Abstract
ASTERIA I was a 40-week, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of subcutaneous omalizumab as add-on therapy for 24 weeks in patients with chronic idiopathic urticaria/spontaneous urticaria (CIU/CSU) who remained symptomatic despite H1 antihistamine treatment at licensed doses. Patients aged 12–75 years with CIU/CSU who remained symptomatic despite treatment with approved doses of H1 antihistamines were randomized (1:1:1:1) in a double-blind manner to subcutaneous omalizumab 75 mg, 150 mg, or 300 mg or placebo every 4 weeks for 24 weeks followed by 16 weeks of follow-up. The primary end point was change from baseline in weekly itch severity score (ISS) at week 12. Among randomized patients (N=319: placebo n=80, omalizumab 75 mg n=78, 150 mg n=80, 300 mg n=81), 262 (82.1%) completed the study. Compared with placebo (n=80), mean weekly ISS was reduced from baseline to week 12 by an additional 2.96 points (95% confidence interval (CI): −4.71 to −1.21; P=0.0010), 2.95 points (95% CI: −4.72 to −1.18; P=0.0012), and 5.80 points (95% CI: −7.49 to −4.10; P<0.0001) in the omalizumab 75-mg (n=77), 150-mg (n=80), and 300-mg groups (n=81), respectively. The omalizumab 300-mg group met all nine secondary end points, including a significant decrease in the duration of time to reach minimally important difference response (⩾5-point decrease) in weekly ISS (P<0.0001) and higher percentages of patients with well-controlled symptoms (urticaria activity score over 7 days (UAS7) ⩽6: 51.9% vs. 11.3% P<0.0001) and complete response (UAS7=0: 35.8% vs. 8.8% P<0.0001) versus placebo. During the 24-week treatment period, 2 (2.9%), 3 (3.4%), 0, and 4 (5.0%) patients in the omalizumab 75-mg, 150-mg, 300-mg, and placebo groups, respectively, experienced a serious adverse event. Omalizumab 300 mg administered subcutaneously every 4 weeks reduced weekly ISS and other symptom scores versus placebo in CIU/CSU patients who remained symptomatic despite treatment with approved doses of H1 antihistamines.
Introduction
Chronic idiopathic urticaria (CIU), also referred to as chronic spontaneous urticaria (CSU) (Zuberbier et al., 2009a), is characterized by itchy wheals (hives), angioedema, or both that recur for >6 weeks and have no apparent external trigger. H1 antihistamines are licensed as first-line treatment for CIU/CSU (Zuberbier et al., 2009b), although many patients continue to experience symptoms despite receiving these drugs at up to four times higher than the approved dose (Asero, 2007; Staevska et al., 2010). Recommended add-on therapy for patients unresponsive to up-dosing of H1 antihistamines includes leukotriene receptor antagonists (LTRAs) and cyclosporine A (Zuberbier et al., 2009b; Maurer et al., 2013a). However, these agents have not received regulatory approval for this indication. Oral corticosteroids are used to treat CIU/CSU exacerbations, but they are not recommended for long-term treatment because of potential safety concerns with chronic use (Zuberbier et al., 2009b).
Cutaneous mast cells, blood basophils, and immunoglobulin E (IgE) have been implicated in the pathophysiology of chronic urticaria (Vonakis and Saini, 2008). Omalizumab, a humanized IgE monoclonal antibody licensed for the treatment of moderate-to-severe (US; patients aged ⩾12 years) and severe (Europe; patients aged ⩾6 years) allergic asthma, recently received approval from the European Medicines Agency and the US FDA (Food and Drug Administration) for the treatment of CIU/CSU (European Medicines Agency, 2014; Genentech, Inc. and Novartis Pharmaceuticals Corporation, 2014). Omalizumab may have a beneficial role in CIU/CSU by reducing mast cell and basophil activation mediated by IgE and its high-affinity receptor (FcɛRI) (Saini and MacGlashan, 2002; Beck et al., 2004; Ong et al., 2005). Data from proof-of-concept and phase II studies indicated that omalizumab improved symptoms and had an acceptable safety profile in patients with CIU/CSU (Gober et al., 2008; Kaplan et al., 2008; Maurer et al., 2011; Saini et al., 2011).
The phase III clinical trial program for omalizumab consisted of three randomized, double-blind, placebo-controlled studies in patients with moderate-to-severe H1 antihistamine–refractory CIU/CSU: ASTERIA I (ClinicalTrials.gov number: NCT01287117), ASTERIA II (NCT01292473), and GLACIAL (NCT01264939). ASTERIA I and ASTERIA II evaluated the efficacy and safety of omalizumab 75 mg, 150 mg, and 300 mg versus placebo in patients with CIU/CSU who remained symptomatic despite treatment with approved doses of H1 antihistamines. These studies were similar in design, except that the double-blind treatment period was twice as long in ASTERIA I (24 weeks) than in ASTERIA II (12 weeks; Supplementary Table S1 online). ASTERIA I was conducted to evaluate the potential effects of treatment over a longer duration, in part because the symptoms of CIU/CSU wax and wane over time. In this study, the introduction of an additional H1 antihistamine was allowed after week 12 with the aim to reduce patient dropout over the longer treatment period. The GLACIAL study was conducted to evaluate the safety and efficacy of omalizumab 300 mg versus placebo in patients with CIU/CSU who remained symptomatic despite receiving up to four times the approved dosage of H1 antihistamines and either an H2 antihistamine or LTRA, or all three in combination. The primary objective of GLACIAL was to assess the safety of omalizumab in patients who were receiving different add-on therapies for CIU/CSU, although efficacy end points from ASTERIA I and II were also evaluated as secondary end points. Background CIU therapies in the GLACIAL population were reflective of the standard-of-care treatments typically used in clinical practice for patients who were refractory to approved dosages of H1 antihistamines. Results for the GLACIAL safety study and the shorter ASTERIA II study have been reported (Kaplan et al., 2013; Maurer et al., 2013b).
