Abstract
Rheumatologists have long considered OA and PsA as two completely distinct arthropathies. This review highlights how some forms of generalized OA and PsA may afflict the same entheseal-associated anatomical territories. While degeneration or inflammation may be clearly discernible at the two extremes, there may be a group of patients where differentiation is impossible. Misdiagnosis of a primary degeneration-related pathology as being part of the PsA spectrum could lead to apparent failure of disease-modifying agents, including apparent anti-TNF and apparent IL23/17 axis therapy failure. This is not a reflection of poor clinical acumen, but rather a failure to appreciate that the pathological process overlaps in the two diseases. Whether the category of OA–PsA overlap disease exists or whether it represents the co-occurrence of two common arthropathies that afflict the same anatomical territories has implications for the optimal diagnosis and management of both OA and PsA.
Keywords: osteoarthritis, psoriatic arthritis, pathogenesis, therapy, enthesitis
Introduction
It is now recognized that sustained attention and focus on a particular object is paradoxically associated with impaired perception. In more colloquial terms this translates into the scenario whereby the harder you look, the less you see [1].
Indeed, with respect to both OA and PsA, rheumatologists have considered these as two completely distinct arthropathies for well over 60 years. Simplistically put, OA has been conceptualized in relation to joint degeneration, whereas PsA has been primarily viewed in terms of joint inflammation [2, 3]. While we recognize that the characteristic features of OA and PsA exist, it is not within the remit of this article to highlight these distinguishing features. OA and PsA can have a heterogeneous nature of disease classification, therefore this article does not assume all types of OA and PsA are comparable [4]. The purpose of this article is to synthesize clinical observations, epidemiology, emerging imaging data and molecular and experimental data in both OA and PsA to highlight that, in certain circumstances, differentiation may be impossible based on existing clinical, imaging and emerging genetic and molecular criteria. Whether this merely represents the co-occurrence of two fairly common arthropathies or raises the possibility of some type of overlap disease is discussed in relation to diagnosis and management (Fig. 1).
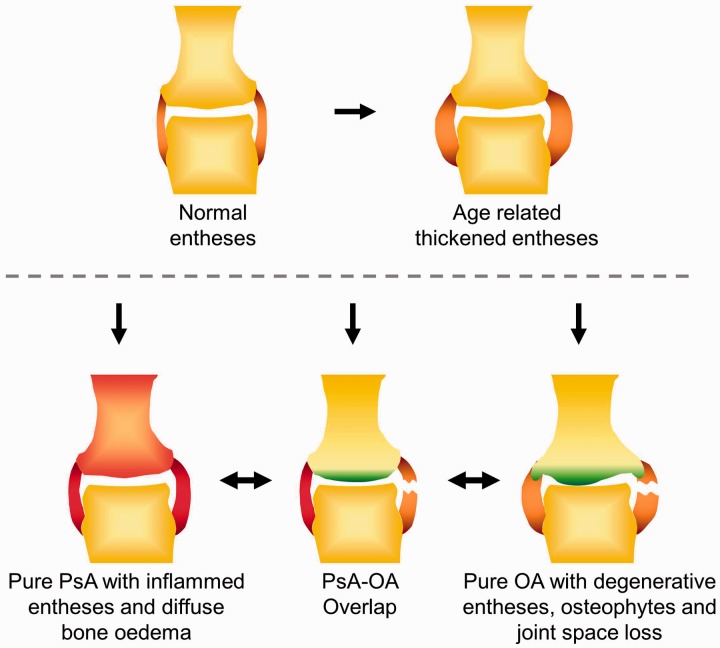
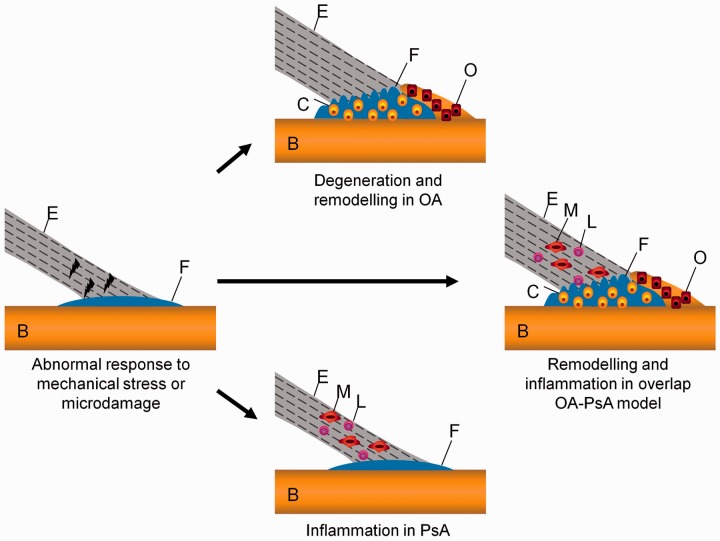
Fig. 1.
The OA and PsA overlap model and variation of the enthesis with pathology
Normal entheses are usually observed in individuals <40 years of age. Thickened entheses are often seen with age with no arthritis, but similar appearances are also evident in early OA or in asymptomatic joints of OA. Classical inflamed entheses are seen in most PsA joints, where there can be diffuse bone oedema on MRI, while classical degenerative entheses are typical of advanced OA joints often accompanied by osteophytes and cartilage loss with joint space narrowing. However, there is a subset of joints where there are overlapping features that can cause a diagnostic challenge, often with some degree of inflammation or degenerative changes that can be accepted as either OA or PsA.
Underpinning pathological concepts for common ground between OA and PsA
It was proposed more than a decade ago that enthesitis may be the primary lesion in PsA and SpA [5, 6]. A number of imaging studies have since emerged showing that enthesopathy is common in psoriasis patients without clinical arthritis [7–10]. Although it is difficult to prove the primacy of enthesitis in human PsA in all patients at all sites of disease, several animal models with features of PsA or SpA can clearly be shown to start at the enthesis [11–14]. Likewise, several experimental studies have shown that spontaneous knee OA can start in the ligament and enthesis and not the articular cartilage, thus providing proof of principle of similar micro-anatomical topography for disease onset [15–17]. Studies in the generalized form of human hand OA have also shown that the ligaments and enthesis are the sites of the earliest discernible pathology [18, 19]. The importance of the enthesis has also emerged in knee OA in man [20–22]. Collectively this has resulted in a mechanistic anatomical classification of OA that recognizes that the generalized form of disease that was previously designated as idiopathic appears to have an enthesis-associated micro-anatomical basis [4].
The different entheses form what is now known as the synovial–entheseal complex, where both degenerative and inflammatory processes can occur [23, 24]. If the pathological processes share the same micro-anatomical territory, then it is worth exploring anew the features that have been historically used to differentiate OA and PsA.
