Abstract
Objectives. Nuclear factor κB (NF-κB) is a critical activator of inflammatory processes and MTX is one of the most commonly prescribed DMARDs for treatment of RA. We sought to determine whether MTX inhibited NF-κB activity in RA and in lymphocytes and fibroblast-like synoviocytes (FLSs) and to define underlying mechanisms of action.
Methods. An NF-κB luciferase reporter plasmid was used to measure NF-κB activation across experimental stimuli. Flow cytometry was used to quantify changes in intracellular protein levels, measure levels of reactive oxygen species and determine apoptosis. Quantitative RT-PCR was used to identify changes in MTX target genes.
Results. In T cell lines, MTX (0.1 μM) inhibited activation of NF-κB via depletion of tetrahydrobiopterin (BH4) and increased Jun-N-terminal kinase (JNK)-dependent p53 activity. Inhibitors of BH4 activity or synthesis also inhibited NF-κB activation and, similar to MTX, increased JNK, p53, p21 and JUN activity. Patients with RA expressed increased levels of phosphorylated or active RelA (p65) compared with controls. Levels of phosphorylated RelA were reduced in patients receiving low-dose MTX therapy. In contrast, inhibition of NF-κB activation by MTX was not mediated via BH4 depletion and JNK activation in FLSs, but rather was completely prevented by adenosine receptor antagonists.
Conclusion. Our findings support a model whereby distinct pathways are activated by MTX in T cells and FLSs to inhibit NF-κB activation.
Keywords: rheumatoid arthritis, methotrexate, Jun-N-terminal kinase, p53, nuclear factor-kappaB, T cell, fibroblast-like synoviocytes
Introduction
RA is the most common serious autoimmune disease, affecting 1% of the world’s population [1]. MTX is the standard of care for the treatment of RA, but the precise mechanism by which MTX exerts its anti-inflammatory effects remains incompletely understood. MTX was originally designed in the 1940s as a folic acid antagonist for the treatment of malignancy. In cancer, folate antagonism via competitive inhibition of dihydrofolate reductase (DHFR) decreases de novo methyl donors tetrahydrofolate and methyltetrahydrofolate, blocking purine and pyrimidine biosynthesis and effectively halting DNA replication and cell proliferation [2]. It was not until the late 1970s and early 1980s that MTX became widely used in RA, but it has since emerged as the basis by which all other therapies for RA are judged [3, 4]. At the time, it was inferred that the anti-inflammatory and immunomodulatory effects of MTX stem from a similar biochemical pathway. However, work spanning the last three decades has indicated that there is still much to learn about the functional role of MTX in the management of RA.
MTX is polyglutamated once taken up by cells. MTX polyglutamates are believed to represent its active form and levels of MTX polyglutamates correlate with clinical efficacy in patients with RA [5]. A prevailing theory has been that anti-inflammatory effects of MTX stem from inhibition of aminoimidazolecarboxamidoribonucleotide (AICAR) transformylase, causing increased intracellular AICAR levels. Increased AICAR levels inhibit adenosine monophosphate deaminase and adenosine deaminase, leading to accumulation and release of adenosine and subsequent A2A and A3 adenosine receptor activation, producing anti-inflammatory properties [6–12]. However, since folate supplementation does not reverse the anti-inflammatory effects of MTX in vivo, the mechanism by which MTX exerts its vulnerary effects in RA may stem from additional biochemical pathways [13]. DHFR also catalyses reduction of dihydrobiopterin (BH2) to tetrahydrobiopterin (BH4), which is inhibited by MTX [14–17]. BH4 is a necessary cofactor of all nitric oxide synthases (NOSs) and loss of BH4 uncouples NOS, leading to loss of NO synthesis and an increase in the synthesis of reactive oxygen species (ROS) such as H2O2. MTX-mediated NOS uncoupling and ROS production activates Jun-N-terminal kinase (JNK) and JNK-dependent induction of p53 and p21 and increased sensitivity to apoptosis via intrinsic and extrinsic pathways. Subjects with RA exhibit reduced levels of JNK, p21 and p53 in peripheral blood mononuclear cells (PBMCs) while subjects with RA receiving MTX possess normal levels of JNK, p21 and p53 in PBMCs, suggesting that MTX-mediated inhibition of reduction of BH2 to BH4 also contributes to the therapeutic effects of MTX in RA, possibly by eliminating self-reactive T cells [18, 19].
Excess TNF-α production plays a central role in RA pathogenesis, as evidenced by the efficacy of therapies that selectively reduce TNF-α levels in vivo. Activation of the transcription factor nuclear factor κB (NF-κB) is a major cellular response to TNF-receptor signalling [20–22]. MTX is also thought to reduce inflammation by lowering levels of TNF-α and/or NF-κB activity [23]. However, mechanistically it is not apparent if and how any of the pathways activated by concentrations of MTX achieved in vivo by standard low-dose therapy might inhibit NF-κB activity. Further, it is unclear if different cells involved in RA pathogenesis, e.g. T lymphocytes and fibroblast-like synoviocytes (FLSs), respond to MTX by activating a single common pathway or multiple pathways. Since these pathways are similarly activated in both primary cells and cell lines, to address these questions we determined whether low concentrations of MTX inhibited NF-κB activation in tissue culture models in both Jurkat T lymphocytes and FLSs and in vivo in subjects with RA. To do so we employed an NF-κB reporter construct in cell-based assays and measured phosphorylation of RelA (p65) as an indicator of NF-κB activity in vivo. Our studies in Jurkat T cells demonstrate that MTX inhibits NF-κB activation via MTX-dependent depletion of BH4, increased ROS synthesis and JNK and p53 activation. Further, we find that a BH4 antagonist or inhibition of BH4 synthesis also stimulates JNK phosphorylation and induces p53, leading to decreased NF-κB activation. In vivo, RA PBMCs exhibit elevated levels of phosphorylated p65 (P-p65) relative to control PBMCs. Levels of P-p65 are near those of control PBMCs in PBMCs from RA subjects taking MTX. In contrast to these results, in FLSs MTX fails to induce ROS synthesis, JNK activation and downstream effects, apparently because FLSs express extremely low levels of NOS enzymes. However, MTX also inhibits NF-κB in FLSs, which appears to be dependent upon adenosine release and activation of A2A and A3 adenosine receptors. Our data are consistent with the notion that two independent pathways activated by MTX target two distinct cell lineages to produce anti-inflammatory effects in RA.