Here, we report results from the longer ASTERIA I study that evaluated the efficacy and safety of three doses of omalizumab over a 24-week treatment period in patients with CIU/CSU who remained symptomatic despite treatment with approved dosages of H1 antihistamines.
Results
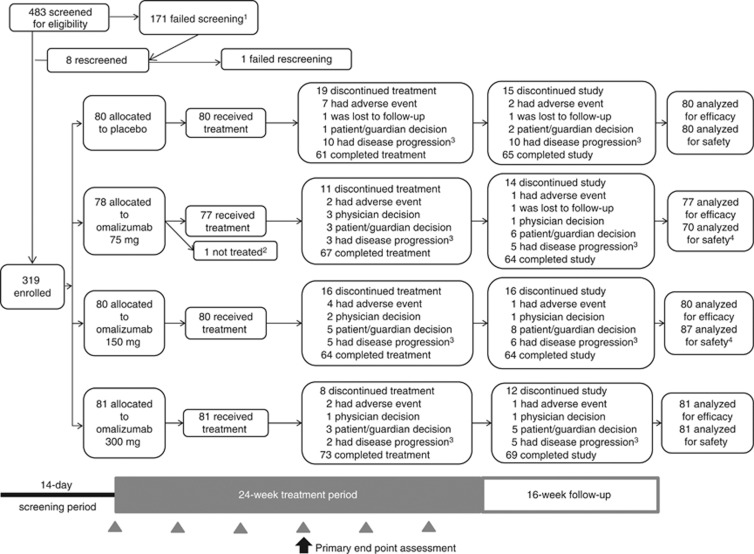
Figure 1 shows the study profile. A total of 319 patients were randomized and 318 received at least one dose of the study drug. Overall, 262 (82.1%) patients completed the 40-week study and 265 (83.1%) patients completed the 24-week treatment period (Figure 1). Baseline characteristics were generally similar across groups (Table 1). Diary compliance was high (>90%) throughout the 40-week study and was similar among groups (Supplementary Table S2 online).
Figure 1.
Patient disposition and study. Gray arrowheads denote study drug treatment on day 1 and at weeks 4, 8, 12, 16, and 20. Black arrow indicates primary end point assessment at week 12. 1Reasons for screen failure: 14.0% of patients were unwilling to give written informed consent, adhere to the visit schedules, and meet study requirements; 10.5% of patients were not diagnosed with chronic idiopathic urticaria/spontaneous urticaria refractory to H1 antihistamines at the time of randomization; and 27.5% of patients were categorized as “Other, not defined.” 2Patient did not receive study drug as a result of not meeting all study eligibility criteria and was therefore not included in the modified intention-to-treat population. 3Defined as either the worsening of or no improvement in symptoms. 4Seven patients randomized to the omalizumab 75-mg group received at least one dose of omalizumab 150 mg during the treatment period and were included in the omalizumab 150-mg group for the safety and pharmacokinetic analyses.
Table 1. Patient demographic and baseline characteristics at randomization1.
|
Omalizumab |
||||
|---|---|---|---|---|
| Characteristic | Placebo (n=80) | 75 mg (n=77) | 150 mg (n=80) | 300 mg (n=81) |
| Age (years) | 40.4 (15.6) | 40.7 (15.2) | 41.1 (14.0) | 42.4 (13.2) |
| Age group, n (%) | ||||
| 12–17 years | 4 (5.0) | 5 (6.5) | 7 (8.8) | 2 (2.5) |
| 18–40 years | 41 (51.3) | 33 (42.9) | 29 (36.3) | 34 (42.0) |
| 41–64 years | 30 (37.5) | 35 (45.5) | 41 (51.3) | 42 (51.9) |
| ⩾65 years | 5 (6.3) | 4 (5.2) | 3 (3.8) | 3 (3.7) |
| Female, n (%) | 52 (65.0) | 55 (71.4) | 64 (80.0) | 60 (74.1) |
| Race, n (%) | ||||
| White | 64 (80.0) | 62 (80.5) | 63 (78.8) | 74 (91.4) |
| Black | 10 (12.5) | 9 (11.7) | 9 (11.3) | 5 (6.2) |
| Other | 6 (7.5) | 6 (7.8) | 8 (10.0) | 2 (2.5) |
| Weight (kg) | 83.0 (20.5) | 81.1 (19.2) | 83.2 (24.4) | 81.6 (19.7) |
| <80 kg, n (%) | 35 (43.8) | 38 (49.4) | 40 (50.0) | 45 (55.6) |
| Body mass index (kg m−2) | 28.7 (6.2) | 29.4 (6.5) | 29.8 (7.7) | 29.3 (6.9) |
| Time since diagnosis of CIU/CSU (years)2 | 7.0 (9.7) | 7.0 (9.7) | 7.6 (9.2) | 6.2 (8.0) |
| CU index test, n (%)3 | 25 (31.3) | 18 (23.4) | 16 (20.3) | 21 (25.9) |
| No. of previous CIU/CSU medications | 5.0 (2.8) | 4.7 (2.8) | 4.5 (3.2) | 4.5 (2.3) |
| Median (range) total IgE level (IU ml−1)4 | 92.0 (1–1,010) | 91.0 (1–2,030) | 71.0 (1–5,000) | 85.5 (1–2,330) |
| In-clinic UAS5 | 5.3 (0.8) | 5.3 (0.8) | 5.3 (0.7) | 5.3 (0.8) |
| UAS76 | 31.1 (6.7) | 31.7 (6.7) | 30.3 (7.3) | 31.3 (5.8) |
| Weekly ISS6 | 14.4 (3.5) | 14.5 (3.6) | 14.1 (3.8) | 14.2 (3.3) |
| ⩾13, n (%) | 54 (67.5) | 49 (63.6) | 54 (67.5) | 53 (65.4) |
| Weekly number of hives score6 | 16.7 (4.4) | 17.2 (4.2) | 16.2 (4.6) | 17.1 (3.8) |
| Overall DLQI score7 | 14.0 (6.6) | 12.8 (6.1) | 13.6 (7.1) | 13.0 (6.7) |
| Angioedema present, n (%)6 | 44 (55.0) | 35 (45.5) | 38 (47.5) | 34 (42.0) |
Abbreviations: CIU/CSU, chronic idiopathic urticaria/chronic spontaneous urticaria; CU, chronic urticaria; DLQI, Dermatology Life Quality Index; IgE, immunoglobulin E; ISS, itch severity score; UAS, urticaria activity score; UAS7, urticaria activity score over 7 days.