Clinical observations of overlapping phenotypes in OA and PsA
From an epidemiological perspective, generalized OA, which can affect multiple small and large joints systemically (Fig. 2), typically commences late in the fifth decade, particularly in women, which coincides with the age of menopause, when there is a relative depletion of oestrogen [25]. In a similar vein, PsA typically commences at ∼50 years of age [26, 27]. These clinical observations take on a clearer meaning when interpreted in relation to recent imaging studies. Of particular note, MRI and US studies have both shown that there is an age-related thickening of the normal enthesis and ligaments, often observed after the age of 40 [18, 28], which broadly links entheseal age-related changes and the epidemiology of these arthropathies.
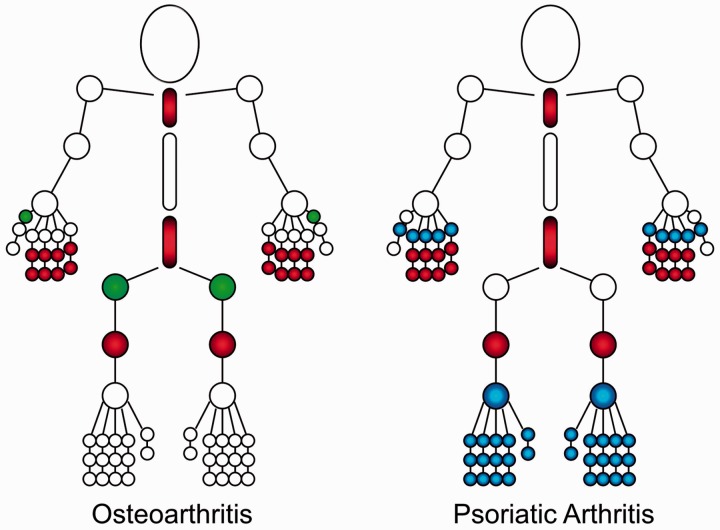
Fig. 2.
Joints commonly affected in OA and PsA
Both OA and PsA can affect similar regions of joints (shaded red), such as the cervical and lumbar spine, distal and PIP joints of the hands, the knees and the first MTP joints. In addition, OA tends to affect the hips and CMC joints (shaded green), while PsA has a predilection for MCP joints, ankles and toes (shaded blue) [110].
Compared with RA, PsA shows a remarkable propensity for disease of the DIP joints, which are typical generalized OA target sites as well [29, 30]. Other joints commonly involved in both OA and PsA include the PIP joints and the large joints of the lower limbs, including the knees (Fig. 2). In the case of axial involvement in PsA, unlike AS, which typically starts from the sacroiliac joints, psoriatic spinal disease has a proclivity for involvement of the cervical spine [31, 32], which is also a common site of degenerative arthritis. In common with SpA in general, OA quite often has propensity for enthesopathies, including lateral epicondylitis, calcaneal enthesophytosis and greater trochanteric involvement with trochanteric bursitis, a type of enthesopathy [33].
Furthermore, a clinical pattern of dactylitis is characteristic of PsA, but in some situations it can be incomplete and characterized by fusiform joint swelling [3]. US and MRI studies of both finger and toe dactylitis have established that SpA-associated dactylitis is predominantly due to flexor tenosynovitis and that enlargement of the joint capsule is not an indispensable condition for the sausage-like feature [34–36]. Although true dactylitis is rare in OA, many patients with OA of the hand often have a diffuse fusiform swelling of the PIP region that rheumatologists historically have called a periarthritis, which in modern parlance equates with extracapsular peri-entheseal pathology [21]. Psoriatic nail changes are well known in PsA [27]; there is also evidence that dystrophic nail changes are more common in OA patients compared with healthy subjects [37, 38]. On nail capillarosopy examination, shorter and tortuous loops of capillaries can be seen in both OA and PsA when compared with healthy individuals [39, 40].
Traditionally, chronic OA can be differentiated from PsA on plain radiograph where there are distinct radiographic features for each [41]. Both arthropathies have the propensity for new bone formation (osteophytes in OA, syndesmophytes and periostitis in PsA) and bone erosions occur in both. However, radiographic imaging occasionally shows enthesophytic bone formation in OA at several sites, including the peripheral joints and the spine [42, 43]. With respect to the enthesophytes in the spine, these tend to be flatter in OA compared with PsA, but an OA variant, diffuse idiopathic skeletal hyperostosis (DISH) [44, 45], is characterized by large flowing osteophytes that are not dissimilar from the pattern of syndesmophytes occasionally noticed in PsA (Fig. 3) [45, 46], therefore it can be difficult to differentiate between the two arthropathies. Indeed, the co-occurrence of the cardinal skeletal manifestation of SpA, namely enthesopathy, and the cardinal feature of OA, namely osteophytes, is firmly established in aged normal skeletons [47]. We previously drew attention to the radiographic appearances of Heberden’s nodes, which are a hybrid combination of distal joint enthesophytes and proximal joint osteophytes both lined by cartilage that combine to form what is in effect a neoarticular surface [19]. At a molecular level, animal models have implicated regulation of the Wnt protein as the target for both these new bone formation processes [48, 49].
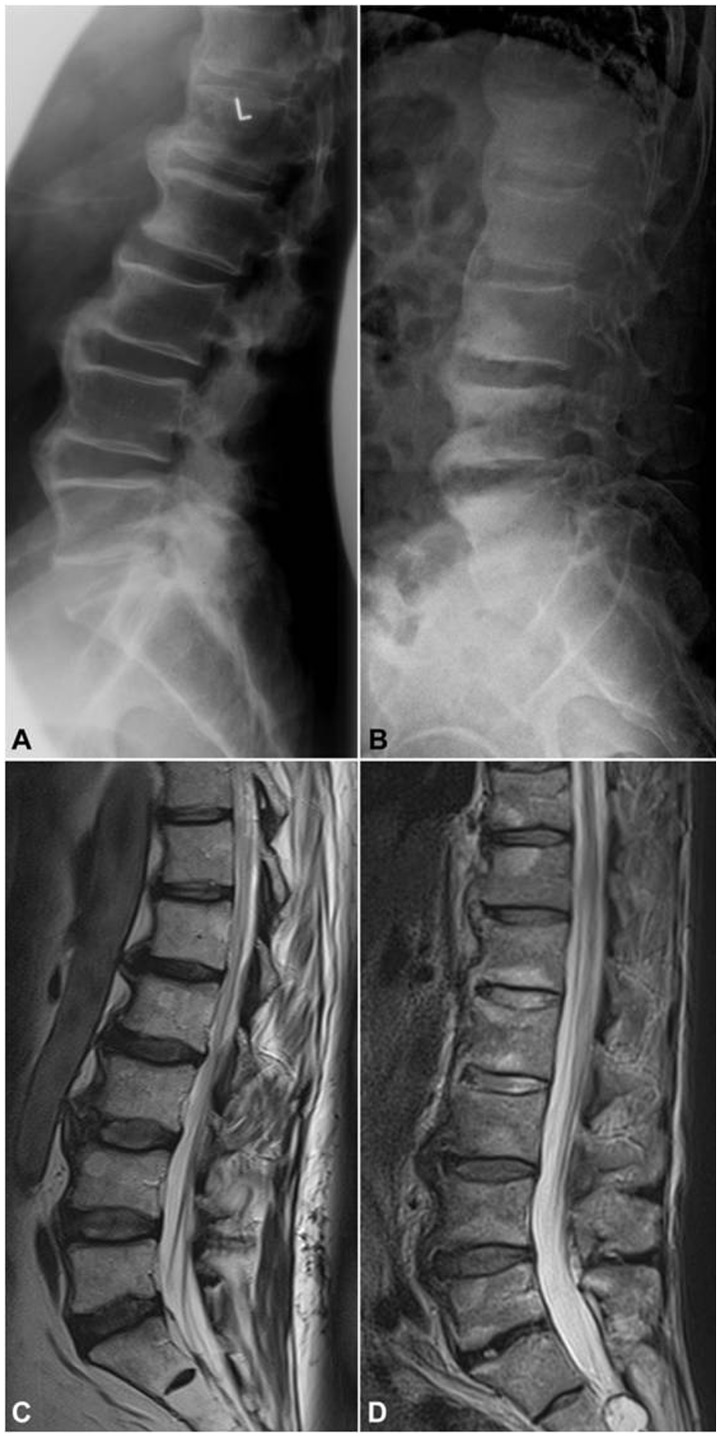
Fig. 3.
