TO THE EDITOR
Familial keratoacanthomas (KAs) are characterized by the appearance of multiple epithelial tumors that are believed to arise from adjoining hair follicles (Schwartz, 1994). These lesions, which phenotypically and histologically resemble squamous cell carcinomas (SCCs) (Cribier et al., 1999), have a fast evolution and spontaneous regression. To date, four familial forms of multiple KAs have been described: (1) multiple self-healing squamous epithelioma (MSSE; Smith, 1948); (2) the Grzybowski syndrome (Grzybowski, 1950); (3) the Witten–Zak syndrome (Witten and Zak, 1952); and 4) the Muir-Torre syndrome (Muir et al., 1967). The Muir-Torre syndrome (MIM#158320) is caused by mutations in the DNA-repair genes MSH2/MLH1 (Bapat et al., 1996; Kruse et al., 1998), and MSSE (MIM#132800) is due to mutations in TGFBR1 (Goudie et al., 2011), but the causative genes underlying the other two familial KAs are still not known.
We describe here, in a five-generation Tunisian family comprising 27 affected individuals with autosomal dominant transmission of palmoplantar KAs, an unreported syndrome that we have named multiple self-healing palmoplantar carcinoma (MSPC) (Figure 1). MSPC has the distinctive feature of mainly affecting epithelial tissues that are devoid of hair follicles, such as palmoplantar skin and the conjunctival epithelium.
Figure 1.
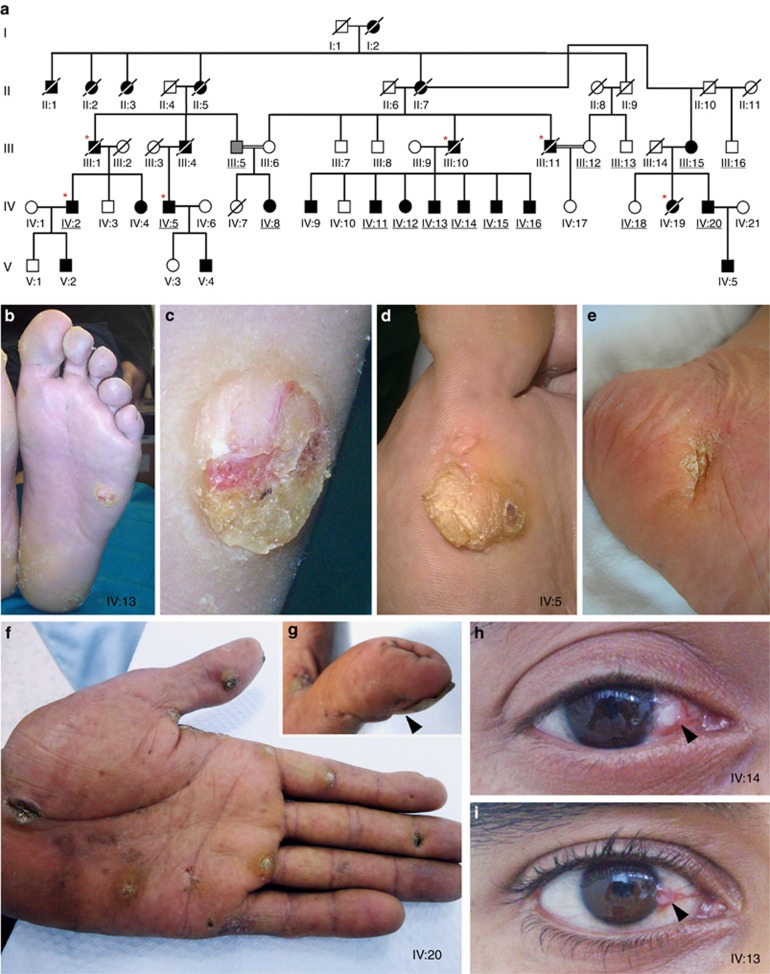
Clinical manifestations of autosomal dominant multiple self-healing palmoplantar carcinoma (MSPC). (a) Pedigree of a five-generation Tunisian family segregating autosomal dominant MSPC (▪ affected man, □: healthy man, ○: healthy woman, ●: affected woman,  : unknown phenotype, *: propositi having developed cancer, underlined: genotyped individual). Clinical histories and physical examinations were undertaken after obtaining informed written consent from all patients and Institutional Review Board approval. (b) Six-week-old primary plantar keratoacanthoma (KA) with a ulcerative-nodular aspect (IV:13). (c) Higher magnification of tumor (20 mm). (d) Evolution of primary KA toward a verrucous tumor (IV:5). (e) Atrophic scar after complete regression of primary KA (IV:5). (f) Multiple lesions in palmar skin showing over 12 KAs at various phases of development ranging from maturation, regression, to final pitted-scar stage (IV:20). (g) Subungual lesion on the thumb. (h, i) Unilateral conjunctival KA (IV:13 and IV:14). Photographs are reproduced with permission from patients.