Analyses are based on the modified intention-to-treat population. Data are presented as mean (standard deviation) unless otherwise stated.
Placebo, n=78; omalizumab 75 mg, n=76; omalizumab 150 mg, n=78; omalizumab 300 mg, n value as stated.
Determined using the CU index test (Viracor-IBT Laboratories, Lee's Summit, MO); n values reflect those for the modified intention-to-treat population except for omalizumab 150 mg, n=79.
Placebo, n=77; omalizumab 75 mg, n=75; omalizumab 150 mg, n=74; omalizumab 300 mg, n=80.
Defined as the largest value from the day −14 screening visit, day −7 screening visit, and day 1 visit.
Based on data collected in a patient daily diary in the 7 days before the first treatment date.
n values reflect those for the modified intention-to-treat population except for placebo, n=79, and omalizumab 75 mg, n=75.
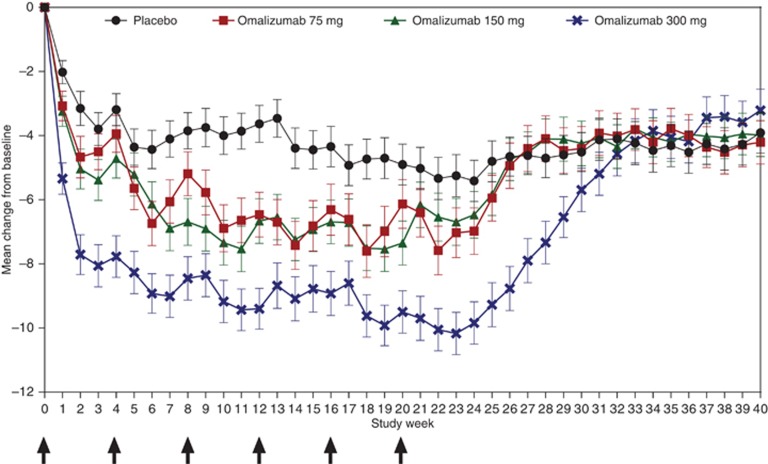
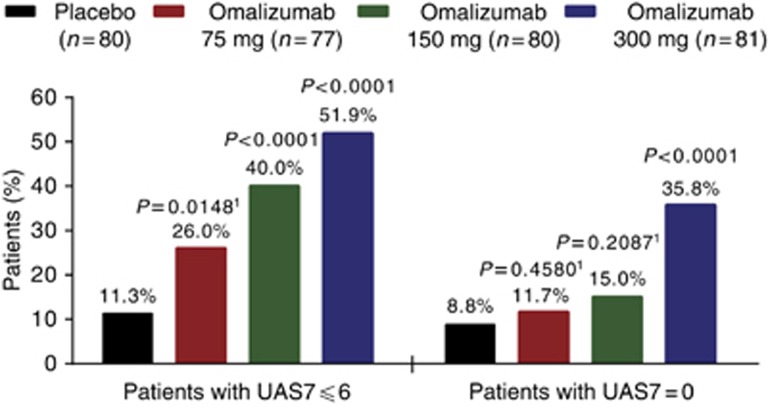
At week 12, mean weekly itch severity score (ISS) decreased from baseline (primary end point) by 6.46, 6.66, and 9.40 points in the omalizumab 75-mg, 150-mg, and 300-mg groups, respectively, versus the placebo group (3.63 points; Table 2). The difference between each omalizumab group and placebo was significant in favor of omalizumab (75 mg, P=0.0010; 150 mg, P=0.0012; 300 mg, P<0.0001). Reductions from baseline in weekly ISS were observed as early as week 1 in all groups (placebo, 2.02 points; omalizumab 75 mg, 3.07 points; omalizumab 150 mg, 3.24 points; omalizumab 300 mg, 5.34 points; Figure 2). Median time to reach the minimally important difference (MID) in weekly ISS was 3, 2, and 1 weeks in the omalizumab 75-mg, 150-mg, and 300-mg groups, respectively, versus 4 weeks in the placebo group (Table 2). Improvements in urticaria activity score (UAS) over 7 days (UAS7; Supplementary Figure S1 online) and weekly number of hives score (Supplementary Figure S2 online) over time were similar to those observed for weekly ISS (Figure 2). Proportions of patients who achieved well-controlled disease (UAS7⩽6) and those with complete response (UAS7=0) at week 12 were more than four times higher in the omalizumab 300-mg group than in the placebo group (Figure 3; both P<0.0001). Significant improvements compared with placebo were observed on all nine end points in the omalizumab 300-mg group, six of nine end points in the omalizumab 150-mg group, and two of nine end points in the omalizumab 75-mg group (Table 2; Supplementary Table S3 online). Sensitivity analyses of the primary end point using different methods for handling missing data confirmed those of the primary analysis (see Supplementary Material online).