Spinal similarities in OA and PsA as seen on imaging
A 69-year-old male with chronic low back pain and gradual stiffening of the spine showing excessive osteophytosis at the lumbar spine as depicted by (A) lateral X-ray and (C) T2-weighted sagittal MRI, compatible with diffuse idiopathic skeletal hyperostosis (DISH), which is an OA variant. In contrast, the 65-year-old male depicted by (B) X-rays and (D) T2-weighted MRI suffers from long-standing psoriatic spondylitis and presents with so called parasyndesmophytes along with inflammatory discal lesions at the lumbar spine. Of note, disc height is well preserved in images of both patients. (B) and (C) courtesy of Dr S. Hermann, Rheumatology, Charité Medical School, Berlin, Germany.
Emerging lessons from MRI pattern of enthesitis in OA and PsA
MRI of peripheral joints has long demonstrated a capsular pattern of inflammation in PsA as well as evidence for diffuse enthesitis related to pathology in a proportion of patients [50] (Fig. 4). Also, it may be impossible to clearly differentiate OA from PsA DIP disease, with both seeming to have prominent abnormalities in the entheses and ligaments [51] (Fig. 4). Even using dynamic contrast enhanced MRI, synovitis assessment as determined by gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) uptake between the two arthropathies was comparable [52]. Studies have noted that enthesitis-related bone oedema seen in PsA is also common in OA [51]. With respect to spinal disease, an association was evident, with bone oedema at the enthesis attachment observed occasionally in healthy subjects, OA and SpA [53].
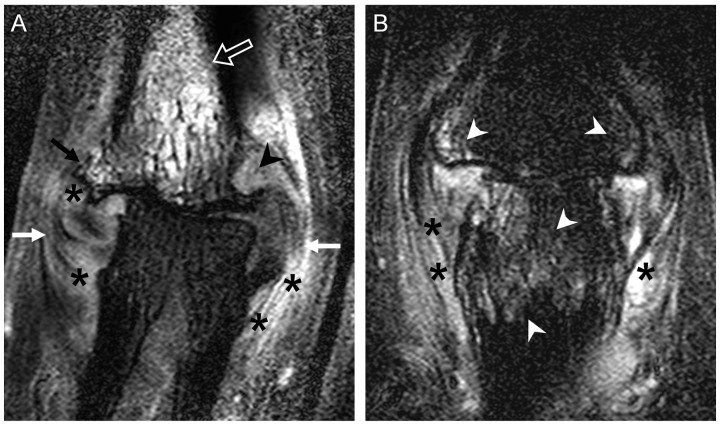
Fig. 4.
MRI of a PIP joint in OA and PsA demonstrating overlapping features as recently reported [51]
(A) A 52-year-old female with a 1.5 year history of OA. Striking collateral ligament and enthesis thickening (white arrows), disrupted with high signal from gadolinium contrast enhancement indicating inflammation (asterisks). There was also striking bone oedema in a diffuse pattern (open arrow) in this OA joint, but this is more commonly seen in PsA. The degenerate joint is subluxed, with loss of cartilage, an osteophyte (black arrow) and a peri-entheseal erosion also noted (black arrowhead). (B) A 45-year-old male with active PIP PsA with inflammatory changes including peri-entheseal bone oedema (white arrowheads) and high signal around the ligaments and soft tissue (asterisks). There was also loss of joint cartilage, reflecting the long disease duration.
Recently we performed MRI studies of 69 patients with PIP disease in RA, OA and PsA [54]. While it was possible to discriminate between the OA and RA, and PsA and RA, it was actually surprisingly difficult to differentiate between OA and PsA, likely because of a shared micro-anatomical basis. Consequently, even state-of-the-art MRI was unable to discriminate between clinically defined PsA and OA but was relatively robust in its ability to distinguish between RA and OA. In a similar vein, diffuse bone oedema was observed commonly in PsA [51], which is similar to foot neuropathic joint disease, an especially destructive form of OA [55].
A reappraisal of inflammation in PsA and OA in relation to enthesopathy
While established PsA is an inflammatory arthropathy, evidence for inflammation in the preclinical disease phase may be absent. Evidence for a lack of association between inflammation and PsA is the presence of radiological sacroiliitis without concurrent clinical signs [56, 57], which is also observed in subjects not known to have PsA [58]. Degenerative changes at the SI joints can also mimic the appearance of inflammatory sacroiliitis, which causes a diagnostic challenge in identifying inflammatory low back pain [59]. Moreover, imaging studies in psoriasis have consistently shown entheseal thickening without changes suggestive of inflammation, including a relative lack of Doppler enhancement on US [9, 10]. Asymptomatic areas of PsA also show thickening of the enthesis, suggesting a common site-specific tissue dysregulation as a precursor to disease [60]. Furthermore, patients labelled as PsA who have normal CRP tend to have less responsiveness to anti-TNF therapy [61], suggesting the possibility of non-inflammatory pathways in pain in some of these cases, although this could also represent misdiagnosis of PsA, as discussed later.
Thus far we have emphasized how the early phases of PsA may not be completely dominated by inflammation. Conversely, it has clearly emerged that inflammation may be very important in early OA, a fact that further blurs the different historical concepts of the two diseases. In fact, OA is recognized as an inflammatory disease [62, 63], with most studies focusing on the interaction between synovium and articular cartilage as the underlying driver for this process. In some cases the degree of inflammation in OA almost resembles that in RA [64, 65]. Arthroscopic, US and MRI studies with contrast enhancement provide evidence for inflammation in acute disease in the vast majority of cases, suggesting a strong inflammatory component in OA [66–69]. From the clinical perspective, patients with so called inflammatory OA often manifest with joint swelling and tend to be erosive [70], but in addition to this they can have protracted stiffness in the morning in a pattern that can be reminiscent of inflammatory arthritis [71]. The erosive OA phenotype is linked to synovio-entheseal complex (SEC) dysfunction, as is the erosive phenotype in PsA [72]. Moreover, other evidence for inflammation in OA, including elevated CRP [73–75], the emerging role of pro-inflammatory cytokines including IL1-β and TNF-α, which are potential targets for therapy [62, 76, 77], and response to IA steroids [78], could be related to the potential primary changes taking place in entheses in the OA joint.