: unknown phenotype, *: propositi having developed cancer, underlined: genotyped individual). Clinical histories and physical examinations were undertaken after obtaining informed written consent from all patients and Institutional Review Board approval. (b) Six-week-old primary plantar keratoacanthoma (KA) with a ulcerative-nodular aspect (IV:13). (c) Higher magnification of tumor (20 mm). (d) Evolution of primary KA toward a verrucous tumor (IV:5). (e) Atrophic scar after complete regression of primary KA (IV:5). (f) Multiple lesions in palmar skin showing over 12 KAs at various phases of development ranging from maturation, regression, to final pitted-scar stage (IV:20). (g) Subungual lesion on the thumb. (h, i) Unilateral conjunctival KA (IV:13 and IV:14). Photographs are reproduced with permission from patients.
Phenotypically, the development of skin lesions begins with a progressive appearance of multiple ulcerative-nodular tumors on plantar skin (Figure 1b–e). The average age of onset was 8.8 years for the 15 propositi studied and ranged from 1 to 25 years (Supplementary Figure S1a online). Palmoplantar tumors (5–50 mm in diameter, 8–12 in number) grew over a period of 3 months and spontaneously regressed after 6 months, leaving atrophic scars consistent with KAs (Figure 1e). Histological examination of a primary plantar lesion and peri-lesional skin of patient IV:2 showed a massive epidermal hyperkeratosis overlying an endophytic squamoproliferative tumor with prominent keratin cyst formation and crypt abscesses and a dense stromal inflammatory infiltrate at the base (Supplementary Figure S1b–f online). Notably, the granular layer, which is prominent in normal palmoplantar epidermis, was nearly absent. Histological findings are suggestive of a verrucous carcinoma, a low-grade SCC.
In addition to palmoplantar tumors, 80% of studied propositi also developed conjunctival lesions, which appeared in the second decade of life (Figure 1h and i). All were surgically removed, and histological examination of one such tumor revealed squamous epithelial lobules with a dyskeratotic center, suggestive of a conjunctival KA (data not shown). The use of retinoids (Etretinate or Acitretin) halted the progression of the palmoplantar tumors but also stopped their self-regression to a final pitted-scar stage. In cases of resistance to retinoid treatment, palmoplantar tumors were operated upon. Despite these precautions, 5 out of 15 (33%) affected patients whose medical records were available had developed malignant tumors in the lungs (III:10) and head and neck (III:11 and IV:19), SCC on the nose (IV:5), and SCC with bone metastasis (IV:2) (Supplementary Figure S1a online). Patient IV:2 developed both labial (Figure 2a) and heel SCC, which infiltrated the calcaneus bone (Figure 2b–e), requiring bilateral leg amputation.
Figure 2.