Table 2. Primary and selected secondary efficacy end points1.
|
Omalizumab |
||||
|---|---|---|---|---|
| Placebo (n=80) | 75 mg (n=77) | 150 mg (n=80) | 300 mg (n=81) | |
| Primary end point | ||||
| Change from baseline in ISS to week 12 | ||||
| Mean (SD) | −3.63 (5.22) | −6.46 (6.14) | −6.66 (6.28) | −9.40 (5.73) |
| Median (range) | −2.3 (−18.5 to 7.5) | −6.0 (−21.0 to 4.0) | −6.0 (−21.0 to 5.0) | −10.0 (−19.5 to 0) |
| LSM treatment difference versus placebo (95% CI) | – | −2.96 (−4.71 to −1.21) | −2.95 (−4.72 to −1.18) | −5.80 (−7.49 to −4.10) |
| P-value versus placebo2 | – | 0.0010 | 0.0012 | <0.0001 |
| Selected secondary end points | ||||
| Change from baseline in UAS7 to week 12 | ||||
| Mean (SD) | −8.01 (11.47) | −13.82 (13.26) | −14.44 (12.95) | −20.75 (12.17) |
| Median (range) | −4.0 (−39.0 to 14.5) | −13.0 (−42.0 to 7.0) | −14.8 (−40.0 to 4.5) | −22.0 (−40.0 to 1.0) |
| LSM treatment difference versus placebo (95% CI) | – | −5.75 (−9.59 to −1.92) | −6.54 (−10.33 to −2.75) | −12.80 (−16.44 to −9.16) |
| P-value versus placebo2 | – | 0.0035 | 0.0008 | <0.0001 |
| Time to MID in weekly ISS (weeks) | ||||
| Median (95% CI) | 4.0 (2.0–6.0) | 3.0 (2.0–5.0) | 2.0 (2.0–3.0) | 1.0 (1.0–2.0) |
| Minimum, maximum | 1.0, 12.0+ | 0+, 12.0+ | 1.0, 12.0+ | 0+, 12.0+ |
| Hazard ratio versus placebo (95% CI)3 | – | 1.39 (0.95–2.03) | 1.49 (1.04–2.14) | 2.34 (1.63–3.36) |
| P-value versus placebo | – | 0.0879 | 0.0301 | <0.0001 |
| Patients with MID in weekly ISS at week 12, n (%) | 29 (36.3) | 43 (55.8) | 45 (56.3) | 61 (75.3) |
| P-value versus placebo | – | 0.01184 | 0.0226 | <0.0001 |
Abbreviations: CI, confidence interval; ISS, itch severity score; LSM, least-squares mean; MID, minimally important difference; SD, standard deviation; UAS7, urticaria activity score over 7 days.
Analyses are based on the modified intention-to-treat population with missing week 12 scores imputed using the baseline weekly score.
Derived from an analysis of covariance t-test.
In this analysis, the hazard ratio represents the likelihood that a patient in a given omalizumab dose group will achieve MID response in weekly ISS relative to patients in the placebo group at any point in time up to week 12.
Not evaluated for statistical significance in accordance with the type 1 error control plan.
Figure 2.
Mean weekly itch severity score by study week. Error bars represent standard error of the mean. Arrows indicate study drug injection day. Analyzed on the basis of the modified intention-to-treat population with missing weekly scores imputed using baseline weekly itch severity scores.
Figure 3.
Responder analysis. P-value versus placebo derived from the Cochran–Mantel–Haenszel χ2-test stratified by baseline UAS7 (<median, ⩾median) and baseline weight (<80 kg, ⩾80 kg). 1Not evaluated for statistical significance in accordance with the type I error control plan. UAS7, urticaria activity score over 7 days.
Magnitudes of mean changes from baseline to week 24 for weekly ISS, UAS7, weekly number of hives score, and weekly size of largest hive score for each omalizumab group (Supplementary Table S3 online) were similar to those observed at week 12 (Table 2 and Supplementary Table S2 online). However, in the placebo group, magnitudes of mean changes in these outcomes were greater from baseline to week 24 than to week 12, resulting in generally smaller treatment effects at week 24. The omalizumab 300-mg group showed sustained improvements from baseline in symptom scores at week 24 (Supplementary Table S3 online). After week 24 (follow-up period), mean weekly ISS in the omalizumab groups increased to values similar to those in the placebo group, but did not return to baseline mean values for the duration of follow-up (Figure 2).
During the treatment period, the percentage of patients with a new onset of antihistamine was numerically lower in the omalizumab 300-mg group (6.2%, n=5) than in the placebo (13.8%, n=11), omalizumab 75-mg (16.9%, n=13), or omalizumab 150-mg (12.5%, n=10) groups. At week 12, a reduction in rescue medication use from baseline was observed in the omalizumab 150- and 300-mg groups versus placebo (P<0.03; Supplementary Table S3 online).