Tissue micro-anatomical studies of normal non-diseased aged cadaveric entheses have shown the potential importance of OA features including cartilage fissuring, fibrillation and chondrocyte hypertrophy, and micro-inflammation at insertions [19]. These pathological processes manifesting in SECs thus offer a novel mechanism for synovitis in OA [24]. Indeed, histological studies show that normal entheses have evidence of age-related micro-inflammation at these sites, as well as microfractures at the enthesis fibrocartilage–bone interface [24, 79].
Could there be an OA–PsA overlap?
Clearly some patients present with a new diagnosis of PsA on a pre-existing OA background and some patients undoubtedly develop secondary OA in a PsA setting. Nevertheless, the existing evidence as discussed above leads to some new questions. Specifically, might there be hitherto overlooked overlapping OA–PsA patient groups. The immunopathology of some forms of OA, and generalized OA in particular, and PsA may be played out in the same enthesis-associated micro-anatomical territory (Fig. 5). Common triggers, including but not limited to injury and microdamage, may be important disease drivers, since trauma/injuries is a common recognized OA triggering factor, and similarly a relationship between trauma and the development of PsA, traditionally reported as a deep Koebner’s effect, is well reported [80].
Fig. 5.
Common enthesis-related anatomical factors in PsA and some forms of OA
It is proposed that common initiating factors, including abnormal responses to mechanical stress or microdamage at the enthesis, in the appropriate genetic and environmental background, can result in either predominantly degenerative changes leading to OA or inflammatory changes leading to PsA. As a natural extension of this, a subgroup has both degenerative and remodelling changes alongside inflammation, thus making the distinction between OA and PsA difficult, and this likely represents a true overlap between PsA and OA. E: enthesis; B: bone; F: fibrocartilage; C: chondrocytes; O: osteoblasts; M: macrophage; L: lymphocyte.
Both OA and PsA also share common risk factors; e.g. obesity has been linked to the development of PsA and OA [81, 82], although the actual pathogenesis of either obesity-related metabolic inflammation or mechanical load as a direct cause is less clear [83, 84]. However, for enthesiogenic or ligamentogenic OA, age-related decay or degeneration with aberrant modelling may underscore the OA disease process. At extremes of both inflammation and aberrant degeneration the disease may readily be distinguished clinically, but it is possible that there is an interface at which this is not possible (Figs. 1 and 5).
Therefore both PsA and some types of enthesiogenic OA may start in the same anatomical region, namely the enthesis, with genes and environment modifying the clinical phenotype (Fig. 6). In younger subjects, genes associated with dysregulation in inflammation-related pathways are likely to manifest as an inflammatory arthropathy. Recently, two rare monogenic forms of psoriasis and PsA with mutations in the caspase recruitment domain family, member 14 (CARD14) or IL-36 receptor antagonist (IL36RA) genes, which result in an increased propensity for inflammation, have been defined [85, 86].
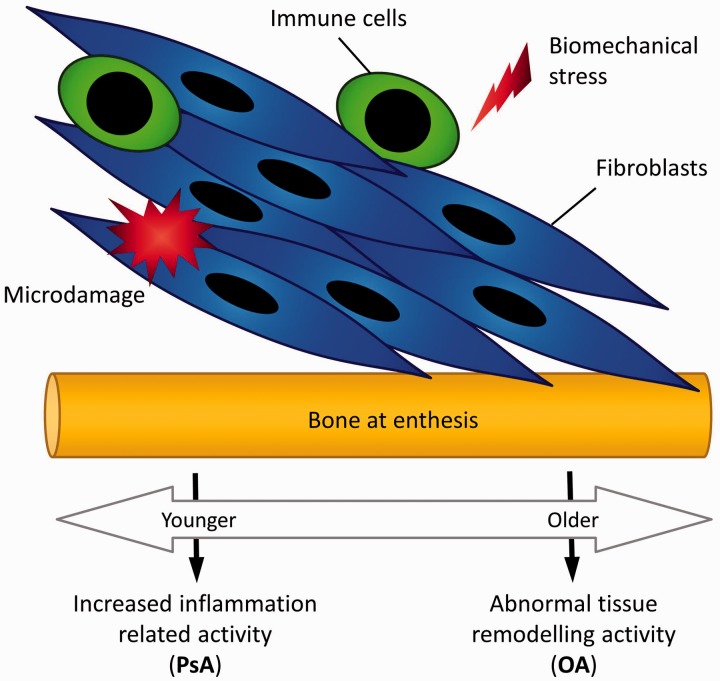
Fig. 6.
Proposed molecular basis for putative overlap between PsA and OA
A common factor is biomechanical stress, microdamage or both at the entheses in some types of OA and also in PsA. For pure PsA, with gain of function in immune system (e.g. CARD14 or IL36RA) genes, this may manifest as an exaggerated inflammatory response instead of a physiological level of inflammation involved in tissue repair with cells of the innate and adaptive immune system driving disease. At the other end of the spectrum, usually in older subjects, the same inciting events may manifest in a dominant tissue repair response in the same territories with pronounced remodelling. The proposal is that there may be overlap with involvement of both aberrant inflammation and remodelling pathways, giving a mixed phenotype or a scenario whereby it is impossible to state with certainty whether it is OA or PsA. IL36RA: interleukin-36 receptor antagonist; CARD: caspase recruitment domain family, member 14.
Conversely, non-dysregulation in inflammation-related genes, but dysregulation in genes associated with tissue remodelling and repair are more likely to manifest as OA in an older age group (Fig. 6). Thus far the genetic basis for generalized OA and hand disease is poorly defined, so there is no concrete genetic evidence to define the OA phenotype at this end of the spectrum. It is our contention that mutations or single nucleotide polymorphisms (SNPs) in proteins associated with both inflammation and tissue repair would be expected to lead to bona fide overlap phenotypes.
Clinical implications of overlapping features of OA and PsA
It may be genuinely difficult or even impossible to tell OA and PsA apart in a significant group of cases. Strategies including imaging may show erosion or periosteal new bone reaction in both, and MRI may not tell them apart (Fig. 4). As an example of this, a recent study in subjects with psoriasis indicated the frequent occurrence of DISH, which is a degenerative disorder of the spine, which could very well have been ankylosis of the spine due to PsA [87]. Furthermore, IA or local steroid injection may lead to some temporary relief in both OA and PsA or true overlaps. These findings are particularly important in the area of biologic therapy, where anti-TNF therapies are efficacious for PsA [88–91], but where there is no evidence that such therapies lead to significant symptomatic relief from OA [92, 93]. Nevertheless, in OA where there are clinically swollen joints, such strategies are associated with erosion progression retardation [94].
