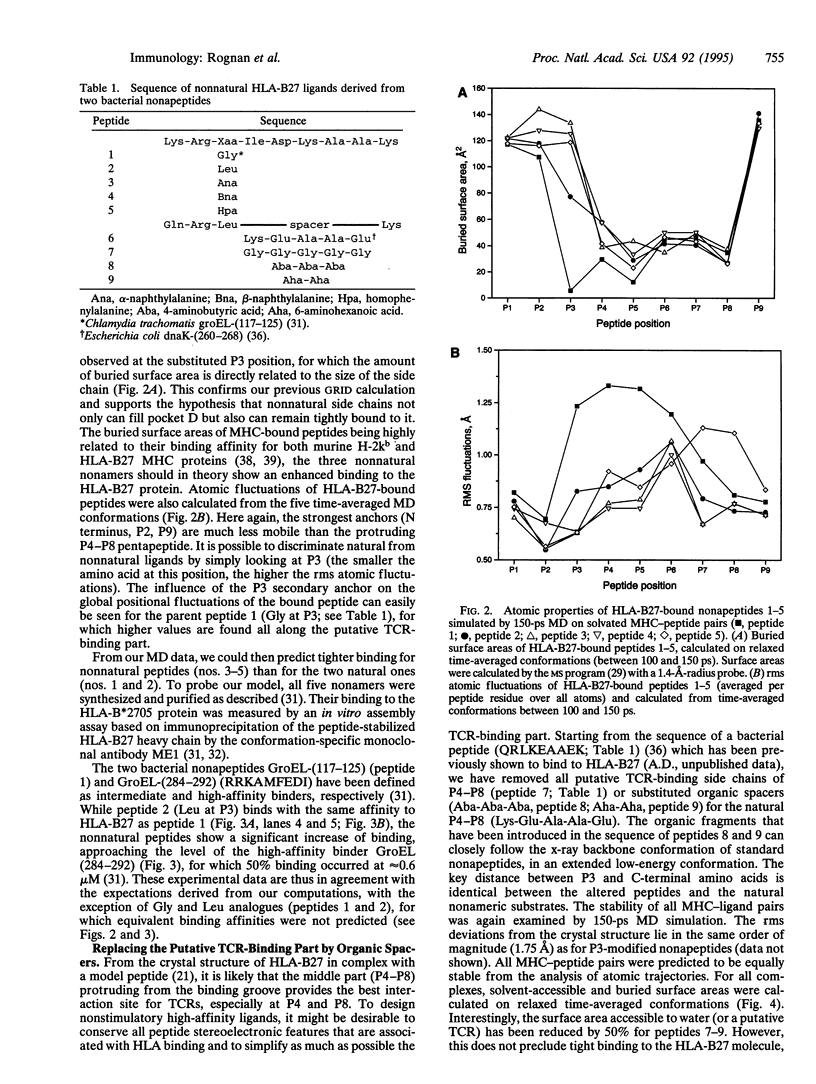
Abstract
From the three-dimensional structure of the class I major histocompatibility complex (MHC) HLA-B*2705 protein, several nonnatural peptides were designed either to optimize the interactions of one peptide amino acid (position 3) with its HLA binding pocket (pocket D) or to simplify the T-cell receptor-binding part by substitution with organic spacers. The stability of each MHC-ligand complex was simulated by 150-ps molecular dynamics in a water environment and compared with that of the natural complexes. All peptides were synthesized and tested for binding to the class I MHC protein in an in vitro assembly assay. As predicted from the computed atomic fluctuations and buried surface areas of MHC-bound ligands, bulky hydrophobic side chains at position 3 enhance the binding of a nonameric peptide to the HLA-B27 protein. Furthermore, it was possible to simplify half of the peptide sequence (residues 4-8) by replacement with organic fragments without altering the affinity of the designed ligands for the class I MHC protein. This study constitutes an initial step toward the rational design of nonpeptide class I MHC ligands for use in the selective immunotherapy of autoimmune diseases associated with particular HLA alleles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Muller S., Cardinaux F., Lehmann P. V., Falcioni F., Nagy Z. A. In vivo competition between self peptides and foreign antigens in T-cell activation. Nature. 1988 Aug 18;334(6183):623–625. doi: 10.1038/334623a0. [DOI] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R., Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990 Apr;11(4):137–142. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Brewerton D. A., Hart F. D., Nicholls A., Caffrey M., James D. C., Sturrock R. D. Ankylosing spondylitis and HL-A 27. Lancet. 1973 Apr 28;1(7809):904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- Daser A., Urlaub H., Henklein P. HLA-B27 binding peptides derived from the 57 kD heat shock protein of Chlamydia trachomatis: novel insights into the peptide binding rules. Mol Immunol. 1994 Apr;31(5):331–336. doi: 10.1016/0161-5890(94)90110-4. [DOI] [PubMed] [Google Scholar]
- De Magistris M. T., Alexander J., Coggeshall M., Altman A., Gaeta F. C., Grey H. M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992 Feb 21;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Ellis S. A., Taylor C., McMichael A. Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum Immunol. 1982 Aug;5(1):49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Erickson J., Neidhart D. J., VanDrie J., Kempf D. J., Wang X. C., Norbeck D. W., Plattner J. J., Rittenhouse J. W., Turon M., Wideburg N. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990 Aug 3;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Sloan-Lancaster J., Allen P. M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993 Dec;14(12):602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Goodford P. J. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J Med Chem. 1985 Jul;28(7):849–857. doi: 10.1021/jm00145a002. [DOI] [PubMed] [Google Scholar]