Methods
Drugs and reagents
MTX, (6R)-5,6,7,8-BH4, caffeine, theophylline, BI-78D3, 2,4-diamino-6-hydroxypyrimidine (DAHP) and 3-[4,5-dimethylthiazol-2-yl]-2,5-dipehyltetrazolium bromide (MTT) were from Sigma-Aldrich (St Louis, MO, USA). 4-amino-7,8-dihydro-l-biopterin (4-ABH4) was from Schircks Laboratories (Jona, Switzerland). L-JNKi1 was from Enzo Life Sciences (Farmingdale, NY, USA). Recombinant human TNF-α was from Becton Dickinson (BD) Biosciences (Bedford, MA, USA). The following primary antibodies were used: monoclonal rabbit anti-phospho-NF-κB p65 (Ser536) (93H1) (3033; Cell Signalling Technologies, Danvers, MA, USA), polyclonal anti-phospho-JNK pT183/pY185 (558268; BD), polyclonal rabbit anti-p53 (NB200-171; Novus Biologicals, Littleton, CO, USA), polyclonal rabbit anti-p21 (ab7960; Abcam, Cambridge, UK), monoclonal mouse TRAILR1 (ab18362; Abcam) and monoclonal rabbit anti-PUMA (ab33906; Abcam). Fluorescein isothiocyanate-conjugated goat anti-rabbit Ig (554020; BD) was used as secondary antibody. For studies with human PBMCs, the following cell surface markers were used from BD Biosciences: Pacific Blue mouse anti-human CD4 (558116), Alexa Fluor 700 mouse anti-human CD8 (557945), APC mouse anti-human CD19 (555415) and PE mouse anti-human CD14 (562691). The NF-κB-luciferase reporter (NF-κB-luc) construct containing five κB elements was a gift from Dr Dean W. Ballard (Vanderbilt University, Nashville, TN, USA). JNK-1 and JNK-2 dominant negative (DN) mutants were from the laboratory of Dr Roger J. Davis (University of Massachusetts Medical School, Worcester, MA, USA). The p53-DN construct was from the laboratory of Dr William Kaelin, Jr (Harvard Medical School, Boston, MA, USA). Plasmids were obtained from the Addgene repository (Cambridge, MA, USA).
Cell culture
Cells were cultured in RPMI 1640 medium (1 μg/ml folic acid) supplemented with fetal bovine serum (FBS) at 10% (v/v; Jurkat, human PBMCs) or 20% (v/v; FLSs), 1% (v/v) penicillin/streptomycin and 1% (v/v) l-glutamine at 37°C in a humidified atmosphere of 5% CO2. Jurkat T cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). FLSs from patients with RA were a generous gift from Dr James W. Thomas (Vanderbilt University). FLSs were isolated from patients with RA undergoing joint replacement surgery. Experiments were performed using established methodologies found in previous studies [24, 25]. Isolated PBMCs were activated with plate-bound anti-CD3 (10 μg/ml; OKT3 Clone; ATCC) in complete RPMI 1640 medium supplemented with soluble anti-CD28 (1 μg/ml; BD Biosciences) for 72 h.
Transient transfections and luciferase measurements
Jurkat T cells were transfected using diethylaminoethyl (DEAE)–dextran. Cells were incubated for 10 min at room temperature with 1 μg of NF-κB-luc construct per 1.0 × 106 cells in a solution of 0.5 mg/ml DEAE–dextran in Tris-buffered saline. Cells were resuspended in complete RPMI 1640 culture medium with 100 μM chloroquine diphosphate and incubated at 37°C for 1 h. Immediately following chloroquine treatment, cells were washed and resuspended in complete culture medium and incubated overnight prior to further experimental treatment. FLS and PBMC cultures were transfected using Lipofectamine-2000 (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s protocol. Plasmid amounts were equalized across transfections. Luciferase was measured using Steady-Glo (E2510; Promega, Madison, WI, USA) according to the manufacturer’s protocol on a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA, USA).
RNA isolation, cDNA synthesis and real-time PCR
Total RNA was isolated using Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA), purified with the RNeasy MinElute Cleanup Kit (Qiagen, Germantown, MD, USA) and quantified using a Nano Drop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was reverse transcribed from 5 μg of total RNA using the SuperScript III First-Strand Synthesis Kit (Life Technologies) using oligo(dT) as the primer and purified using the Qiagen QiaQuick PCR purification kit. Real-time qPCR (ABI-7300 Real Time PCR System; Applied Biosystems, Carlsbad, CA, USA) was performed in duplicate using TaqMan gene expression assays in volumes of 25 μl with 50 ng of cDNA and TaqMan Gene Expression Master Mix (Applied Biosystems). Fold change expression levels were determined by the ΔΔCT method comparing expression of test genes with that of GAPDH.