A study involving the Danish registry showed that failure to respond to anti-TNF therapy in PsA was associated with normal CRP as measured by conventional assays [61]. Therefore it is possible that many failures of anti-TNF in PsA, at least in part, reflect the genuine inability of clinicians to differentiate OA from PsA. This may also be relevant for recent studies that have shown the apparent inefficacy of MTX in well-established PsA [95], whereas studies in early PsA patients who had a much greater elevation of CRP showed good evidence of efficacy [96].
A recent study showed that anti-IL-12/23 p40 subunit therapy with ustekinumab was associated with apparently much better responses for dactylitis and isolated enthesitis compared with synovial joint swelling in PsA [97]. To us this is somewhat perplexing, given the primacy of enthesitis in synovial joints in animal models and its frequency in and adjacency to swollen synovial joints on imaging. Investigators need to consider whether the apparent relative lack of efficacy in hand disease may reflect this difficulty in telling secondary OA from primary PsA at that site, which may reflect the underlying overlap process.
Beyond these therapeutic studies, these findings also have considerable implications for outcome measure development in PsA, as it is possible that some of the things being measured are not modifiable by potential anti-inflammatory or biologic therapies. Indeed, we feel that this is potentially such a large problem that strategies assessing the efficacy of biologic agents in PsA should a priori select patients with clear-cut clinical arthritis without clinical evidence of OA to help eliminate this confounding factor. This is all the more important as many TNF failures may not have had a biologic therapy failure, but an inaccurate diagnosis [61]. This is not a reflection of poor clinical acumen, but rather a failure to appreciate that the pathological process overlaps in the two diseases.
These findings also suggest that physicians need to seriously consider the possibility of the presence of psoriasis modulating the underlying OA phenotype, making it more inflammatory in nature. This has implications for searching carefully using epidemiological and genetic studies to see whether an association can be found. This is particularly relevant because the heritability of familial PsA and OA is much greater compared with RA [98, 99].
Immunopathological implications for the OA–PsA overlap model
Historically, joint degeneration, remodelling and inflammation were viewed as different processes, but it is now clear that molecules related to degeneration or remodelling may also have potent pro-inflammatory effects. At the molecular level, the key role played by bone morphogenetic proteins (BMPs) in joint homeostasis is well recognized and the emerging role of BMPs in joint inflammation is now appreciated [100]. As an example of this, Lories et al. [101] have shown that noggin, a BMP antagonist, can prevent the onset of experimental PsA-like arthropathy. This pathway has been shown to be a potential therapeutic target not only for SpA, including PsA, but also for degenerative arthritis [11, 101]. Another prominent example of how inflammation and remodelling can be aberrantly regulated comes from functional studies showing that blockade of Dickkopf-1 (DKK-1), a Wnt pathway antagonist, is associated with the emergence of a prominent bone remodelling phenotype in the face of active inflammation [48]. Elevated concentrations of DKK-1 have been detected in both OA and PsA [102, 103].
As highlighted earlier, the classical pro-inflammatory cytokines, including IL-1 and TNF, that are commonly released in PsA are also involved in OA [62, 76, 77]. In addition, regulatory-type cytokines such as TGF-β play key roles in tissue repair and also facilitate pro-inflammatory reactions [104, 105]. Inflammation in OA may be triggered by danger signals released on tissue damage, including hyaluronin fragments, fibronectin and necrotic fragments of tissue [106], and similar danger signals may be initiators of PsA, but this is less well defined. Therefore common triggering mechanisms at the enthesis could result in either a predominant degenerative or a predominant inflammatory phenotype or a mixture of the two. In turn, this difference is likely to be controlled by genetic factors, including the IL-17/23 axis. The field is in its infancy, but the presence of novel lymphocyte populations raises new research avenues for experimental OA models, not just PsA models [14].
Conclusions
This perspective highlights how some forms of generalized OA and PsA may afflict the same anatomical territory. While degeneration or inflammation may be clearly discernible at the two extremes, there may be a group of patients where differentiation is impossible. Whether this represents the effect of one disease modifying the other or whether there are other determining factors awaits confirmation. It is clear that genetic factors, including TRAF3IP2, TNAFAIP3 and the IL-12/23 axis, may help in defining PsA; however, this will only likely hold for a subset of cases [107–109]. What is already emerging is that there is clinical difficulty in telling them apart and this may in turn lead to apparent failure of disease modifying agents or biologic therapy [95]. This has not been properly considered or addressed, thus no data on the prevalence of such an overlap are available to date, and this has implications for the optimal diagnosis and management of both OA and PsA.
Rheumatology key messages.
Some forms of OA and PsA share similar features that can complicate diagnosis.
Enthesopathy may be the unifying pathology in the OA–PsA overlap entity.
Appreciating OA–PsA overlap disease helps with optimal management and apparent biologic therapy failure.
Acknowledgements
Professor McGonagle's work on OA is funded through WELMEC, a Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC, under grant number WT 088908/Z/09/Z.
Funding: None.
Disclosure statement: K.G.A.H. has received lecture fees from AbbVie, MSD, Pfizer and UCB. All other authors have declared no conflicts of interest.