At baseline, mean Dermatology Life Quality Index (DLQI) scores ranged from 12.8 to 14.0 across groups (Table 1; higher score=greater impairment). At week 12, mean (standard deviation) DLQI score decreased from baseline by 10.29 (7.23) points in the omalizumab 300-mg group versus 6.13 (6.25) points in the placebo group (Supplementary Table S3 online). The difference between these groups was significant in favor of omalizumab (P<0.0001). Improvement in the Chronic Urticaria Quality-of-Life Questionnaire overall score at week 12 was consistent with that observed for the DLQI (Supplementary Table S3 online). Patients in the omalizumab 300-mg group experienced a greater mean proportion of angioedema-free days during weeks 4 to 12 (96.1% (95% confidence interval (CI): 93.5–98.7)) versus the placebo group (88.2% (95% CI: 83.5–93.0); P<0.0001; Supplementary Table S3 online). The proportion of patients with angioedema decreased in all groups over the 24-week treatment period (Supplementary Table S5 online). Overall, results of the prespecified subgroup and exploratory analyses (Supplementary Figure S3 online; Supplementary Table S4 online) were generally consistent with those of the primary analyses.
Safety
During the 24-week treatment period, the proportions of patients who experienced one or more treatment-emergent adverse events (AEs) ranged from 57 to 69% in the omalizumab groups versus 51% in the placebo group (Table 3; Supplementary Table S6 online). Headaches, arthralgia, and injection-site reactions were more common in the omalizumab groups than in the placebo group. The majority of AEs were mild or moderate in intensity (Supplementary Table S7 online).
Table 3. Summary of AEs according to study group over the 40-week study period1.
|
Omalizumab |
||||
|---|---|---|---|---|
| Patients, n (%) | Placebo (n=80) | 75 mg (n=70) | 150 mg (n=87) | 300 mg (n=81) |
| Any AE | 53 (66.3) | 55 (78.6) | 72 (82.8) | 57 (70.4) |
| Any AE during treatment period | 41 (51.3) | 41 (58.6) | 60 (69.0) | 46 (56.8) |
| Any AE during follow-up period | 32 (40.0) | 36 (51.4) | 45 (51.7) | 38 (46.9) |
| Any AE leading to discontinuation of study drug | 7 (8.8) | 2 (2.9) | 4 (4.6) | 2 (2.5) |
| Early discontinuation from study owing to AE | 2 (2.5) | 0 | 2 (2.3) | 1 (1.2) |
| Any serious AE | 5 (6.3) | 2 (2.9) | 5 (5.7) | 2 (2.5) |
| Serious AE during treatment period | 4 (5.0) | 2 (2.9) | 3 (3.4) | 0 |
| Serious AE during follow-up period | 1 (1.3) | 0 | 2 (2.3) | 2 (2.5) |
| Death | 0 | 0 | 0 | 0 |
| Any AE suspected to be caused by study drug | 4 (5.0) | 6 (8.6) | 9 (10.3) | 14 (17.3) |
| Any severe AE | 8 (10.0) | 7 (10.0) | 8 (9.2) | 13 (16.0) |
| Severe AE during treatment period | 8 (10) | 5 (7.1) | 5 (5.7) | 3 (3.7) |
| Severe AE during follow-up period | 1 (1.3) | 3 (4.3) | 4 (4.6) | 10 (12.3) |
Abbreviation: AE, adverse event.
Data are for the safety population.
The proportion of patients with AEs reported as suspected to be caused by study drug increased as the dose of omalizumab increased (Table 3). The dose-dependent trend was not clustered by a specific AE type. The largest numerical differences occurred between the omalizumab 300-mg and placebo groups for headache (4 vs. 0 patients) and injection-site reactions (3 vs. 1 patient). The majority of AEs suspected to be caused by study drug were mild (34/50) or moderate in intensity (14/50); 10 of the moderate events were in the omalizumab 300-mg group. Two severe events were reported, one each in the omalizumab 150-mg and 300-mg groups. During the treatment period, 2 (2.9%), 3 (3.4%), and 0 patients in the omalizumab 75-mg, 150-mg, and 300-mg groups, respectively, experienced a serious AE compared with 4 (5.0%) patients in the placebo group (Table 3; Supplementary Table S8 online). None of these was assessed by the investigator to be related to the study drug. There were no deaths during the study.
There were no observations of treatment-emergent AEs considered to be malignancies in the omalizumab groups. At database lock, an event of cervical dysplasia was reported in one patient (placebo group); however, after receiving the pathology report for this patient post-database lock, it was determined that the patient experienced cervical adenocarcinoma in situ (see Supplementary Material online). Three patients with suspected anaphylaxis (two during omalizumab treatment and one 142 days post final dose of study drug) were referred for blinded external adjudication. The two events during omalizumab treatment were judged to be not anaphylaxis and anaphylaxis not attributed to study drug, respectively. The event 142 days post treatment was judged to be dipryone-induced anaphylaxis (see Supplementary Material online).
Discussion
ASTERIA I is one of the largest clinical studies in patients with H1 antihistamine–refractory CIU/CSU to date. Strengths of this study include its size and high rates of diary compliance; the main results were confirmed by the pre-planned sensitivity analyses. Efficacy results in ASTERIA I were consistent with those from ASTERIA II and GLACIAL (Kaplan et al., 2013; Maurer et al., 2013b). The baseline characteristics of patients were generally similar among the three studies except for the number of previous medications used for CIU/CSU, which was slightly higher in GLACIAL (median, 6) than in ASTERIA I and ASTERIA II (median, 4 for both). In ASTERIA II, significant improvements from baseline in weekly ISS at week 12 compared with placebo were observed in the omalizumab 150-mg and 300-mg groups, but not in the 75-mg group, which differs from the results of the current study. It is possible that this difference may have resulted from the higher placebo effect in ASTERIA II compared with ASTERIA I (placebo mean change from baseline: –5.1 and –3.6, respectively). In both studies, dose-dependent effects were observed for most of the prespecified secondary end points, with omalizumab 300 mg affording greatest efficacy (Maurer et al., 2013b). A return of symptoms was observed in both the current study and ASTERIA II (Maurer et al., 2013b) during follow-up, reaching mean values similar to those in the placebo group but not returning to baseline values. One possible explanation for this observation that occurred in all treatment groups is that some patients may have experienced spontaneous remission of their disease. The safety data in ASTERIA I confirm those observed in ASTERIA II (Maurer et al., 2013b) and were consistent with the established profile of omalizumab in allergic asthma (Maurer et al., 2011; Saini et al., 2011; Maurer et al., 2013b; European Medicines Agency, 2014; Genentech, Inc. and Novartis Pharmaceuticals Corporation, 2014). Improvements across all end points for the omalizumab 300-mg group were maintained to week 24 in both ASTERIA I and GLACIAL, and similar safety data were observed in these studies (Kaplan et al., 2013).