Flow cytometry
Cells were suspended in PBS with 10% FBS and 0.1% sodium azide. For intracellular protein determinations, cells were fixed with BD Cytofix Buffer, permeabilized using BD Phospho Perm/Wash Buffer (BD Biosciences) and labelled with primary antibodies overnight at 4°C. The following morning, cells were washed and incubated with an FITC-labelled secondary antibody and cell surface marker (where noted) at 4°C for 1 h as described previously [18, 19]. Cells were analysed using a three-laser BD LSRII flow cytometer at the Vanderbilt Medical Center Flow Cytometry Core facility (Nashville, TN, USA). Supplemental analysis was performed using FlowJo (TreeStar, Ashland, OR, USA).
Study populations
The study group consisted of control subjects with no current chronic or acute infection and no family history of autoimmune disease and patients meeting the ACR/European League Against Rheumatism (EULAR) classification criteria for RA. Table 1 summarizes the demographic and clinical characteristics of the subject populations. PBMCs were isolated using sodium heparin cell preparation tubes (BD Biosciences) according to the supplied protocol. The study was approved by the Vanderbilt University Medical Center and Penn State Milton S. Hershey Medical Center Institutional Review Boards. Written informed consent was obtained at the time of blood draw.
Table 1.
Demographic characteristics of the RA patients and healthy controls and clinical characteristics of the RA patients
| Controls (n = 29) | RA, MTX treatment (n = 8) | RA, no MTX treatment (n = 8) | |
|---|---|---|---|
| Age, mean (s.d.), years | 38 (11) | 49 (11) | 52 (15) |
| Female | 100 | 88 | 100 |
| Ethnicity | |||
| Caucasian | 93 | 75 | 88 |
| African American | 0 | 0 | 12 |
| Hispanic | 7 | 25 | 0 |
| Asian | 0 | 0 | 0 |
| Clinical characteristics | |||
| Disease duration, mean (s.d.), years | — | 12 (4) | 11 (7) |
| Active diseasea | — | 63 | 88 |
| Early RA (disease duration <1 year) | — | 13 | 25 |
| Treatment | |||
| HCQ | — | 50 | 38 |
| Steroids | — | 38 | 63 |
| TNF inhibitors | — | 25 | 13 |
Except where indicated otherwise, values are given as a percentage. aDefined as the presence of at least three of the following: morning stiffness >45 min, >3 swollen joints, >6 tender joints and ESR >28 mm/h. The mean disease activity score for RA subjects is 4.7 (s.d. 0.2) with no MTX and 4.9 (s.d. 0.4) with MTX.
Statistical analysis
Data are expressed as the mean (s.d.) of three or more independent experiments. Significance was determined by Student’s t-test using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). P-values <0.05 were considered significant.
Results
MTX reduces NF-κB activity in Jurkat cells
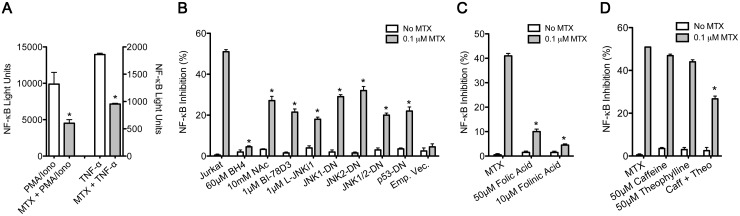
To determine the effects of MTX on NF-κB activation, an NF-κB reporter construct was transfected into Jurkat T cells. Transfected cells were treated for 48 h with 0.1 μM MTX and stimulated with either phorbol 12-myristate 13-acetate (PMA, 50 nM) and ionomycin (1 μM) or 5 ng of TNF-α for 24 h. We found that MTX markedly reduced stimulation of NF-κB activity in response to either PMA/ionomycin or TNF-α (Fig. 1A). MTX-mediated inhibition of NF-κB activation by TNF-α was significantly reversed by supplementation of cultures with BH4, the free radical scavenger N-acetyl cysteine (NAc), JNK inhibitors, BI-78D3 or l-JNKi1 or by transient transfection of JNK1-, JNK2- or p53-DN expression vectors (Fig. 1B). JNK inhibitors BI-78D3 and pepJIP1 (L-JNKi1) target the JNK-JNK-interacting protein 1 (JIP1) binding site and prevent JNK phosphorylation [26, 27]. Consistent with our previous studies, we concluded that MTX-mediated inhibition of NF-κB activation by TNF-α resulted from MTX-dependent BH4 depletion, leading to increased ROS production, JNK activation and JNK-dependent induction of p53, which is the final mediator of inhibition of NF-κB activation.
Fig. 1.
Inhibition of NF-κB activation by MTX
(A–D) Jurkat (JKT) cells containing an NF-κB-luciferase reporter were cultured with 0.1 μM MTX for 48 h and stimulated with (A) PMA (50 nM) and ionomycin (1 μM) 6 h or (A–D) TNF-α (5 ng) 24 h prior to luciferase measurements. (B) MTX-treated JKT cells were cultured with or without BH4, N-acetyl-l-cysteine (NAC) or JNK inhibitors BI-78D3 or L-JNKi1. JNK1-DN, JNK2-DN, p53-DN or empty vector plasmids with a green fluorescent protein (GFP) plasmid were transiently transfected into JKT cells. (C and D) MTX-treated JKT cells were treated with (C) folic or folinic acid or (D) adenosine receptor antagonists caffeine and/or theophylline. Values are mean (s.d.). (A) *P < 0.05 vs PMA/ionomycin- or TNF-α-treated cultures. (B–D) *P < 0.05 vs cultures stimulated with MTX alone. Iono: ionomycin; PMA: phorbol 12-myristate 13-acetate; Theo: theophylline; NF-κB: nuclear factor κB; JNK: Jun-N-terminal kinase.