References
- 1.Ling S, Carrasco M. When sustained attention impairs perception. Nat Neurosci. 2006;9:1243–5. doi: 10.1038/nn1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radin EL. The physiology and degeneration of joints. Semin Arthritis Rheum. 1972;2:245–57. doi: 10.1016/0049-0172(72)90010-8. [DOI] [PubMed] [Google Scholar]
- 3.Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- 4.McGonagle D, Tan AL, Carey J, Benjamin M. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J Anat. 2010;216:279–91. doi: 10.1111/j.1469-7580.2009.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGonagle D, Khan MA, Marzo-Ortega H, et al. Enthesitis in spondyloarthropathy. Curr Opin Rheumatol. 1999;11:244–50. doi: 10.1097/00002281-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 6.McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352:1137–40. doi: 10.1016/S0140-6736(97)12004-9. [DOI] [PubMed] [Google Scholar]
- 7.Aydin SZ, Ash ZR, Tinazzi I, et al. The link between enthesitis and arthritis in psoriatic arthritis: a switch to a vascular phenotype at insertions may play a role in arthritis development. Ann Rheum Dis. 2013;72:992–5. doi: 10.1136/annrheumdis-2012-201617. [DOI] [PubMed] [Google Scholar]
- 8.Emad Y, Ragab Y, Gheita T, et al. Knee enthesitis and synovitis on magnetic resonance imaging in patients with psoriasis without arthritic symptoms. J Rheumatol. 2012;39:1979–86. doi: 10.3899/jrheum.120301. [DOI] [PubMed] [Google Scholar]
- 9.Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis. 2012;71:553–6. doi: 10.1136/annrheumdis-2011-200478. [DOI] [PubMed] [Google Scholar]
- 10.Gisondi P, Tinazzi I, El-Dalati G, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30. doi: 10.1136/ard.2007.075101. [DOI] [PubMed] [Google Scholar]
- 11.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–9. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lories RJ, Matthys P, de Vlam K, Derese I, Luyten FP. Ankylosing enthesitis, dactylitis, and onychoperiostitis in male DBA/1 mice: a model of psoriatic arthritis. Ann Rheum Dis. 2004;63:595–8. doi: 10.1136/ard.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques P, Venken K, Van Beneden K, et al. Invariant natural killer T cells are natural regulators of murine spondylarthritis. Arthritis Rheum. 2010;62:988–99. doi: 10.1002/art.27324. [DOI] [PubMed] [Google Scholar]
- 14.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med. 2012;18:1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 15.Quasnichka HL, Anderson-MacKenzie JM, Tarlton JF, et al. Cruciate ligament laxity and femoral intercondylar notch narrowing in early-stage knee osteoarthritis. Arthritis Rheum. 2005;52:3100–9. doi: 10.1002/art.21340. [DOI] [PubMed] [Google Scholar]
- 16.Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 17.Anderson-MacKenzie JM, Billingham ME, Bailey AJ. Collagen remodeling in the anterior cruciate ligament associated with developing spontaneous murine osteoarthritis. Biochem Biophys Res Commun. 1999;258:763–7. doi: 10.1006/bbrc.1999.0713. [DOI] [PubMed] [Google Scholar]
- 18.Tan AL, Grainger AJ, Tanner SF, et al. High-resolution magnetic resonance imaging for the assessment of hand osteoarthritis. Arthritis Rheum. 2005;52:2355–65. doi: 10.1002/art.21210. [DOI] [PubMed] [Google Scholar]
- 19.Tan AL, Toumi H, Benjamin M, et al. Combined high-resolution magnetic resonance imaging and histological examination to explore the role of ligaments and tendons in the phenotypic expression of early hand osteoarthritis. Ann Rheum Dis. 2006;65:1267–72. doi: 10.1136/ard.2005.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toumi H, Larguech G, Filaire E, Pinti A, Lespessailles E. Regional variations in human patellar trabecular architecture and the structure of the quadriceps enthesis: a cadaveric study. J Anat. 2012;220:632–7. doi: 10.1111/j.1469-7580.2012.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGonagle D, Tan AL, Grainger AJ, Benjamin M. Heberden’s nodes and what Heberden could not see: the pivotal role of ligaments in the pathogenesis of early nodal osteoarthritis and beyond. Rheumatology. 2008;47:1278–85. doi: 10.1093/rheumatology/ken093. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Molina G, Guermazi A, Niu J, et al. Central bone marrow lesions in symptomatic knee osteoarthritis and their relationship to anterior cruciate ligament tears and cartilage loss. Arthritis Rheum. 2008;58:130–6. doi: 10.1002/art.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007;56:2482–91. doi: 10.1002/art.22758. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin M, McGonagle D. Histopathologic changes at “synovio-entheseal complexes” suggesting a novel mechanism for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum. 2007;56:3601–9. doi: 10.1002/art.23078. [DOI] [PubMed] [Google Scholar]
- 25.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 26.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarpa R, Oriente P, Pucino A, et al. Psoriatic arthritis in psoriatic patients. Br J Rheumatol. 1984;23:246–50. doi: 10.1093/rheumatology/23.4.246. [DOI] [PubMed] [Google Scholar]
- 28.Yu TY, Tsai WC, Cheng JW, et al. The effects of aging on quantitative sonographic features of rotator cuff tendons. J Clin Ultrasound. 2012;40:471–8. doi: 10.1002/jcu.21919. [DOI] [PubMed] [Google Scholar]
- 29.Roberts ME, Wright V, Hill AG, Mehra AC. Psoriatic arthritis. Follow-up study. Ann Rheum Dis. 1976;35:206–12. doi: 10.1136/ard.35.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plato CC, Norris AH. Osteoarthritis of the hand: age-specific joint-digit prevalence rates. Am J Epidemiol. 1979;109:169–80. doi: 10.1093/oxfordjournals.aje.a112672. [DOI] [PubMed] [Google Scholar]
- 31.Salvarani C, Macchioni P, Cremonesi T, et al. The cervical spine in patients with psoriatic arthritis: a clinical, radiological and immunogenetic study. Ann Rheum Dis. 1992;51:73–7. doi: 10.1136/ard.51.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkinson T, Armas J, Evison G, et al. The cervical spine in psoriatic arthritis: a clinical and radiological study. Br J Rheumatol. 1994;33:255–9. doi: 10.1093/rheumatology/33.3.255. [DOI] [PubMed] [Google Scholar]
- 33.Falsetti P, Frediani B, Fioravanti A, et al. Sonographic study of calcaneal entheses in erosive osteoarthritis, nodal osteoarthritis, rheumatoid arthritis and psoriatic arthritis. Scand J Rheumatol. 2003;32:229–34. doi: 10.1080/03009740310003721. [DOI] [PubMed] [Google Scholar]
- 34.Olivieri I, Salvarani C, Cantini F, et al. Fast spin echo-T2-weighted sequences with fat saturation in dactylitis of spondylarthritis. No evidence of entheseal involvement of the flexor digitorum tendons. Arthritis Rheum. 2002;46:2964–7. doi: 10.1002/art.10633. [DOI] [PubMed] [Google Scholar]
- 35.Healy PJ, Groves C, Chandramohan M, Helliwell PS. MRI changes in psoriatic dactylitis—extent of pathology, relationship to tenderness and correlation with clinical indices. Rheumatology. 2008;47:92–5. doi: 10.1093/rheumatology/kem315. [DOI] [PubMed] [Google Scholar]
- 36.Kane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O. Ultrasonography in the diagnosis and management of psoriatic dactylitis. J Rheumatol. 1999;26:1746–51. [PubMed] [Google Scholar]
