones of anti-miR-181a+KLF6 (+) group. In addition, the permeability coefficient values of LY and 4 kDa FITC-dextrans were increased in the three clones of pre-miR-181a+KLF6 (−) group and anti-miR-181a+KLF6 (−) group compared with the three clones of pre-miR-181a+KLF6 (+) group and anti-miR-181a+KLF6 (+) group, respectively. There was no significant change in the permeability to 70 kDa FITC-dextran. These results showed that overexpression of miR-181a could impair the integrity and increase the permeability of BTB by downregulating KLF6.
Figure 5.
MicroRNA-181a (miR-181a) regulated the integrity and permeability of blood–tumor barrier (BTB), and the expression of tight junction-related proteins in glioma endothelial cells (GECs) by regulating Kruppel-like factor 6 (KLF6). (A) The transendothelial electric resistance (TEER) values of BTB with the expression of miR-181a and KLF6 changed. (B) The permeability coefficient values of Lucifer Yellow (LY) (457 Da), 4 kDa fluorescein isothiocyanate (FITC)-dextrans, and 70 kDa FITC-dextran in BTB with the expression of miR-181a and KLF6 changed. (C) Quantitative RT-PCR analysis of tight junction-related proteins in GECs with the expression of miR-181a and KLF6 changed. (D) Western blot analysis of tight junction-related proteins in GECs with the expression of miR-181a and KLF6 changed, using GAPDH as an endogenous control. Representative protein expression and their integrated light density values (IDVs) are shown. Data represent means±s.d. (n=5, each). *P<0.01 versus control group, ▴P<0.05 versus pre-miR-181a+KLF6 (+) group, #P<0.05 versus anti-miR-181a+KLF6 (+) group, △P<0.05 versus pre-miR-181a+KLF6(−) (#1) group, †P<0.05 versus anti-miR-181a +KLF6(+) (#1) group, and ‡P<0.05 versus anti-miR-181a+KLF6(−) (#1) group.
Owing to the fact that the effects of miR-181a agomir (or miR-181a antagomir) cotransfected with KLF6 (+) (#1) and KLF6 (−) (#1) were most significant among other clones in the TEER and permeability assays, the clones of KLF6 (+) (#1) and KLF6 (−) (#1) were used in subsequent experiments and abbreviated as KLF6 (+) and KLF6 (−). As shown in Figure 5C, the mRNA expression levels of ZO-1, occludin, and claudin-5 were decreased in the pre-miR-181a+KLF6 (−) group but increased in anti-miR-181a+KLF6 (+) group compared with the control group. The mRNA expression levels of them were decreased in the pre-miR-181a+KLF6 (−) group and anti-miR-181a+KLF6 (−) group compared with the pre-miR-181a+KLF6 (+) group and anti-miR-181a+KLF6 (+) group, respectively. The same changes were also observed in detection of the protein expression levels of ZO-1, occludin, and claudin-5 (Figure 5D). Collectively, these results suggested that miR-181a could reduce the mRNA and protein expression of tight junction-related proteins in GECs by downregulating KLF6.