We also tested the ability of folic and folinic acid to reverse MTX-mediated inhibition of NF-κB activation by TNF-α. Supplementation of cultures with either folic acid or folinic acid blocked inhibition of NF-κB activation by MTX (Fig. 1C). BH2 and folate are converted to BH4 through a salvage pathway regulated by DHFR expression [28, 29]. Blockade of DHFR by MTX depletes tetrahydrofolate levels and decreases cellular amounts of BH4. Supplementation of MTX-treated cultures with folic acid and/or folinic acid increases intracellular BH4 bioavailability [17]. MTX also has been shown to stimulate the release of adenosine and activate adenosine receptors. Therefore we examined the ability of two non-selective adenosine receptor antagonists, caffeine and theophylline, to reverse the effects of MTX. Treatment of cells with MTX and either caffeine or theophylline alone at pharmacological concentrations did not reverse MTX-mediated inhibition of NF-κB activation (Fig. 1D). However, incubation of cells with MTX and the combination of caffeine and theophylline significantly reduced the inhibitory effects of MTX. We interpret these results to suggest that the release of adenosine and adenosine receptor activation also contributed to MTX-mediated inhibition of NF-κB activation.
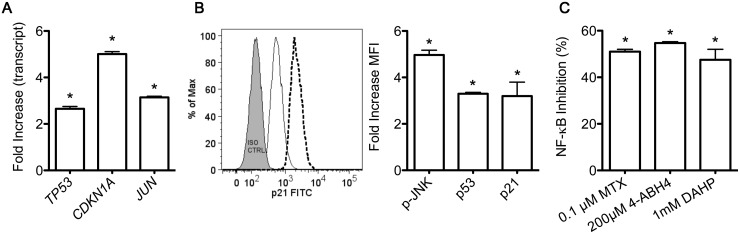
Induction of TP53, CDKN1A and JUN by the 4-amino analogue of BH4
Given our findings that MTX inhibits NF-κB through blockade of BH4 biosynthesis, we investigated whether pterin-site inhibitors of NOS also inhibited NF-κB activation. One such inhibitor is 4-aminotetrahydrobiopterin (4-ABH4). Jurkat cells treated for 48 h with 4-ABH4 show increased TP53, CDKN1A and JUN expression levels and corresponding increases in phosphorylated JNK, p53 and p21 protein (Fig. 2A and B), closely mirroring the stimulatory activity of MTX. We also determined whether 4-ABH4 inhibited TNF-dependent NF-κB activation in T cells. We found that 4-ABH4 decreased TNF-induced NF-κB activity to a level similar to MTX (Fig. 2C). As an additional experimental comparator, we used diamino-hydroxypyrimidine (DAHP), which inhibits guanosine triphosphate (GTP) cyclohydrase 1, the rate-limiting enzyme in BH4 synthesis [30], and found that DAHP also significantly reduced NF-κB activation. Thus both a BH4 antagonist and an inhibitor of BH4 synthesis stimulated a pathway in T cells similar to that stimulated by MTX, leading to inhibition of activation of NF-κB by TNF-α.
Fig. 2.
Increased expression of p-JNK, p53 and p21 and inhibition of NF-κB activation by a BH4 antagonist or an inhibitor of BH4 synthesis
(A–C) Jurkat cells were treated with the BH4 antagonist 4-ABH4 (200 μM) for 48 h and (A) transcript levels of TP53, CDKN1A and JUN were measured by quantitative PCR. Results are expressed as fold induction relative to GAPDH. (B) Levels of p-JNK, p53 and p21 protein were determined by flow cytometry. A representative flow diagram shows background fluorescence (grey) and results obtained with untreated (solid line) or 4-ABH4-treated (dashed line) Jurkat cells. (C) MTX-, 4-ABH4- or DAHP-treated Jurkat cells were stimulated with TNF-α for 24 h prior to luciferase measurements. Results are expressed as percentage inhibition of TNF-α-stimulated NF-κB activity in relative light units. Values are mean (s.d.). *P < 0.05 vs untreated cells. NF-κB: nuclear factor κB; JNK: Jun-N-terminal kinase; DAHP: 2,4-diamino-6-hydroxypyrimidine; BH4: tetrahydrobiopterin.
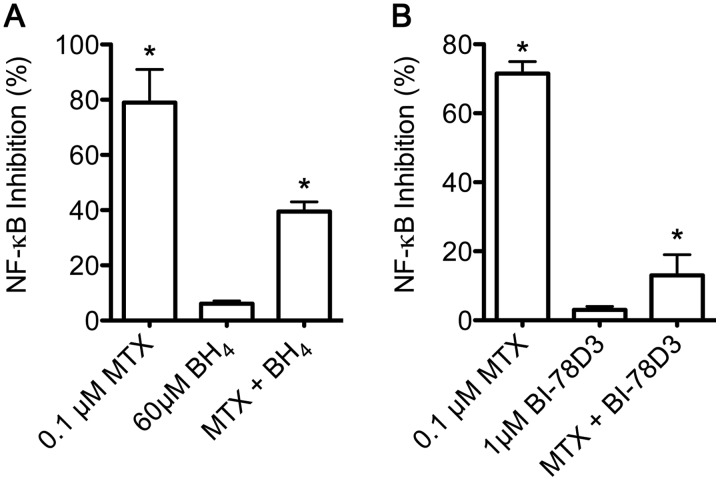
Inhibition of NF-κB activity by MTX in activated T cells
In Jurkat cells, MTX decreases TNF-α-dependent activation of NF-κB. This effect is reversed by supplementing cultures with BH4 or inhibiting activation of JNK using specific JNK inhibitors (Fig. 1B). We further examined these effects in activated human T cells (see Methods). T cells were treated with MTX in the presence or absence of BH4 (Fig. 3A) or the JNK inhibitor BI-78D3 (Fig. 3B). In T cells we found that MTX reduced TNF-α-dependent activation of NF-κB. In contrast to the Jurkat cell experiments, the effects of MTX on NF-κB were more pronounced in activated T cells, with inhibition averaging >70%. Mirroring the Jurkat cell findings, the inhibitory effects of MTX on NF-κB activation were abrogated by the addition of BH4 or BI-78D3 to the MTX-treated PBMC cultures (Fig. 3A and B). Thus, in both the Jurkat T cell line and primary human T cells, we conclude that MTX reduced NF-κB luciferase activity via loss of BH4 and activation of JNK.