- Guo H. C., Madden D. R., Silver M. L., Jardetzky T. S., Gorga J. C., Strominger J. L., Wiley D. C. Comparison of the P2 specificity pocket in three human histocompatibility antigens: HLA-A*6801, HLA-A*0201, and HLA-B*2705. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8053–8057. doi: 10.1073/pnas.90.17.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V., Elvin J., Willis A. C., Aidoo M., Allsopp C. E., Gotch F. M., Gao X. M., Takiguchi M., Greenwood B. M., Townsend A. R. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992 Dec 3;360(6403):434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- Huet S., Nixon D. F., Rothbard J. B., Townsend A., Ellis S. A., McMichael A. J. Structural homologies between two HLA B27-restricted peptides suggest residues important for interaction with HLA B27. Int Immunol. 1990;2(4):311–316. doi: 10.1093/intimm/2.4.311. [DOI] [PubMed] [Google Scholar]
- Ishioka G. Y., Adorini L., Guery J. C., Gaeta F. C., LaFond R., Alexander J., Powell M. F., Sette A., Grey H. M. Failure to demonstrate long-lived MHC saturation both in vitro and in vivo. Implications for therapeutic potential of MHC-blocking peptides. J Immunol. 1994 May 1;152(9):4310–4319. [PubMed] [Google Scholar]
- Jameson B. A., McDonnell J. M., Marini J. C., Korngold R. A rationally designed CD4 analogue inhibits experimental allergic encephalomyelitis. Nature. 1994 Apr 21;368(6473):744–746. doi: 10.1038/368744a0. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kingsley G., Sieper J. Current perspectives in reactive arthritis. Immunol Today. 1993 Aug;14(8):387–391. doi: 10.1016/0167-5699(93)90139-C. [DOI] [PubMed] [Google Scholar]
- Latron F., Pazmany L., Morrison J., Moots R., Saper M. A., McMichael A., Strominger J. L. A critical role for conserved residues in the cleft of HLA-A2 in presentation of a nonapeptide to T cells. Science. 1992 Aug 14;257(5072):964–967. doi: 10.1126/science.1380181. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general mechanism for tight peptide binding to MHC. Cell. 1992 Sep 18;70(6):1035–1048. doi: 10.1016/0092-8674(92)90252-8. [DOI] [PubMed] [Google Scholar]
- Parham P., Brodsky F. M. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981 Dec;3(4):277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- Parker K. C., Bednarek M. A., Coligan J. E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994 Jan 1;152(1):163–175. [PubMed] [Google Scholar]