Kruppel-Like Factor 6 Upregulated the Promoter Activities and Bound to the Promoters of Tight Junction-Related Proteins in Glioma Endothelial Cells
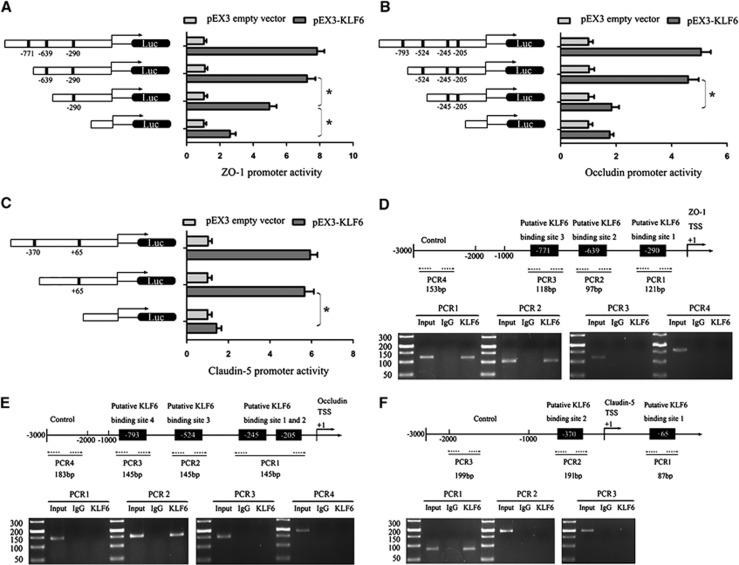
To test whether KLF6 is required for the promoter activity of tight junction-related proteins in GECs, a series of constructs were generated and luciferase assays were performed. Kruppel-like factor 6 binds to ‘CACCC' and ‘GC box' DNA sequences, which are promoter elements in target genes.22,23 The sequences of ZO-1, occludin, and claudin-5 promoters were set according to the database of DBTSS HOME (http://dbtss.hgc.jp/). Positions of transcription start site (TSS) of ZO-1 and claudin-5 were predicted by DBTSS HOME and the TSS position of occludin was set according to the finding of Ando-Akatsuka et al.24 By analyzing these DNA sequences in the 1,000-bp region upstream of the TSS and its 100 bp downstream sequence, three putative KLF6 binding sites in ZO-1, four putative KLF6 binding sites in occludin, and two putative KLF6 binding sites in claudin-5 were confirmed. So a series of DNA fragments were constructed and ligated into the pGL3 basic vector. Wild-type and deletion constructs and putative KLF6 binding sites were indicated. On cotransfection with pEX3-KLF6, ZO-1 promoter activities were upregulated by 7.83-, 7.23-, 4.96-, and 2.6-fold; occludin promoter activities were upregulated by 5.06-, 4.6-, 1.83-, and 1.76-fold; claudin-5 promoter activities were upregulated by 5.93-, 5.66-, and 1.43-fold. With regard to ZO-1, deletion of the −639 and −290 site regions significantly diminished the promoter activities of ZO-1, suggesting that the important functional elements of ZO-1 that were necessary for high promoter activity were likely to reside in the −639 and −290 site regions (Figure 6A). Moreover, deletion of the −524 and +65 site regions significantly diminished the promoter activities of occludin and claudin-5, respectively. The results indicated that the KLF6-responsive elements resided within −524 and +65 site regions of occludin and claudin-5, respectively (Figures 6B and 6C).
Figure 6.
Kruppel-like factor 6 (KLF6) on promoter activity bind to promoters of tight junction-related proteins in glioma endothelial cells (GECs). (A–C) Schematic depiction of the different reporter constructs used and the luciferase activity: zonula occluden (ZO)-1, occludin, and claudin-5 are shown. The Y-bar shows the position of the deletions on the DNA fragments. X-bar shows the constructed plasmid activity after normalization with the cotransfected reference vector (pRL-TK), and relative to the activity of pEX3 empty vector, which the activity was set to 1. Data represent means±s.d. (n=5, each). (D–F) Schematic representation of the human ZO-1, occludin, and claudin-5 promoter region 3,000 bp upstream of the transcription start site (TSS) which designated as +1. Putative KLF6 binding sites are indicated. Chromatin immunoprecipitation (ChIP) PCR products for binding sites and an upstream region not expected to associate with KLF6 are depicted with bold lines. Dashed arrows represent the primers used for each PCR. GECs were used to conduct ChIP assays. PCR was conducted with the resulting precipitated DNA. Images are representative of independent experiments (n=4).