Fig. 3.
MTX inhibits NF-κB in activated T cells via depletion of BH4 and increased JNK activation
(A and B) Activated T cells were treated with 0.1 μM MTX for 24 h with or without the addition of (A) BH4 or (B) JNK inhibitor BI-78D3. Values are mean (s.d.). *P < 0.05 vs unstimulated cultures or cultures treated with MTX. NF-κB: nuclear factor κB; JNK: Jun-N-terminal kinase; BH4: tetrahydrobiopterin.
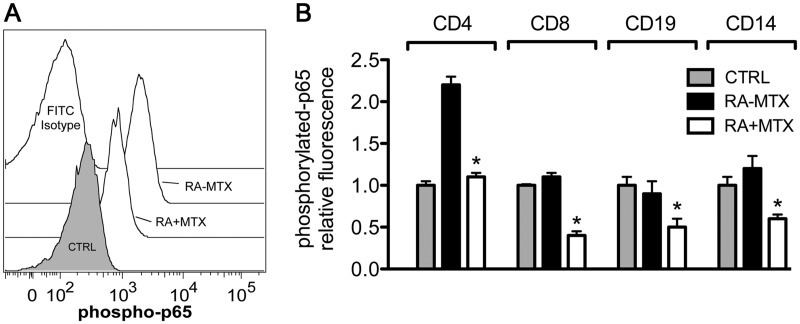
MTX corrects elevated P-p65 levels in RA subjects in vivo
Since MTX reduced activation of NF-κB by TNF-α in T cells, we sought to examine the basal level of NF-κB activation in subjects with RA. For these studies we obtained PBMCs from healthy control subjects and patients fulfilling the ACR/EULAR criteria for RA. Subjects with RA were divided into those receiving or not receiving MTX therapy. PBMCs were isolated, washed, fixed, permeabilized and incubated with a primary antibody specific for P-p65. Gating on the CD4+ T cells, we found in control subjects that the mean fluorescence intensity was only slightly above background (Fig. 4A and B). In contrast, we detected increased P-p65 levels in RA subjects not receiving low-dose MTX as therapy. RA patients receiving once-weekly MTX exhibited decreased P-p65 levels, similar to controls. We further examined additional PBMC populations, including CD8+ T cells, CD19+ B cells and CD14+ monocytes. While RA subjects not receiving MTX did not exhibit increased levels of P-p65 in these cell subsets, the cohort of RA subjects receiving MTX therapy exhibited decreased levels of P-p65 in each of the cell types examined (Fig. 4B). We conclude from these studies that RA subjects not receiving MTX exhibit chronic activation of NF-κB in CD4+ T cells compared with controls. Chronic activation of NF-κB in the CD4+ T cell compartment was markedly reduced in RA subjects on stable MTX therapy to near control values. Further, MTX lowers P-p65 levels in multiple cell types in vivo.
Fig. 4.
Levels of P-p65 in PBMCs from control subjects or subjects with RA receiving or not receiving MTX therapy in vivo
(A and B) P-p65 protein concentrations in healthy control subjects (CTRL) and RA patients receiving MTX (RA + MTX) and not receiving MTX (RA − MTX). PBMCs were fixed, permeabilized and stained for CD4, CD8, CD19 and CD14 cell surface markers and intracellular levels of P-p65 were determined by flow cytometry. (A) A representative flow diagram with gating on CD4+ T cells is shown along with (B) quantification of relative P-p65 fluorescence across the indicated cell populations. Values are mean (s.d.). *P < 0.05. PBMCs: peripheral blood mononuclear cells.