- 37.Lin YC, Wu YH, Scher RK. Nail changes and association of osteoarthritis in digital myxoid cyst. Dermatol Surg. 2008;34:364–9. doi: 10.1111/j.1524-4725.2007.34070.x. [DOI] [PubMed] [Google Scholar]
- 38.Cimmino MA, Seriolo B, Accardo S. Prevalence of nail involvement in nodal osteoarthritis. Clin Rheumatol. 1994;13:203–6. doi: 10.1007/BF02249013. [DOI] [PubMed] [Google Scholar]
- 39.Fioravanti A, Tofi C, Cerase A, Priolo F, Marcolongo R. Capillaroscopic findings in erosive and nodal osteoarthritis of the hands. Clin Rheumatol. 2001;20:174–6. doi: 10.1007/s100670170059. [DOI] [PubMed] [Google Scholar]
- 40.Zaric D, Clemmensen OJ, Worm AM, Stahl D. Capillary microscopy of the nail fold in patients with psoriasis and psoriatic arthritis. Dermatologica. 1982;164:10–4. doi: 10.1159/000250060. [DOI] [PubMed] [Google Scholar]
- 41.Martel W, Stuck KJ, Dworin AM, Hylland RG. Erosive osteoarthritis and psoriatic arthritis: a radiologic comparison in the hand, wrist, and foot. AJR Am J Roentgenol. 1980;134:125–35. doi: 10.2214/ajr.134.1.125. [DOI] [PubMed] [Google Scholar]
- 42.Slobodin G, Rozenbaum M, Boulman N, Rosner I. Varied presentations of enthesopathy. Semin Arthritis Rheum. 2007;37:119–26. doi: 10.1016/j.semarthrit.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Rogers J, Shepstone L, Dieppe P. Is osteoarthritis a systemic disorder of bone? Arthritis Rheum. 2004;50:452–7. doi: 10.1002/art.20136. [DOI] [PubMed] [Google Scholar]
- 44.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 45.Mader R, Sarzi-Puttini P, Atzeni F, et al. Extraspinal manifestations of diffuse idiopathic skeletal hyperostosis. Rheumatology. 2009;48:1478–81. doi: 10.1093/rheumatology/kep308. [DOI] [PubMed] [Google Scholar]
- 46.McEwen C, DiTata D, Lingg C, et al. Ankylosing spondylitis and spondylitis accompanying ulcerative colitis, regional enteritis, psoriasis and Reiter’s disease. A comparative study. Arthritis Rheum. 1971;14:291–318. doi: 10.1002/art.1780140302. [DOI] [PubMed] [Google Scholar]
- 47.Rogers J, Shepstone L, Dieppe P. Bone formers: osteophyte and enthesophyte formation are positively associated. Ann Rheum Dis. 1997;56:85–90. doi: 10.1136/ard.56.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a masterregulator of joint remodeling. Nat Med. 2007;13:156–63. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 49.Schett G, Zwerina J, David JP. The role of Wnt proteins in arthritis. Nat Clin Pract Rheumatol. 2008;4:473–80. doi: 10.1038/ncprheum0881. [DOI] [PubMed] [Google Scholar]
- 50.Jevtic V, Watt I, Rozman B, et al. Distinctive radiological features of small hand joints in rheumatoid arthritis and seronegative spondyloarthritis demonstrated by contrast-enhanced (Gd-DTPA) magnetic resonance imaging. Skeletal Radiol. 1995;24:351–5. doi: 10.1007/BF00197064. [DOI] [PubMed] [Google Scholar]
- 51.Tan AL, Grainger AJ, Tanner SF, Emery P, McGonagle D. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis Rheum. 2006;54:1328–33. doi: 10.1002/art.21736. [DOI] [PubMed] [Google Scholar]
- 52.Schraml C, Schwenzer NF, Martirosian P, et al. Assessment of synovitis in erosive osteoarthritis of the hand using DCE-MRI and comparison with that in its major mimic, the psoriatic arthritis. Acad Radiol. 2011;18:804–9. doi: 10.1016/j.acra.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 53.Arnbak B, Leboeuf-Yde C, Jensen TS. A systematic critical review on MRI in spondyloarthritis. Arthritis Res Ther. 2012;14:R55. doi: 10.1186/ar3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braum LS, McGonagle D, Bruns A, et al. Characterisation of hand small joints arthropathy using high-resolution MRI—limited discrimination between osteoarthritis and psoriatic arthritis. Eur Radiol. 2013;23:1686–93. doi: 10.1007/s00330-012-2739-0. [DOI] [PubMed] [Google Scholar]
- 55.Halstead J, Bergin D, Keenan AM, Madden J, McGonagle D. Ligament and bone pathologic abnormalities more frequent in neuropathic joint disease in comparison with degenerative arthritis of the foot and ankle: implications for understanding rapidly progressive joint degeneration. Arthritis Rheum. 2010;62:2353–8. doi: 10.1002/art.27547. [DOI] [PubMed] [Google Scholar]
- 56.Hanly JG, Russell ML, Gladman DD. Psoriatic spondyloarthropathy: a long term prospective study. Ann Rheum Dis. 1988;47:386–93. doi: 10.1136/ard.47.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung YY, Ho KW, Tam LS, et al. Evaluation of spinal mobility measurements in predicting axial psoriatic arthritis. Clin Rheumatol. 2011;30:1157–62. doi: 10.1007/s10067-011-1717-2. [DOI] [PubMed] [Google Scholar]
- 58.Slobodin G, Croitoru S, Starikov N, et al. Incidental computed tomography sacroiliitis: clinical significance and inappropriateness of the New York radiological grading criteria for the diagnosis. Clin Rheumatol. 2012;31:425–8. doi: 10.1007/s10067-011-1871-6. [DOI] [PubMed] [Google Scholar]
- 59.O’Shea FD, Boyle E, Salonen DC, et al. Inflammatory and degenerative sacroiliac joint disease in a primary back pain cohort. Arthritis Care Res. 2010;62:447–54. doi: 10.1002/acr.20168. [DOI] [PubMed] [Google Scholar]
- 60.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–10. doi: 10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glintborg B, Ostergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor alpha therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011;63:382–90. doi: 10.1002/art.30117. [DOI] [PubMed] [Google Scholar]
- 62.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 63.Ehrlich GE. Inflammatory osteoarthritis. I. The clinical syndrome. J Chronic Dis. 1972;25:317–28. doi: 10.1016/0021-9681(72)90026-4. [DOI] [PubMed] [Google Scholar]
- 64.Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52:870–5. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haraoui B, Pelletier JP, Cloutier JM, Faure MP, Martel-Pelletier J. Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis. In vivo effects of antirheumatic drugs. Arthritis Rheum. 1991;34:153–63. doi: 10.1002/art.1780340205. [DOI] [PubMed] [Google Scholar]
- 66.Loeuille D, Sauliere N, Champigneulle J, et al. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011;19:1433–9. doi: 10.1016/j.joca.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Keen HI, Wakefield RJ, Grainger AJ, et al. An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum. 2008;59:1756–63. doi: 10.1002/art.24312. [DOI] [PubMed] [Google Scholar]
- 68.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Kortekaas MC, Kwok WY, Reijnierse M, Huizinga TW, Kloppenburg M. In erosive hand osteoarthritis more inflammatory signs on ultrasound are found than in the rest of hand osteoarthritis. Ann Rheum Dis. 2013; 72:930–4. doi: 10.1136/annrheumdis-2012-201458. [DOI] [PubMed] [Google Scholar]
- 71.Thomas E, Peat G, Mallen C, et al. Predicting the course of functional limitation among older adults with knee pain: do local signs, symptoms and radiographs add anything to general indicators? Ann Rheum Dis. 2008;67:1390–8. doi: 10.1136/ard.2007.080945. [DOI] [PubMed] [Google Scholar]