Finally, to determine whether KLF6 directly associated with the promoters of ZO-1, occludin, and claudin-5 in GECs, ChIP assays were performed. The putative KLF6 binding sites were indicated. Because the first two binding sites of occludin were located close to each other at −205 to −245 positions, primers were designed to bind sequences flanking these two putative KLF6-binding sites. As an NC, PCR was conducted to amplify the 1,000-bp upstream region of the putative KLF6 binding site that was not expected to associate with KLF6. The results showed that there were associations of KLF6 with putative binding sites 1 and 2 of ZO-1, putative binding site 3 of occludin, and putative binding site 1 of claudin-5. There was no association of KLF6 with all of the control regions (Figures 6D–6F).
Discussion
In the present study, we have found that the miR-181a expression was increased in GECs. Overexpression of miR-181a resulted in an impaired and permeability increased BTB, and the decreased expression of ZO-1, occludin, and claudin-5. In contrast to the expression of miR-181a, KLF6 expression was decreased in GECs, indicating that it might be a key factor in regulating BTB permeability. We further showed that miR-181a could inhibit the expression of KLF6 by targeting its 3′-UTR. Furthermore, it was also confirmed that KLF6 was involved in the miR-181a-mediated regulation of the integrity and permeability of BTB, as well as the expression of ZO-1, occludin, and claudin-5. In addition, KLF6 could upregulate the promoter activities and bind to the promoters of ZO-1, occludin, and claudin-5 directly in GECs, which might contribute to the transcriptional regulation of these genes. We showed for the first time that overexpression of miR-181a could increase the BTB permeability by targeting KLF6, which could directly regulate the expression of tight junction-related proteins.
In this study, we have found that miR-181a was expressed in hCMEC/D3 cells (ECs), an immortalized human cerebral microvascular EC line, which recapitulated most properties of the BBB in situ and was often used as an in vitro model of human BBB.25 In vitro BTB model was established successfully so as to clarify the function and molecular mechanism of miR-181a in BTB. We found that the expression of miR-181a in GECs was upregulated compared with that in ECs, which suggested that miR-181a might involve in the regulation of BTB function. Consistent with our results, others researchers have found that miR-181a was upregulated in gastric, pancreatic, and ovarian cancers, indicating that it was an oncomir.10,26,27 However, another study found that it functioned as a tumor suppressor in human glioma cells.28 The reason for this was that certain miRNA may be oncogenic in one cell or tissue type but tumor suppressive in another, depending on the cancer type and cellular context.29
Further, we showed that overexpression of miR-181a typically reduced the basal TEER value, indicating a key role of miR-181a in regulating BTB integrity. In addition, permeability assays also found that overexpression of miR-181a increased the permeability to LY and 4 kDa FITC-dextrans, suggesting that it increased small magnitude permeability of BTB. Other studies have revealed that tight junction was mainly responsible for the structural integrity of BBB and the regulation of paracellular pathway.30 To clarify the potential molecular mechanisms responsible for the miR-181a-induced regulation of BTB function, the expression of tight junction-related proteins in BTB model was detected. Results showed that overexpression of miR-181a reduced the mRNA and protein expression levels of ZO-1, occludin, and claudin-5. In addition, our immunofluorescence results showed that overexpression of miR-181a resulted in discontinuous distribution of ZO-1 and occludin, and decreased expression of claudin-5, which were consistent with the above results as well. Therefore, our data showed that overexpression of miR-181a resulted in an impaired and permeability increased BTB, as well as the decreased expression of ZO-1, occludin, and claudin-5 in GECs. However, our experiments did not rule out the possibility that miR-181a could also affect other associated proteins to regulate BTB permeability. An earlier report had described that peripheral blood mononuclear cells transfected with miR-181a mimics led to a reduction in growth factors hepatocyte growth factor and granulocyte colony-stimulating factor levels.31 Hepatocyte growth factor can enhance the barrier function in primary cultures of rat brain microvascular ECs.32 Granulocyte colony-stimulating factor leads to neurologic functional improvement by reducing brain edema, BBB permeability, neuronal death, and apoptosis.33 But whether the above factors are also involved in the miR-181a-dependent regulation of BTB permeability needs to be further investigated.