The results of this study may not be generalizable to all patients with CIU/CSU encountered in clinical practice or to patients with inducible urticaria. One limitation is that safety and efficacy have not been studied in patients with treatment longer than 24 weeks; however, the long-term safety profile of omalizumab has been well established in allergic asthma (Tan and Corren, 2011).
In this study, omalizumab significantly reduced symptoms after 12 weeks of treatment in patients with CIU/CSU who remained symptomatic despite treatment with approved doses of H1 antihistamines. Median time to MID in weekly ISS was shortest in the omalizumab 300-mg group and suggests a relationship between omalizumab dose and time to onset. Our current findings not only confirm those from ASTERIA II, but also extend them beyond 12 weeks of treatment and show a sustained treatment effect of omalizumab 300 mg for up to 24 weeks on CIU/CSU symptom scores in patients with H1 antihistamine–refractory CIU/CSU. The safety profile for omalizumab over 24 weeks of treatment in patients with CIU/CSU receiving approved doses of H1 antihistamines was consistent with the established safety profile in allergic asthma and with previous observations in CIU/CSU.
Materials and Methods
Study design and participants
This was a global phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study to investigate the efficacy and safety of omalizumab in adult and adolescent patients with CIU/CSU who remained symptomatic despite treatment with approved doses of H1 antihistamines. It was carried out at 53 centers in Denmark, France, Germany, Italy, Poland, Spain, Turkey, and the US between February 2011 and October 2012. The study comprised a 14-day screening period, 24-week double-blind treatment period, and 16-week follow-up period during which omalizumab was not administered (Figure 1). For the first 12 weeks of the treatment period, participants were required to maintain stable doses of their prerandomization H1 antihistamine treatment. During weeks 13 to 24 of the treatment period, patients were allowed to add one additional H1 antihistamine. Patients were permitted to take diphenhydramine 25 mg as needed for itch relief (up to a maximum of three doses per 24 hours, or less if required by local regulations) throughout the entire study period.
Patients aged 12–75 years (18–75 years in Germany) with a diagnosis of CIU/CSU for ⩾6 months who had hives and itching for ⩾8 consecutive weeks at any time before enrollment despite H1 antihistamine treatment were eligible for study inclusion. Participants were required to meet the following criteria during the screening period: the use of an approved dosage of an H1 antihistamine for CIU/CSU for ⩾3 consecutive days immediately before day −14 with documented use on the day of the initial screening visit; in-clinic physician-assessed UAS ⩾4 (range 0–6; see Procedures) on one or more screening days (day −14, −7, or 1); UAS7 (range 0–42) of ⩾16 and itch component of UAS7 (range 0–21) of ⩾8 during the 7 days before randomization; willing and able to complete a symptom diary with an electronic hand-held device (eDiary; Urticaria Patient Daily Diary) twice daily during the study; and no missing eDiary entries during the 7 days before randomization. Exclusion criteria included the following: clearly defined underlying etiology for chronic urticaria (e.g., cold, pressure); the presence of a disease with symptoms of urticaria or angioedema, including hereditary or acquired angioedema; routine doses (daily or every other day for ⩾5 consecutive days) of systemic steroids, hydroxychloroquine, methotrexate, cyclosporine, cyclophosphamide, or intravenous immunoglobulin⩽30 days of day −14; use of H2 antihistamines or LTRAs⩽7 days of day −14; use of H1 antihistamines at greater than approved doses⩽3 days of day −14; history of malignancy; weight <20 kg; hypersensitivity to omalizumab; or previous treatment with omalizumab within the previous year.
The study protocol was approved by the institutional review board or ethics committee at each center. The study was conducted in accordance with US FDA regulations, the International Conference on Harmonization E6 Guideline for Good Clinical Practice, Declaration of Helsinki, and any other applicable country laws. The study is registered with ClinicalTrials.gov, NCT01287117. There was one amendment to the protocol based on feedback from the US FDA, Genentech, Inc. staff, and external advisors. The protocol was amended before the study began and registration at ClinicalTrials.gov (see Supplementary Material online). An independent data monitoring committee was established to monitor study conduct and to review blinded and unblinded safety data at 6-month intervals. A blinded external anaphylaxis review committee adjudicated suspected cases of anaphylaxis. Written informed consent was obtained from each participant or, if the participant was aged <18 years, their parent or legal guardian.
Randomization and masking
On day 1, patients were randomized in a 1:1:1:1 ratio using an interactive voice and web response system to receive subcutaneous doses of omalizumab 75 mg, 150 mg, or 300 mg or placebo at intervals of 4 weeks during the 24-week treatment period (six doses). Randomization to treatment group was stratified by baseline weekly ISS (<13, ⩾13), baseline weight (<80 kg, ⩾80 kg), and study site (see Supplementary Material online). All patients, evaluating physician(s), the sponsor and its agents, and study personnel were masked to treatment assignment, except for the site pharmacist(s) who prepared the study drug but who did not interact with the patients.