- 72.Grainger AJ, Farrant JM, O’Connor PJ, et al. MR imaging of erosions in interphalangeal joint osteoarthritis: is all osteoarthritis erosive? Skeletal Radiol. 2007;36:737–45. doi: 10.1007/s00256-007-0287-5. [DOI] [PubMed] [Google Scholar]
- 73.Sharif M, Shepstone L, Elson CJ, Dieppe PA, Kirwan JR. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59:71–4. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pearle AD, Scanzello CR, George S, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:516–23. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Sturmer T, Brenner H, Koenig W, Gunther KP. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann Rheum Dis. 2004;63:200–5. doi: 10.1136/ard.2003.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calich AL, Domiciano DS, Fuller R. Osteoarthritis: can anti-cytokine therapy play a role in treatment? Clin Rheumatol. 2010;29:451–5. doi: 10.1007/s10067-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 78.Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005328. doi: 10.1002/14651858.CD005328.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Benjamin M, Toumi H, Suzuki D, et al. Microdamage and altered vascularity at the enthesis-bone interface provides an anatomic explanation for bone involvement in the HLA-B27-associated spondylarthritides and allied disorders. Arthritis Rheum. 2007;56:224–33. doi: 10.1002/art.22290. [DOI] [PubMed] [Google Scholar]
- 80.Olivieri I, Padula A, D’Angelo S, Scarpa R. Role of trauma in psoriatic arthritis. J Rheumatol. 2008;35:2085–7. doi: 10.3899/jrheum.080668. [DOI] [PubMed] [Google Scholar]
- 81.Canete JD, Mease P. The link between obesity and psoriatic arthritis. Ann Rheum Dis. 2012;71:1265–6. doi: 10.1136/annrheumdis-2012-201632. [DOI] [PubMed] [Google Scholar]
- 82.Vuolteenaho K, Koskinen A, Moilanen T, Moilanen E. Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis. Ann Rheum Dis. 2012;71:1912–3. doi: 10.1136/annrheumdis-2011-201242. [DOI] [PubMed] [Google Scholar]
- 83.Yusuf E. Metabolic factors in osteoarthritis: obese people do not walk on their hands. Arthritis Res Ther. 2012;14:123. doi: 10.1186/ar3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol Aging Age Relat Dis. 2012;2:17470. doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due tomutations in CARD14. Am J Hum Genet. 2012;90:784–95. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74: 187–92. doi: 10.1016/j.jdermsci.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 87.De Marco G, Cattaneo A, Battafarano N, et al. Not simply a matter of psoriatic arthritis: epidemiology of rheumatic diseases in psoriatic patients. Arch Dermatol Res. 2012;304:719–26. doi: 10.1007/s00403-012-1281-x. [DOI] [PubMed] [Google Scholar]
- 88.Antoni C, Dechant C, Hanns-Martin Lorenz PD, et al. Open-label study of infliximab treatment for psoriatic arthritis: clinical and magnetic resonance imaging measurements of reduction of inflammation. Arthritis Rheum. 2002;47:506–12. doi: 10.1002/art.10671. [DOI] [PubMed] [Google Scholar]
- 89.Salvarani C, Cantini F, Olivieri I, et al. Efficacy of infliximab in resistant psoriatic arthritis. Arthritis Rheum. 2003;49:541–5. doi: 10.1002/art.11201. [DOI] [PubMed] [Google Scholar]
- 90.Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 91.Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–90. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 92.Verbruggen G, Wittoek R, Vander Cruyssen B, Elewaut D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: a double blind, randomised trial on structure modification. Ann Rheum Dis. 2012;71:891–8. doi: 10.1136/ard.2011.149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magnano MD, Chakravarty EF, Broudy C, et al. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol. 2007;34:1323–7. [PubMed] [Google Scholar]
- 94.Fioravanti A, Fabbroni M, Cerase A, Galeazzi M. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: a pilot study. Rheumatol Int. 2009;29:961–5. doi: 10.1007/s00296-009-0872-0. [DOI] [PubMed] [Google Scholar]
- 95.Kingsley GH, Kowalczyk A, Taylor H, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology. 2012;51:1368–77. doi: 10.1093/rheumatology/kes001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scarpa R, Peluso R, Atteno M, et al. The effectiveness of a traditional therapeutical approach in early psoriatic arthritis: results of a pilot randomised 6-month trial with methotrexate. Clin Rheumatol. 2008;27:823–6. doi: 10.1007/s10067-007-0787-7. [DOI] [PubMed] [Google Scholar]
- 97.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–9. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 98.Hirsch R, Lethbridge-Cejku M, Hanson R, et al. Familial aggregation of osteoarthritis: data from the Baltimore Longitudinal Study on Aging. Arthritis Rheum. 1998;41:1227–32. doi: 10.1002/1529-0131(199807)41:7<1227::AID-ART13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 99.Moll JM, Wright V. Familial occurrence of psoriatic arthritis. Ann Rheum Dis. 1973;32:181–201. doi: 10.1136/ard.32.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lories RJ, Luyten FP. Bone morphogenetic protein signaling in joint homeostasis and disease. Cytokine Growth Factor Rev. 2005;16:287–98. doi: 10.1016/j.cytogfr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 101.Lories RJ, Daans M, Derese I, et al. Noggin haploinsufficiency differentially affects tissue responses in destructive and remodeling arthritis. Arthritis Rheum. 2006;54:1736–46. doi: 10.1002/art.21897. [DOI] [PubMed] [Google Scholar]
- 102.Dalbeth N, Pool B, Smith T, et al. Circulating mediators of bone remodeling in psoriatic arthritis: implications for disordered osteoclastogenesis and bone erosion. Arthritis Res Ther. 2010;12:R164. doi: 10.1186/ar3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luyten FP, Tylzanowski P, Lories RJ. Wnt signaling and osteoarthritis. Bone. 2009;44:522–7. doi: 10.1016/j.bone.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Mantel PY, Schmidt-Weber CB. Transforming growth factor-β: recent advances on its role in immune tolerance. Methods Mol Biol. 2011;677:303–38. doi: 10.1007/978-1-60761-869-0_21. [DOI] [PubMed] [Google Scholar]
- 105.van Lent PL, Blom AB, van der Kraan P, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004;50:103–11. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]
- 106.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–72. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 107.Bluett J, Barton A. What have genome-wide studies told us about psoriatic arthritis? Curr Rheumatol Rep. 2012;14:364–8. doi: 10.1007/s11926-012-0255-5. [DOI] [PubMed] [Google Scholar]
- 108.Villanova F, Di Meglio P, Nestle FO. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii104–10. doi: 10.1136/annrheumdis-2012-203037. [DOI] [PubMed] [Google Scholar]
- 109.Chandran V. The genetics of psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44:149–56. doi: 10.1007/s12016-012-8303-5. [DOI] [PubMed] [Google Scholar]
- 110.McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology. 2003;42:778–83. doi: 10.1093/rheumatology/keg217. [DOI] [PubMed] [Google Scholar]