Kruppel-like factor 6, a transcription factor of the Krüppel-like family, was confirmed as one of the target candidates of miR-181a. We have found that KLF6 was expressed in both ECs and GECs, and displayed a decreased expression level in GECs, indicating that KLF6 acted as a tumor suppressor in BTB. Consistent with our results, others researchers have found that KLF6 was decreased in a number of human cancers including glioma.17 But whether the expression of KLF6 in BTB was also reduced in vivo needs to be further investigated. Moreover, it was confirmed that miR-181a inhibited the mRNA and protein expression levels of KLF6 by directly targeting its 3′-UTR. MicroRNAs can regulate gene expression by inducing direct cleavage of the targeted mRNAs or inhibiting translation through perfect or nearly perfect complementarity to targeted mRNAs at the 3′-UTRs in animals.34 The results indicated that miR-181a might regulate the KLF6 expression by cleaving its mRNA. More recently, KLF6 has been reported to be expressed in human umbilical vein ECs and can activate ALK1 gene after wire-induced endothelial denudation.35 In response to vascular injury, KLF6 can also activate the endoglin and urokinase promoters in vascular repair.22,36 Thus, these findings and our results of reduced KLF6 level in GECs have suggested that KLF6 might be involved in the regulation of vascular ECs. We further investigated whether KLF6 was also involved in the miR-181a-mediated regulation of BTB function. Results showed that the overexpression of miR-181a impaired the integrity and increased the permeability of BTB by downregulating KLF6. Mechanistically, the mRNA and protein expression levels of ZO-1, occludin, and claudin-5 were decreased. These results indicated that the impaired and increased permeability of BTB might be due to the decreased expression of ZO-1, occludin, and claudin-5.
Kruppel-like factors are a large family characterized by a DNA-binding domain with conserved three tandem C2H2-type zinc finger motifs at the carboxy terminus, which recognizes the GT/GC box or CACCC element sites on promoter regions.37 To further clarify the direct relationship between KLF6 and promoters of ZO-1, occludin, and claudin-5, we performed luciferase assays and ChIP experiments. Results showed that KLF6 could promote the promoter activities and bind to putative binding sites 1 and 2 of ZO-1, putative binding site 3 of occludin, and putative binding site 1 of claudin-5 in GECs, indicating that there was a direct interaction between KLF6 and promoters of tight junction-related proteins. Thus, miR-181a-mediated downregulation of KLF6 in GECs could directly reduce the expression of tight junction-related proteins so as to increase the permeability of BTB. However, our experiments could not rule out the possibility that KLF6 could also affect other associated proteins to regulate BTB permeability. Previous studies showed that KLF6 could transcriptionally activate the human inducible nitric-oxide synthase and insulin-like growth factor I promoters.38,39 Furthermore, KLF6 regulates inducible pro- and anti-inflammatory gene expression in macrophages.40 Therefore, whether the above factors are also involved in the KLF6-dependent regulation of BTB permeability needs to be further investigated.
In conclusion, we have shown for the first time that overexpression of miR-181a results in an impaired and permeability increased BTB by targeting KLF6. The reduced expression of KLF6 dampens the expression of tight junction-related proteins through binding to the promoter regions of ZO-1, occludin, and claudin-5 directly in GECs. In this study, we have showed the possibility that overexpression of miR-181a contributes to the increased permeability of BTB by altering the expression of tight junction-related proteins, thereby revealing potential therapeutic targets for the treatment of brain gliomas.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work is supported by grants from the Natural Science Foundation of China (81172197, 81171131, 81272564, 81272795 and 81372484, 81372682), the Natural Science Foundation of Liaoning Province in China (No. 201102300), Liaoning Science and Technology Plan Projects (No. 2011225020), and Shenyang Science and Technology Plan Projects (nos. F11-264-1-15, F12-277-1-05, F13-318-1-16, F13-318-1-19, and F13-220-9-15).