Procedures
Patient-reported outcomes were recorded in the Urticaria Patient Daily Diary using an electronic hand-held device. Itch severity, number of hives, and size of largest hive (all scored 0–3) were recorded every morning and evening. Sleep interference (range 0−3), activity interference (range 0–3), rescue medication use (range 0–9), angioedema (yes, no), angioedema treatment, and healthcare contacts were recorded once daily (see Supplementary Material online). Urticaria Patient Daily Diary compliance was evaluated throughout the study. The UAS is a composite eDiary-recorded score with severity intensity ratings (range: 0=none to 3=intense/severe) for the number of hives and intensity of itch, measured twice daily (morning and evening). The daily UAS is the average of the morning and evening scores (range 0–6 points per day), and the UAS7 is the sum of the daily UAS scores over 7 days (total score 0–42) (Mathias et al., 2010). Health-related quality of life was assessed using the DLQI questionnaire (range 0–30; higher score represents greater impairment) (Finlay and Khan, 1994).
The primary end point was change from baseline in weekly ISS (i.e., sum of the daily ISS for 7 days; range 0–21) at week 12. Secondary end points, all evaluated at week 12, included changes from baseline in UAS7 and weekly number of hives score; time to MID response (⩾5-point decrease) in weekly ISS; the proportion of patients with UAS7⩽6; the proportion of weekly ISS MID responders; changes from baseline in weekly size of largest hive score and overall DLQI score; the proportion of angioedema-free days during weeks 4 to 12; and the proportion of patients with complete response (UAS7=0). Subgroup analyses were performed based on demographic and baseline variables to evaluate the consistency of the primary efficacy results. Exploratory end points included the assessment of efficacy end points at week 24, the proportion of itch-free days and/or hive-free days at weeks 12 and 24, and change from baseline to week 12 in Chronic Urticaria Quality-of-Life Questionnaire score (see Supplementary Material online). In addition, time to relapse (loss of UAS7⩽6) during the follow-up period was assessed in patients with UAS7 response⩽6 at week 24. The proportion of patients with UAS7⩽6 at week 24 who maintained their response at week 40 also was evaluated. Safety was evaluated by recording and monitoring incidence and severity of AEs and serious AEs and changes in vital signs and clinical laboratory values. The presence of antibodies against omalizumab was evaluated from blood samples at week 40.
Statistical analysis
For the primary end point, analysis of covariance models stratified by baseline weekly ISS (<13, ⩾13) and baseline weight (<80 kg, ⩾80 kg) were used to generate least-squares means of the differences between each of the omalizumab groups and the placebo group. Similar models were used for the secondary end points that measured change from baseline. Efficacy analyses were conducted using data from the modified intention-to-treat population (i.e., randomized patients who received one or more doses of study drug). Missing data at week 12 were imputed with the baseline observation carried forward in analyses of end points evaluating change from baseline to week 12 (except for the DLQI, for which no imputation was performed). All statistical tests were two-sided with an overall significance level of 0.05. Adjustments for multiple comparisons were performed according to the type I error control plan (see Supplementary Material online).
The sample size for this study was based on safety and regulatory considerations. The estimation of power for efficacy assumed a mean change from baseline to week 12 in weekly ISS of 9 points in the omalizumab groups and 3.5 points in the placebo group, with a common standard deviation of 6 points and an early discontinuation rate of 15% by week 12; enrollment of 300 patients (75 in each group) would yield ∼98% power to detect a treatment effect for the primary end point.
Acknowledgments
The ASTERIA I study was funded by Genentech, Inc., South San Francisco, CA, and Novartis Pharma AG, Basel, Switzerland. Medical writing support for this manuscript was provided by Alison Gagnon of Excel Scientific Solutions and funded by Genentech, Inc., and Novartis Pharma AG. The trial was registered with ClinicalTrials.gov, number NCT01287117. This project was funded by Genentech, Inc. and Novartis Pharma AG. ASTERIA I Investigators: Denmark: Carsten Bindslev-Jensen, Tonny Karlsmark; France: Jean-Jacques Grob, Ziad Reguiai, Alain Taieb; Germany: Randolf Brehler, Thomas Hoelting, Marcus Maurer, Franziska Rueff, Knutt Schaekel; Italy: Adriano Mari; Poland: Piotr Kuna, Krystyna Obtulowicz, Ewa Trebas-Pietras, Jolanta Weglowska; Spain: Ana Maria Gimenez Arnau, Esther Serra Baldrich; Turkey: Emel Bülbül Baskan; US: Oral Alpan, Robert Chrzanowski, Pramila Daftary, Daniel Ein, Sandra M. Gawchik, Pinkus Goldberg, Alan Goldsobel, Michael Kaplan, Alan Kaufman, Adina Knight, Phillip E. Korenblat, Bobby Lanier, Miguel Lanz, Fu-Tong Liu, Patricia Lugar, Michael Marcus, Donald McNeil, Isaac Melamed, Steven Meltzer, Anthony Montanaro, Mark Moss, Thomas Murphy, Andrew Pedinoff, Syed Rehman, David Riester, Ronald Saff, Sarbjit Saini, Joseph Shapiro, Dareen Siri, David Skoner, Ricardo Tan, Kay Walker, Steven M. Weinstein, Hugh Windom.