MTX-mediated inhibition of NF-κB activation in FLSs
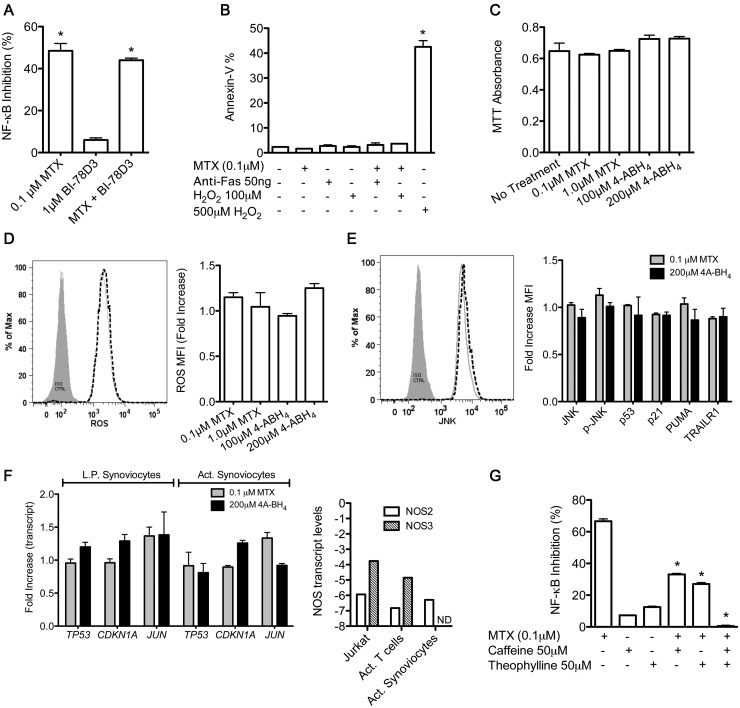
Both lymphocytes and FLSs are key effector cells in RA pathogenesis. Therefore we determined if MTX also inhibited TNF-α-mediated NF-κB activation low-passage FLSs. FLSs were transfected with the NF-κB luciferase reporter construct using lipofectamine and treated with 0.1 μM MTX for 48 h. As in T cells, MTX inhibited TNF-α-dependent activation of NF-κB by ∼50% (Fig. 5A). However, in contrast to T cells, JNK inhibition did not attenuate inhibition of NF-κB activation by MTX. In T cells, MTX induces cell cycle checkpoints and increases sensitivity to apoptosis via a JNK-dependent pathway [18, 19]. To examine these responses in FLSs, we measured apoptosis by annexin V labelling. FLSs were treated with MTX for 48 h and exposed to either anti-Fas or H2O2 for an additional 24 h as extrinsic or intrinsic mediators of apoptosis, respectively. In contrast to T cells, FLSs did not exhibit increased levels of apoptosis after treatment with MTX followed by either anti-Fas or H2O2 (Fig. 5B). Further, treatment of FLSs with MTX or 4-ABH4 did not reduce cell numbers as measured by the MTT assay (Fig. 5C). Levels of ROS or pro-apoptotic proteins as assessed by flow cytometry were also not increased in FLSs by either MTX or 4A-BH4 (Fig. 5D and E). Finally, we performed mRNA measurements of low-passage or activated FLSs and found that MTX or 4-ABH4 did not increase transcript levels of TP53, CDKN1A or JUN, which is in marked contrast to the effects seen in T cells reported previously (Fig. 5F, left panel) [19]. One possible explanation for the failure of MTX to activate these ROS-/JNK-dependent pathways in FLSs as is seen in both Jurkat and primary T cells is if FLSs had significantly lower levels of NOS enzymes compared with T cells. To test this possibility, we compared NOS2 and NOS3 transcript levels among Jurkat T cells, primary activated T cells from healthy donors and FLSs. We found that Jurkat T cells and primary T cells expressed much higher NOS2 and NOS3 transcripts compared with FLSs (Fig. 5F, right panel). Therefore failure to activate these ROS-/JNK-dependent pathways in FLSs is probably due to low levels of NOS enzymes in FLSs, thus preventing sufficient NOS-dependent ROS synthesis to activate JNK-dependent pathways.
Fig. 5.
Reversal of MTX-mediated inhibition of NF-κB activation by adenosine receptor antagonists in FLSs
(A–G) FLSs were treated with the indicated concentrations of MTX or 4-ABH4 for 48 h. (A) FLSs were transfected with an NF-κB luciferase reporter construct and treated with MTX ± BI-78D3. (B) FLSs were cultured with MTX followed by culture with anti-Fas antibody or H2O2 for an additional 6 h. The percentage of annexin V–positive cells was determined by flow cytometry. (C) FLS proliferation evaluated by MTT assay. (D) Synthesis of ROS was determined by labelling FLSs with 5-(and 6)-chloromethyl-2',7'-dichlorohydrofluorescein diacetate (CM-H2DCFDA) and flow cytometry. (E) Levels of JNK, p-JNK, p53, p21, PUMA and TRAILR1 in MTX- and 4A-BH4-treated FLSs were determined by flow cytometry and are reported as the fold increase compared with untreated FLSs. (F) Transcript measurements: (left panel) TP53, CDKN1A and JUN in MTX- or 4-ABH4-treated low-passage FLSs or activated FLSs stimulated with IL-1β (2 ng/ml), TNF-α (50 ng/ml) and LPS (1 μg/ml) for 24 h; (right panel) NOS2 or NOS3 mRNA levels for the indicated cell type. (G) FLSs transiently transfected with an NF-κB luciferase reporter were treated with MTX ± adenosine receptor antagonists caffeine and/or theophylline for 48 h and stimulated with TNF-α 24 h prior to luciferase measurements. Values are mean (s.d.). (A–F) *P < 0.05 vs untreated FLSs. (G) *P < 0.05 vs MTX-treated FLSs. FLS: fibroblast-like synoviocyte; NF-κB: nuclear factor κB; JNK: Jun-N-terminal kinase; BH4: tetrahydrobiopterin.
Given these findings, we sought alternative explanations for decreased NF-κB activation in MTX-treated FLSs. Therefore we examined the effects of adenosine receptor antagonists caffeine and/or theophylline at pharmacological concentrations. We found that the combination of caffeine and theophylline markedly abrogated inhibition of NF-κB activation by MTX (Fig. 5G). Thus, in contrast to T cells, MTX appears to mediate inhibition of NF-κB activation in FLSs by stimulating adenosine release.
Discussion
Our current studies coupled with previous studies indicate that MTX-mediated inhibition of DHFR initiates two parallel anti-inflammatory pathways: (i) inhibition of BH2 reduction to BH4 leading to iNOS uncoupling, ROS production, JNK activation and downstream effects and (ii) AICAR-dependent adenosine release and activation of adenosine receptors. Both pathways ultimately lead to inhibition of stimulus-dependent activation of NF-κB, which most likely is a major contributor to the activity of MTX in RA. Interestingly, these two pathways seem to be activated in a cell-type-specific manner. The BH2/BH4 pathway seems to predominate in lymphocytes, while the adenosine receptor activation pathway is the predominant pathway in FLSs (see supplementary Fig. S1, available at Rheumatology Online). A major difference between the two cell lineages is that FLSs express extremely low levels of NOS enzymes compared with T cells, which could explain the failure to activate the BH2/BH4 pathway in FLSs.