Supplementary Material
References
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Black KL, Ningaraj NS. Modulation of brain tumor capillaries for enhanced drug delivery selectively to brain tumor. Cancer Control. 2004;11:165–173. doi: 10.1177/107327480401100304. [DOI] [PubMed] [Google Scholar]
- Czupalla CJ, Liebner S, Devraj K. In vitro models of the blood-brain barrier. Methods Mol Biol. 2014;1135:415–437. doi: 10.1007/978-1-4939-0320-7_34. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Paolinelli R, Corada M, Orsenigo F, Dejana E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery. Pharmacol Res. 2011;63:165–171. doi: 10.1016/j.phrs.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279–289. doi: 10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- Parikh A, Lee C, Peronne J, Marchini S, Baccarini A, Kolev V, et al. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nat Commun. 2014;5:2977. doi: 10.1038/ncomms3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P, et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- He Q, Zhou X, Li S, Jin Y, Chen Z, Chen D, et al. MicroRNA-181a suppresses salivary adenoid cystic carcinoma metastasis by targeting MAPK-Snai2 pathway. Biochim Biophys Acta. 2013;1830:5258–5266. doi: 10.1016/j.bbagen.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Sossey-Alaoui K, Thompson CL, Danielpour D, Schiemann WP. TGF-beta upregulates miR-181a expression to promote breast cancer metastasis. J Clin Invest. 2013;123:150–163. doi: 10.1172/JCI64946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippo MR, Olivieri F, Monsurro V, Prattichizzo F, Albertini MC, Procopio AD. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol. 2014;56:154–163. doi: 10.1016/j.exger.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010;116:2395–2401. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- Andreoli V, Gehrau RC, Bocco JL. Biology of Kruppel-like factor 6 transcriptional regulator in cell life and death. IUBMB Life. 2010;62:896–905. doi: 10.1002/iub.396. [DOI] [PubMed] [Google Scholar]
- Camacho-Vanegas O, Narla G, Teixeira MS, DiFeo A, Misra A, Singh G, et al. Functional inactivation of the KLF6 tumor suppressor gene by loss of heterozygosity and increased alternative splicing in glioblastoma. Int J Cancer. 2007;121:1390–1395. doi: 10.1002/ijc.22809. [DOI] [PubMed] [Google Scholar]
- Das A, Fernandez-Zapico ME, Cao S, Yao J, Fiorucci S, Hebbel RP, et al. Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J Biol Chem. 2006;281:39105–39113. doi: 10.1074/jbc.M607720200. [DOI] [PubMed] [Google Scholar]
- Jeong KH, Kim SK, Kim SY, Cho KO. Immunohistochemical localization of Kruppel-like factor 6 in the mouse forebrain. Neurosci Lett. 2009;453:16–20. doi: 10.1016/j.neulet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Chiambaretta F, De Graeve F, Turet G, Marceau G, Gain P, Dastugue B, et al. Cell and tissue specific expression of human Kruppel-like transcription factors in human ocular surface. Mol Vis. 2004;10:901–909. [PubMed] [Google Scholar]
- Ma J, Wang P, Liu Y, Zhao L, Li Z, Xue Y. Kruppel-Like Factor 4 Regulates Blood-Tumor Barrier Permeability via ZO-1, Occludin and Claudin-5. J Cell Physiol. 2014;229:916–926. doi: 10.1002/jcp.24523. [DOI] [PubMed] [Google Scholar]
- Kojima S, Hayashi S, Shimokado K, Suzuki Y, Shimada J, Crippa MP, et al. Transcriptional activation of urokinase by the Kruppel-like factor Zf9/COPEB activates latent TGF-beta1 in vascular endothelial cells. Blood. 2000;95:1309–1316. [PubMed] [Google Scholar]