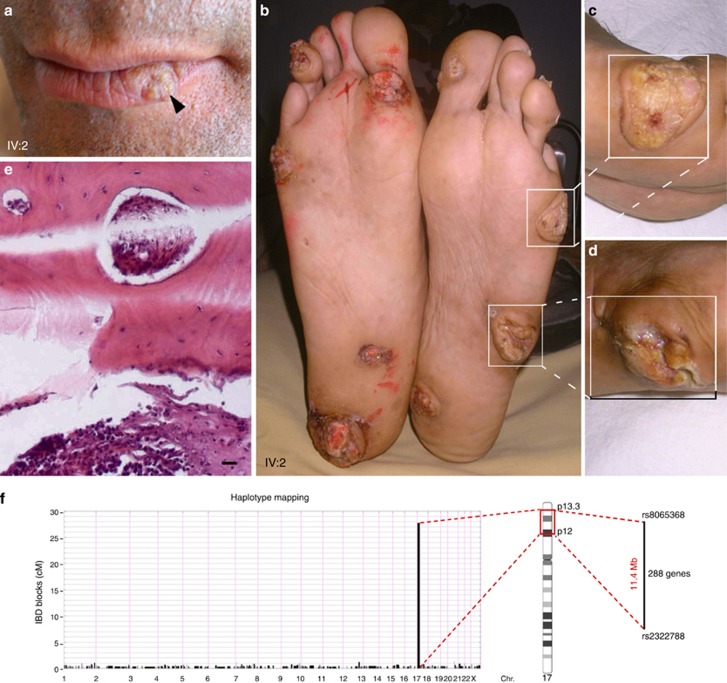
Cancer development in patient IV:2 and mapping of multiple self-healing palmoplantar carcinoma (MSPC) to Chr. 17p. (a) Squamous cell carcinoma (SCC) of the lower lip in patient IV:2 (20 mm). (b–d) Plantar ulceroburgeoning tumor after malignant transformation in patient IV:2 (50 mm). (e) After metastases and bilateral leg amputations, histological section of the tibia revealed SCC infiltration to the bone tissue (bar=50 μm). (f) Identical-by-descent (IBD) mapping following single-nucleotide polymorphism genotyping delineates one locus on chromosome 17p containing 288 genes shared by all 11 genotyped propositi.
On the basis of its autosomal dominant transmission and multiple self-regressive presentations, we initially hypothesized that this syndrome could be a clinically heterogeneous form of MSSE. However, neither Sanger sequencing of all nine exons of TGFBR1 nor haplotype mapping of its locus on chromosome 9q22.33 revealed any common allele shared by all propositi. No DNA-repair defects were observed consistent with the patient's normal karyotypes and absence of chromatin fragility in patients' B-lymphocytes, arguing against a phenotype related to the Muir-Torre syndrome.
To unambiguously map the causative allele for this uncommon genodermatosis, we obtained DNA from blood samples from 11 affected and 5 unaffected members after obtaining informed written consent and Institutional Review Board approval. Members were single-nucleotide polymorphism genotyped using human 660W-Quad BeadChips from Illumina (Figure 1a), and identity-by-descent mapping was performed (Reversade et al., 2009). Assuming autosomal dominant transmission, only one identity-by-descent block on chromosome 17p13.3-p12 was found to be shared by all genotyped probands and not by any of the five unaffected family members tested (Figure 2f). Meeting genome-wide significance, parametric and non-parametric linkage analyses yielded a maximum LOD score of 1.69 (Abecasis et al., 2002) and 4.21 (manual calculation), respectively. This 11.4 Mb locus bracketed by single-nucleotide polymorphisms rs8065368 and rs2322788 contains 288 genes (GRCh37/hg19: chr17:1541109-12964181) (Figure 2f) including TP53, a prominent tumor suppressor often mutated in skin cancer (Giglia-Mari and Sarasin, 2003). Sanger sequencing excluded mutations in TP53, arguing that a hitherto unidentified causative gene for MSPC lies within this haplotype.
The combination of clinical presentations primarily affecting palmoplantar and conjunctival skin and autosomal dominant genetic transmission argues that this syndrome is an inherited skin disease that to our knowledge is previously unreported. Six characteristics distinguish MSPC from the other four familial KA syndromes: (1) the earlier age of onset varying between 1 and 25 years; (2) the primary lesions that do not arise in sun-exposed areas but rather at points of pressure and friction; (3) the presence of conjunctival KA; (4) the ulcerative-nodular aspects of tumors; (5) the larger size of tumors, which vary between 5 and 50 mm; and (6) the tendency to transform into SCC with metastases, a feature only seen in MSSE patients subjected to radiotherapy (Chakrabarty and Perks, 1996). These differences, together with the presence of exclusive linkage to chromosome chr.17p13.3-p12, argue that MSPC is clinically and genetically distinct from MSSE, other inherited multiple KA, or punctate palmoplantar-specific genodermatosis (Pohler et al., 2012). To the best of our knowledge, only one similar case with multiple self-healing palmoplantar lesions has been reported in the literature (Feldman and Maize, 2007). Unlike KAs, which are believed to originate from hair follicles, the presence of lesions on hairless palmoplantar skin and on conjunctival epithelia argues instead that MSPC can originate from interfollicular keratinocyte progenitors.