- Parker K. C., Biddison W. E., Coligan J. E. Pocket mutations of HLA-B27 show that anchor residues act cumulatively to stabilize peptide binding. Biochemistry. 1994 Jun 21;33(24):7736–7743. doi: 10.1021/bi00190a029. [DOI] [PubMed] [Google Scholar]
- Rognan D., Scapozza L., Folkers G., Daser A. Molecular dynamics simulation of MHC-peptide complexes as a tool for predicting potential T cell epitopes. Biochemistry. 1994 Sep 27;33(38):11476–11485. doi: 10.1021/bi00204a009. [DOI] [PubMed] [Google Scholar]
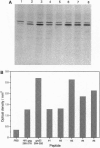
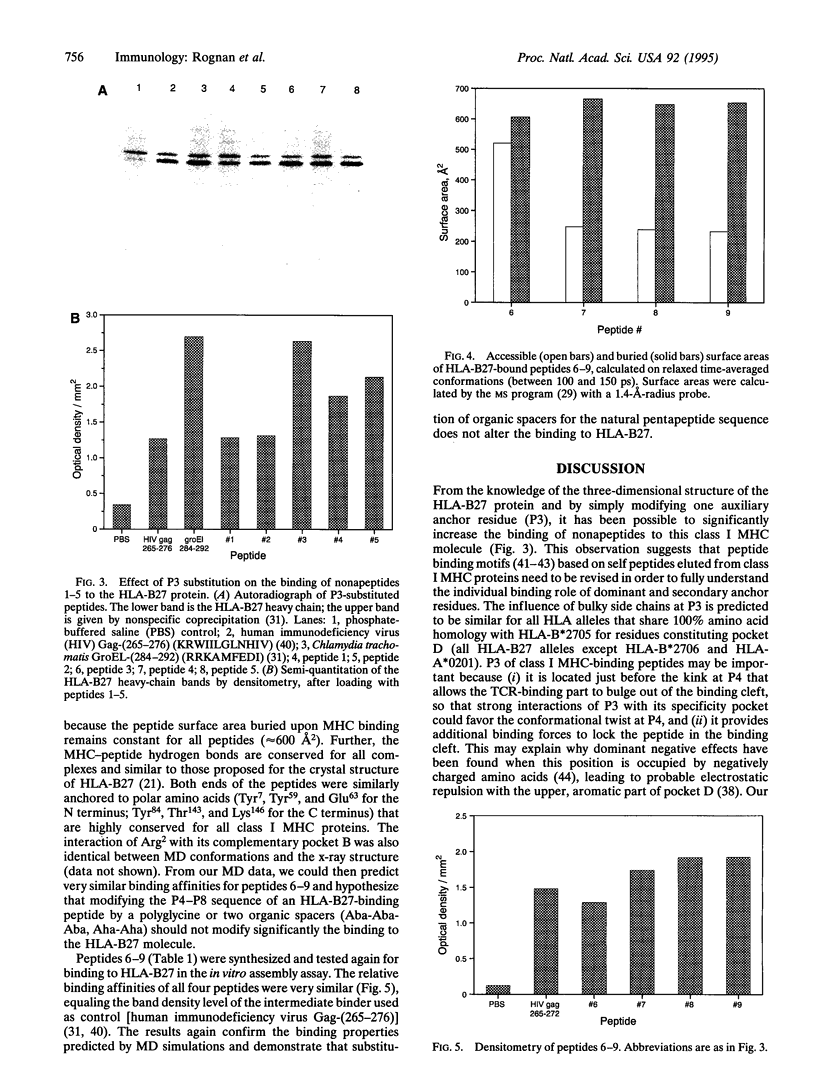
- Rognan D., Zimmermann N., Jung G., Folkers G. Molecular dynamics study of a complex between the human histocompatibility antigen HLA-A2 and the IMP58-66 nonapeptide from influenza virus matrix protein. Eur J Biochem. 1992 Aug 15;208(1):101–113. doi: 10.1111/j.1432-1033.1992.tb17163.x. [DOI] [PubMed] [Google Scholar]
- Ruppert J., Sidney J., Celis E., Kubo R. T., Grey H. M., Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993 Sep 10;74(5):929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Stevanović S., Gnau V., Jung G., Rammensee H. G. Dominant aromatic/aliphatic C-terminal anchor in HLA-B*2702 and B*2705 peptide motifs. Immunogenetics. 1994;39(1):74–77. doi: 10.1007/BF00171803. [DOI] [PubMed] [Google Scholar]
- Saito Y., Peterson P. A., Matsumura M. Quantitation of peptide anchor residue contributions to class I major histocompatibility complex molecule binding. J Biol Chem. 1993 Oct 5;268(28):21309–21317. [PubMed] [Google Scholar]
- Scofield R. H., Warren W. L., Koelsch G., Harley J. B. A hypothesis for the HLA-B27 immune dysregulation in spondyloarthropathy: contributions from enteric organisms, B27 structure, peptides bound by B27, and convergent evolution. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9330–9334. doi: 10.1073/pnas.90.20.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. A., Lopez M. T., McDevitt H. O. Autoimmune diseases: the failure of self tolerance. Science. 1990 Jun 15;248(4961):1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Evavold B. D., Allen P. M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993 May 13;363(6425):156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- Soares L. R., Sercarz E. E., Miller A. Vaccination of the Leishmania major susceptible BALB/c mouse. I. The precise selection of peptide determinant influences CD4+ T cell subset expression. Int Immunol. 1994 May;6(5):785–794. doi: 10.1093/intimm/6.5.785. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. Antigenic peptide binding by class I and class II histocompatibility proteins. Structure. 1994 Apr 15;2(4):245–251. doi: 10.1016/s0969-2126(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Stewart J. J. MOPAC: a semiempirical molecular orbital program. J Comput Aided Mol Des. 1990 Mar;4(1):1–105. doi: 10.1007/BF00128336. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]