A BH4 antagonist or inhibition of BH4 synthesis also stimulates ROS production, JNK activation and downstream effector pathways similarly to MTX. In activated T cells, inhibition of BH4 synthesis also decreases production of the pro-inflammatory Th1-associated cytokine IFN-γ and increases production of the anti-inflammatory Th2 cytokine IL-4 [30, 31]. MTX induces a similar shift and reduces expression of pro-inflammatory cytokines IL-1, IL-2, IL-6 and IFN-γ and increases expression of anti-inflammatory cytokines such as IL-4 and IL-10 in subjects with RA [32]. Thus we would argue that inhibition of BH4 synthesis by MTX might explain the pro-inflammatory to anti-inflammatory shift found in RA.
Of particular interest is the association between MTX, p53 and NF-κB. While NF-κB is well established as a transcription factor that promotes cell proliferation and inflammation and p53 is well established as a transcription factor that inhibits cell proliferation and induces apoptosis, it is becoming increasingly apparent that p53 also inhibits inflammation [33–37]. RA is characterized by both p53 deficiency and elevated NF-κB activity. MTX both in vivo and in cell culture reverses these defects, and inhibition of NF-κB by MTX is dependent on p53 induction in cell culture [35, 38–44]. Whether or not p53 deficiency and elevated NF-κB activity in RA are mechanistically linked is not known. Thus p53 deficiency may contribute to elevated NF-κB activity in RA. The opposite is also possible, elevated NF-κB activity in RA may contribute to depressed p53 activity in RA. In this regard, longitudinal studies in RA that examine responses to MTX, TNF-α inhibitors and their combination would be informative.
Importantly, MTX also inhibits NF-κB activity in FLSs. MTX inhibits production of inflammatory mediators such as metalloproteinases and IL-6, and expression of these proteins is known to require activation of NF-κB [45]. In contrast to T cells, inhibition of NF-κB activity in FLSs is independent of p53 induction but appears dependent upon adenosine release and activation of adenosine receptors. Thus these results are consistent with a model whereby MTX derives its anti-inflammatory effects, at least in part, from its ability to inhibit activity of the pro-inflammatory transcription factor NF-κB. Therefore distinct cell-type-specific pathways are utilized to achieve this beneficial outcome in RA.
Apart from RA, connections between p53 and NF-κB have been experimentally demonstrated in cancer models and in mouse endotoxaemia. Overexpression of wild-type p53 in human colon cancer cells reduces endogenous levels of NF-κB activity and restores the ability of these cells to undergo apoptosis [34]. Further, glucocorticoids, which inhibit inflammation, block NF-κB activity through a p53-dependent process. Mortality from LPS-induced endotoxaemia, a process critically dependent upon NF-κB activity, is markedly increased in mice that lack p53. The conclusion from these studies, closely mirroring our own work, is that p53 mediates repression of NF-κB [46].
Rheumatology key messages.
MTX inhibits NF-κB activity in T cells via BH4 depletion and activation of JNK and p53.
MTX inhibits NF-κB activity in fibroblast-like synoviocytes via release of adenosine and adenosine receptor activation.
MTX normalizes elevated NF-κB activity in RA in vivo.
Supplementary Material
Acknowledgements
The Vanderbilt Medical Center Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center [National Institutes of Health (NIH) grant P30 CA68485] and the Vanderbilt Digestive Research Center (NIH grant DK058404). We thank Cheri Stewart and members of the Clinical Trials Center at Vanderbilt University Medical Center for assisting with the collection of patient samples. We also thank John Tossberg at Vanderbilt University Medical Center and Carl McAloose at Penn State Milton S. Hershey Medical Center for technical assistance. Author contributions: C.F.S. and T.M.A. conceived and designed the experiments; C.F.S., H.M.G., C.J.B. and B.C.W. performed the experiments; C.F.S, H.M.G., C.J.B., B.C.W. and T.M.A. analysed the data; N.J.O. acquired patient data and participated in preparation of the article; C.F.S. and T.M.A. wrote the paper. All authors read and approved the final document.