We propose that this different syndrome should be classified as an inherited predisposition to skin cancer with primary palmoplantar and conjunctival tumors. We anticipate that the causative gene for MSPC will be rapidly identified as awareness is raised about its existence and correct clinical diagnosis.
Acknowledgments
We thank the MSPC-affected family members and their relatives for their contribution and availability. We thank L Bouzir for helping with sample collection and A Msakni, S Sassi, and M Gaussen for their technical support. We thank B Lane, I Szeverényi, L Lacina, SH Tan, and X Lim for their constructive feedback. O Mamaï is a PhD student at the University of Monastir in Tunisia and is funded by an A*STAR Research Attachment Program. B Reversade is a fellow of the Branco Weiss Foundation, an A*STAR Investigator, and Young EMBO Investigator. This work was partly funded by the Ministry of Higher Education and Scientific Research of Tunisia, the Skin Research Institute of Singapore, and a Strategic Positioning Fund on Genetic Orphan Diseases from A*STAR, Singapore.
Glossary
- KA
keratoacanthoma
- MSPC
multiple self-healing palmoplantar carcinoma
- MSSE
multiple self-healing squamous epithelioma
- SCC
squamous cell carcinoma
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Abecasis GR, Cherny SS, Cookson WO, et al. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Bapat B, Xia L, Madlensky L, et al. The genetic basis of Muir-Torre syndrome includes the hMLH1 locus. Am J Hum Genet. 1996;59:736–739. [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty KH, Perks AG. Ferguson-Smith syndrome: the importance of long term follow-up. Br J Plast Surg. 1996;49:497–498. doi: 10.1016/s0007-1226(96)90041-7. [DOI] [PubMed] [Google Scholar]
- Cribier B, Asch P, Grosshans E. Differentiating squamous cell carcinoma from keratoacanthoma using histopathological criteria. Is it possible? A study of 296 cases. Dermatology. 1999;199:208–212. doi: 10.1159/000018276. [DOI] [PubMed] [Google Scholar]
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77–79. doi: 10.1111/j.1365-4632.2006.02948.x. [DOI] [PubMed] [Google Scholar]
- Giglia-Mari G, Sarasin A. TP53 mutations in human skin cancers. Hum Mutat. 2003;21:217–228. doi: 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- Goudie DR, D'Alessandro M, Merriman B, et al. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat Genet. 2011;43:365–369. doi: 10.1038/ng.780. [DOI] [PubMed] [Google Scholar]
- Grzybowski A case of peculiar generalised epithelial tumors of the skin. Br J Dermatol. 1950;63:310–313. doi: 10.1111/j.1365-2133.1950.tb15666.x. [DOI] [PubMed] [Google Scholar]
- Kruse R, Rutten A, Lamberti C, et al. Muir-Torre phenotype has a frequency of DNA mismatch-repair-gene mutations similar to that in hereditary nonpolyposis colorectal cancer families defined by the Amsterdam criteria. Am J Hum Genet. 1998;63:63–70. doi: 10.1086/301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir E, Bell A, Barlow K. Multiple primary carcinomata of the colon, duodenum, and larynx associated with keratoacanthomata of the face. Br J Surg. 1967;54:191–195. doi: 10.1002/bjs.1800540309. [DOI] [PubMed] [Google Scholar]
- Pohler E, Mamai O, Hirst J, et al. Haploinsufficiency for AAGAB causes clinically heterogeneous forms of punctate palmoplantar keratoderma. Nat Genet. 2012;44:1272–1276. doi: 10.1038/ng.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Escande-Beillard N, Dimopoulou A, et al. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009;41:1016–1021. doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1–19. doi: 10.1016/s0190-9622(94)70001-x. [DOI] [PubMed] [Google Scholar]
- Smith JF. Multiple primary, self-healing squamous epithelioma of the skin. Br J Dermatol Syph. 1948;60:315–318. doi: 10.1111/j.1365-2133.1948.tb10932.x. [DOI] [PubMed] [Google Scholar]
- Witten VH, Zak FG. Multiple, primary, self-healing prickle-cell epithelioma of the skin. Cancer. 1952;5:539–550. doi: 10.1002/1097-0142(195205)5:3<539::aid-cncr2820050316>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.