Glossary
- AE
adverse event
- CIU
chronic idiopathic urticaria
- CSU
chronic spontaneous urticaria
- DLQI
Dermatology Life Quality Index
- IgE
immunoglobulin E
- ISS
itch severity score
- LTRA
leukotriene receptor antagonist
- MID
minimally important difference
- UAS
urticaria activity score
- UAS7
urticaria activity score over 7 days
SSS has received research support from Astra-Zeneca, Genentech, Inc., the National Institutes of Health, and Novartis, and has served as a consultant to Array, Genentech, Inc., Kendle, Medimmune, Novartis, and Pharmacyclics. SSS also serves as an interest section leader for American Academy of Allergy, Asthma & Immunology, section editor for UpToDate, and section editor for the Journal of Investigative Dermatology. CB-J has received research funding from Genentech, Inc., Meda, MSD, Novartis, Schering-Plough, Shire, Stallergenes, and Termo Fisher and has served as a speaker for Faes Farma, MSD, and Termo Fisher. MM has received research funding from Almirall, Faes Farma, Novartis, MSD, UCB, and Uriach and has served as a speaker and/or advisor for Almirall, Genentech, Inc., Merckle Recordati, Moxie, MSD, Novartis, Sanofi-Aventis, Schering-Plough, UCB, and Uriach. J-JG has received funding and/or honoraria for advisor or speaker functions from Almirall, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Meda, Merck, and Roche. SS has received research funding from Array, Astra-Zeneca, Forest, Genentech, Inc., GlaxoSmithKline, Johnson & Johnson, KaloBios, Merck, Mylan, Novartis, Revalesio, Rigel, Roxane, Sanofi-Aventis, Teva, and Vectura. KR is employed by Genentech, Inc. and receives stock/stock options from Roche. AR is employed by Genentech, Inc. and receives stock/stock options from Roche. MSB is employed by Genentech, Inc. and receives stock options from Roche. JC is employed by and receives stock/stock options from Novartis Pharma. PG is employed by and receives stock/stock options from Novartis Pharma AG. The remaining authors declare no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
AUTHOR CONTRIBUTIONS
SSS, MM, SS, CB-J, MSB, JC, and KR participated in the design of the study. SSS, CB-J, MM, J-JG, EBB, OA, and SS participated in patient accrual and data collection. All authors analyzed and interpreted the data. MSB was the study biostatistician responsible for the statistical analyses. All authors were members of the writing group and participated in the development of the report, agreed on the content, reviewed drafts, and approved the final version.
Supplementary Material
References
- Asero R. Chronic unremitting urticaria: is the use of antihistamines above the licensed dose effective? A preliminary study of cetirizine at licensed and above-licensed doses. Clin Exp Dermatol. 2007;32:34–8. doi: 10.1111/j.1365-2230.2006.02278.x. [DOI] [PubMed] [Google Scholar]
- Beck LA, Marcotte GV, MacGlashan D, et al. Omalizumab-induced reductions in mast cell FcɛRI expression and function. J Allergy Clin Immunol. 2004;114:527–30. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency 2014. Summary of product characteristics (omalizumab (Xolair)). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000606/WC500057298.pdf last accessed 3 April 2014
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Genentech, Inc. and Novartis Pharmaceuticals Corporation 2014. Xolair (omalizumab) prescribing information. http://www.gene.com/gene/products/information/pdf/xolair-prescribing.pdf last accessed 3 April 2014
- Gober LM, Sterba PM, Eckman JA, et al. Effect of anti-IgE (omalizumab) in chronic idiopathic urticaria (CIU) patients (abstract) J Allergy Clin Immunol. 2008;121 (suppl 1:S147. [Google Scholar]
- Kaplan A, Ledford D, Ashby M, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101–9. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Kaplan AP, Joseph K, Maykut RJ, et al. Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol. 2008;122:569–73. doi: 10.1016/j.jaci.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Mathias SD, Dreskin SC, Kaplan A, et al. Development of a daily diary for patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2010;105:142–8. doi: 10.1016/j.anai.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Maurer M, Altrichter S, Bieber T, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202–9. doi: 10.1016/j.jaci.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Maurer M, Magerl M, Metz M, et al. Revisions to the international guidelines on the diagnosis and therapy of chronic urticaria. J Dtsch Dermatol Ges. 2013;11:971–978. doi: 10.1111/ddg.12194. [DOI] [PubMed] [Google Scholar]
- Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–35. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- Ong YE, Menzies-Gow A, Barkans J, et al. Anti-IgE (omalizumab) inhibits late-phase reactions and inflammatory cells after repeat skin allergen challenge. J Allergy Clin Immunol. 2005;116:558–64. doi: 10.1016/j.jaci.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Saini S, Rosen KE, Hsieh H-J, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine–refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567–73. doi: 10.1016/j.jaci.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Saini SS, MacGlashan D. How IgE upregulates the allergic response. Curr Opin Immunol. 2002;14:694–7. doi: 10.1016/s0952-7915(02)00404-1. [DOI] [PubMed] [Google Scholar]
- Staevska M, Popov TA, Kralimarkova T, et al. The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J Allergy Clin Immunol. 2010;125:676–82. doi: 10.1016/j.jaci.2009.11.047. [DOI] [PubMed] [Google Scholar]
- Tan RA, Corren J. Safety of omalizumab in asthma. Expert Opin Drug Saf. 2011;10:463–71. doi: 10.1517/14740338.2011.563840. [DOI] [PubMed] [Google Scholar]
- Vonakis BM, Saini SS. New concepts in chronic urticaria. Curr Opin Immunol. 2008;20:709–16. doi: 10.1016/j.coi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbier T, Asero R, Bindslev-Jensen C, Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. et al. EAACI/GA2LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417–1426. doi: 10.1111/j.1398-9995.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- Zuberbier T, Asero R, Bindslev-Jensen C, Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization et al. 2009EAACI/GA2LEN/EDF/WAO guideline: management of urticaria Allergy 641427–1443.19772513 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.