Funding: This work was supported by grants from the National Institutes of Health (R21AR063846, R42AI53948, R01AI044924), the American College of Rheumatology Within Our Reach grant program (ACR124405), the National Center for Advancing Translation Sciences (UL1TR000445) and the National Science Foundation Graduate Research Fellowship Program.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–55. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309:1094–104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- 3.Williams HJ, Willkens RF, Samuelson COJ, et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum. 1985;28:721–30. doi: 10.1002/art.1780280702. [DOI] [PubMed] [Google Scholar]
- 4.Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005;57:163–72. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- 5.Halilova KI, Brown EE, Morgan SL, et al. Markers of treatment response to methotrexate in rheumatoid arthritis: where do we stand? Int J Rheumatol. 2012;2012:978396. doi: 10.1155/2012/978396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan SLC, Cronstein BN. Methotrexate-how does it really work? Nat Rev Rheumatol. 2010;6:175–8. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 7.Montesinos MC, Desai A, Delano D, et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003;48:240–7. doi: 10.1002/art.10712. [DOI] [PubMed] [Google Scholar]
- 8.Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65:168–73. [PubMed] [Google Scholar]
- 9.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–82. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronstein BN. Molecular therapeutics. Methotrexate and its mechanism of action. Arthritis Rheum. 1996;39:1951–60. doi: 10.1002/art.1780391203. [DOI] [PubMed] [Google Scholar]
- 11.Cronstein BN. Molecular mechanism of methotrexate action in inflammation. Inflammation. 1992;16:411–23. doi: 10.1007/BF00918968. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar S, Aggarwal BB. Methotrexate suppresses NF-κB activation through inhibition of IκBα phosphorylation and degradation. J Immunol. 2001;167:2911–20. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- 13.Morgan SL, Baggott JE, Vaughn WH, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med. 1994;121:833–41. doi: 10.7326/0003-4819-121-11-199412010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2005;102:9056–61. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284:12691–700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree MJ, Tatham AL, Al-Wakeel Y, et al. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–44. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009;284:28128–36. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spurlock CF, III, Aune ZT, Tossberg JT, et al. Increased sensitivity to apoptosis induced by methotrexate is mediated by JNK. Arthritis Rheum. 2011;63:2606–16. doi: 10.1002/art.30457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spurlock CF, III, Tossberg JT, Fuchs HA, Olsen NJ, Aune TM. Methotrexate increases expression of cell cycle checkpoint genes via JNK activation. Arthritis Rheum. 2012;64:1780–9. doi: 10.1002/art.34342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atreya I, Atreya R, Neurath MF. NF-κB in inflammatory bowel disease. J Intern Med. 2008;263:591–6. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 21.Oikonomidou O, Vlachoyiannopoulos PG, Kominakis A, et al. Glucocorticoid receptor, nuclear factor κB, activator protein-1 and C-jun N-terminal kinase in systemic lupus erythematosus patients. Neuroimmunomodulation. 2006;13:194–204. doi: 10.1159/000100474. [DOI] [PubMed] [Google Scholar]
- 22.Karin M, Cao Y, Greten FR, Li ZW. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 23.Rudwaleit M, Yin Z, Siegert S, et al. Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor alpha, increase of interleukin 10, and predicted by the initial concentration of interleukin 4. Ann Rheum Dis. 2000;59:311–4. doi: 10.1136/ard.59.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J Immunol. 2005;174:4301–6. doi: 10.4049/jimmunol.174.7.4301. [DOI] [PubMed] [Google Scholar]
- 25.Alvaro-Gracia JM, Zvaifler NJ, Brown CB, Kaushansky K, Firestein GS. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991;146:3365–71. [PubMed] [Google Scholar]
- 26.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 27.Stebbins JL, De SK, Machleidt T, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci USA. 2008;105:16809–13. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L, Chalupsky K, Stefani E, Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol. 2009;47:752–60. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams EA, Morrison JF. Human dihydrofolate reductase: reduction of alternative substrates, pH effects, and inhibition by deazafolates. Biochemistry. 1992;31:6801–11. doi: 10.1021/bi00144a022. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Li L, Brod T, et al. The role of increased guanosine triphosphate cyclohydrolase-1 expression and tetrahydrobiopterin levels upon T cell activation. J Biol Chem. 2011;286:13846–51. doi: 10.1074/jbc.M110.191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 32.Hobl EL, Mader RM, Erlacher L, et al. The influence of methotrexate on the gene expression of the pro-inflammatory cytokine IL-12A in the therapy of rheumatoid arthritis. Clin Exp Rheumatol. 2011;29:963–9. [PubMed] [Google Scholar]
- 33.Ak P, Levine AJ. p53 and NF-κB: different strategies for responding to stress lead to a functional antagonism. FASEB J. 2010;24:3643–52. doi: 10.1096/fj.10-160549. [DOI] [PubMed] [Google Scholar]
- 34.Shao J, Fujiwara T, Kadowaki Y, et al. Overexpression of the wild-type p53 gene inhibits NF-κB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000;19:726–36. doi: 10.1038/sj.onc.1203383. [DOI] [PubMed] [Google Scholar]
- 35.Cooks T, Pateras IS, Tarcic O, et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–46. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisz L, Damalas A, Liontos M, et al. Mutant p53 enhances nuclear factor kappaB activation by tumor necrosis factor alpha in cancer cells. Cancer Res. 2007;67:2396–401. doi: 10.1158/0008-5472.CAN-06-2425. [DOI] [PubMed] [Google Scholar]
- 37.Squatrito M, Brennan CW, Helmy K, et al. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell. 2010;18:619–29. doi: 10.1016/j.ccr.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komarova EA, Krivokrysenko V, Wang KH, et al. p53 is a suppressor of inflammatory response in mice. FASEB J. 2005;19:1030–2. doi: 10.1096/fj.04-3213fje. [DOI] [PubMed] [Google Scholar]
- 39.Seemayer CA, Kuchen S, Neidhart M, et al. p53 in rheumatoid arthritis synovial fibroblasts at sites of invasion. Ann Rheum Dis. 2003;62:1139–44. doi: 10.1136/ard.2003.007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-κB and its relevance to arthritis and inflammation. Rheumatology. 2008;47:584–90. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 42.Handel ML, McMorrow LB, Gravallese EM. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–70. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- 43.Firestein GS. NF-κB: Holy Grail for rheumatoid arthritis? Arthritis Rheum. 2004;50:2381–6. doi: 10.1002/art.20468. [DOI] [PubMed] [Google Scholar]
- 44.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohya N, Yamada H, Hama N, Kikukawa T, Ichikawa Y. Methotrexate inhibits IL-6 and matrix metalloproteinase-2 production by mixed synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:S196. [Google Scholar]
- 46.Murphy SH, Suzuki K, Downes M, et al. Tumor suppressor protein (p)53 is a regulator of NF-κB repression by the glucocorticoid receptor. Proc Natl Acad Sci USA. 2011;108:17117–22. doi: 10.1073/pnas.1114420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.