- Warke VG, Nambiar MP, Krishnan S, Tenbrock K, Geller DA, Koritschoner NP, et al. Transcriptional activation of the human inducible nitric-oxide synthase promoter by Kruppel-like factor 6. J Biol Chem. 2003;278:14812–14819. doi: 10.1074/jbc.M300787200. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, et al. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Federici C, Guillonneau F, Chretien F, Camoin L, Glacial F, et al. Guanine nucleotide-binding protein Galphai2: a new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells. J Cereb Blood Flow Metab. 2012;32:860–873. doi: 10.1038/jcbfm.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Nie Y, Li X, Wu G, Huang Q, Cao J, et al. MicroRNA-181a functions as an oncomir in gastric cancer by targeting the tumour suppressor gene ATM. Pathol Oncol Res. 2014;20:381–389. doi: 10.1007/s12253-013-9707-0. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu D, Wang Q, Zheng D, Jiang X, Xu L. LPS induced miR-181a promotes pancreatic cancer cell migration via targeting PTEN and MAP2K4. Dig Dis Sci. 2014;59:1452–1460. doi: 10.1007/s10620-014-3049-y. [DOI] [PubMed] [Google Scholar]
- Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, et al. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Zheng ZM, Wang X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim Biophys Acta. 2011;1809:668–677. doi: 10.1016/j.bbagrm.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, et al. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and MicroRNA expression in nonhuman primates. Alcohol Clin Exp Res. 2014;38:980–993. doi: 10.1111/acer.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Nakagawa S, Horai S, Tanaka K, Deli MA, Yatsuhashi H, et al. Hepatocyte growth factor enhances the barrier function in primary cultures of rat brain microvascular endothelial cells. Microvasc Res. 2014;92:41–49. doi: 10.1016/j.mvr.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Chu H, Tang Y, Dong Q. Protection of granulocyte-colony stimulating factor to hemorrhagic brain injuries and its involved mechanisms: effects of vascular endothelial growth factor and aquaporin-4. Neuroscience. 2014;260:59–72. doi: 10.1016/j.neuroscience.2013.12.017. [DOI] [PubMed] [Google Scholar]
- de Moor CH, Meijer H, Lissenden S. Mechanisms of translational control by the 3' UTR in development and differentiation. Semin Cell Dev Biol. 2005;16:49–58. doi: 10.1016/j.semcdb.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Garrido-Martin EM, Blanco FJ, Roque M, Novensa L, Tarocchi M, Lang UE, et al. Vascular injury triggers Kruppel-like factor 6 mobilization and cooperation with specificity protein 1 to promote endothelial activation through upregulation of the activin receptor-like kinase 1 gene. Circ Res. 2013;112:113–127. doi: 10.1161/CIRCRESAHA.112.275586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella LM, Sanchez-Elsner T, Sanz-Rodriguez F, Kojima S, Shimada J, Guerrero-Esteo M, et al. Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between Sp1 and KLF6: their potential role in the response to vascular injury. Blood. 2002;100:4001–4010. doi: 10.1182/blood.V100.12.4001. [DOI] [PubMed] [Google Scholar]
- Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Mgbemena V, Segovia JA, Chang TH, Tsai SY, Cole GT, Hung CY, et al. Transactivation of inducible nitric oxide synthase gene by Kruppel-like factor 6 regulates apoptosis during influenza A virus infection. J Immunol. 2012;189:606–615. doi: 10.4049/jimmunol.1102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Idelman G, Plymate SR, Narla G, Friedman SL, Werner H. Transcriptional activation of the insulin-like growth factor I receptor gene by the Kruppel-like factor 6 (KLF6) tumor suppressor protein: potential interactions between KLF6 and p53. Endocrinology. 2004;145:3769–3777. doi: 10.1210/en.2004-0173. [DOI] [PubMed] [Google Scholar]
- Date D, Das R, Narla G, Simon DI, Jain MK, Mahabeleshwar GH. Kruppel-like Transcription Factor 6 Regulates Inflammatory Macrophage Polarization. J Biol Chem. 2014;289:10318–10329. doi: 10.1074/jbc.M113.526749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.