Abstract
Introduction
Hyperlipidemia is a risk factor for cardiovascular diseases such as acute infarction. Inflammation and platelet activation are critical phenomena in acute myocardial infarction (AMI).
Aim
The aim of the study was to assess potential protective effects of aspirin and/or clopidogrel on AMI in hypercholesterolemic rats.
Methods
Forty adult male Wistar rats were divided into five groups (eight rats in each). Group I included normal healthy rats. The other 32 rats were subjected to induction of hypercholesterolemia by high-fat diet for 3 weeks, followed by induction of AMI by subcutaneous injections of isoproterenol (85 mg/kg/day, for 2 days). Rats were divided into the following groups: group II, rats with induced hypercholesterolemia and AMI; group III, hypercholesterolemic rats that received aspirin 30 mg/kg/day orally for 7 days before induction of AMI; group IV, hypercholesterolemic rats that received clopidogrel 10 mg/kg/day orally for 7 days before induction of AMI; and group V, hypercholesterolemic rats treated with both aspirin and clopidogrel in the same doses for 7 days before induction of AMI. Serum levels of pentraxin 3 (PTX3), transforming growth factor-β1 (TGF-β1), creatine kinase (CK), lactate dehydrogenase (LDH), total cholesterol and triglycerides were estimated in all rats.
Results
Isoproterenol-induced AMI in hypercholesterolemic rats was associated with an increase in serum levels of PTX3, TGF-β1, CK and LDH. Aspirin and/or clopidogrel pretreatment for 1 week led to a reduction of their levels as compared with non-treated rats. However, the reduction caused by combination of aspirin and clopidogrel was more than that caused by each drug separately.
Conclusion
Combination of aspirin and clopidogrel could be a therapeutic option for hypercholesterolemic patients to attenuate the complex vascular inflammatory process which is a key step in the setting of AMI.
Introduction
Hyperlipidemia is a major risk factor for many cardiovascular diseases, including atherosclerosis, hypertension, myocardial ischemia and acute infarction [1]. It was established that atherosclerosis is a long-term progressive inflammatory disease that leads too frequently to an acute atherothrombotic event through plaque rupture and formation of a platelet-rich thrombus. The principle clinical manifestations of atherothrombosis are sudden cardiac death, acute myocardial infarction (AMI), ischemic stroke and peripheral arterial ischemia [2]. Atherothrombotic events may be viewed as the end result of a complex inflammatory process to multifaceted pathology. AMI is the most common cause of cardiac remodeling. In the first minutes after injury in the ischemic zone, there is an important augmentation in the synthesis and release of proinflammatory cytokines such as interleukin-6 (IL-6), interleukin-1β (IL-1β) and transforming growth factor-β1 (TGF-β1). This acute release of cytokines could regulate the survival or apoptosis of myocytes in the infarcted zone, and their negative inotropic effect could represent an adaptive response to delimit the injury and to decrease myocardial energy demand [3].
Pentraxins are an essential component of the humoral arm of innate immunity. They are a superfamily of acute phase proteins highly conserved during evolution and can be classified as short pentraxins such as C-reactive protein (CRP) and long pentraxins such as pentraxin 3 (PTX3). The latter has an unrelated, long N-terminal domain coupled to the C-terminal pentraxin domain and differs from CRP in gene organization, cellular source and recognized ligands. PTX3 in humans, like CRP, is a marker of atherosclerosis and correlates with the risk of developing vascular events [4]. The long pentraxin family member PTX3 is induced by acute inflammatory stimuli, and it is increased in the blood of patients with AMI [5].
In addition to their role in initiating thrombus formation at the site of a ruptured atherosclerotic plaque, platelets play a key role in vascular inflammation through release of their own proinflammatory mediators and interaction with other relevant cell types: endothelial cells, smooth muscle cells and leukocytes. Inflammation and platelet activation are critical phenomena in the setting of AMI [6]. This suggests that systemic therapies that reduce vascular inflammation and perhaps stabilize vulnerable plaques over the long term are important complementary adjuncts to local interventions that are used to stabilize a ruptured plaque [7]. The importance of vascular inflammation in both the progressive and acute components of atherothrombotic diseases raises the possibility that antiplatelet agents, which interfere with platelet activation pathways, may have inhibitory effects on the platelet-driven inflammatory cascade [8]. So, pharmacological inhibition of platelet activation may prevent ischemic complications in patients with coronary artery disease.
Aim
The current study was designed to investigate the potential protective effects of two widely used antiplatelet agents (aspirin and clopidogrel) on isoproterenol-induced AMI in hypercholesterolemic rats.
Materials and Methods
The present study was conducted on 40 adult male Wistar (albino) rats with body weight ranging from 180 to 220 g. Rats were housed under the same standard environmental conditions of light and temperature, with free access to water. They were treated according to the ethical guidelines of the Ethics Committee, Faculty of Medicine, Alexandria University. Therefore, this study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rats were divided into five groups (each of eight rats).
Group I: included eight normal healthy rats fed on milk and bread. Three weeks after housing, rats received 1 ml of distilled water subcutaneously for 2 consecutive days and served as normal controls.
The remaining 32 rats were fed on a high-fat diet (HFD)—58 % fat, 25 % protein and 17 % carbohydrates as a percentage of total kilocalories—for a period of 3 weeks for induction of hypercholesterolemia. The composition of the HFD was as follows: 365 g/kg powdered non-pellet diet, 310 g/kg lard, 250 g/kg casein, 10 g/kg cholesterol, 60 g/kg vitamin and mineral mix, 3 g/kg methionine, 1 g/kg of both yeast powder and sodium chloride [9].
Then AMI was induced by subcutaneous injection of isoproterenol (Sigma-Aldrich, Germany) in a daily dose of 85 mg/kg for 2 consecutive days [10] (Fig. 1). The calculated dose of isoproterenol/rat was dissolved in 1 ml of distilled water. Then, grouping of rats continued as follows:
Fig. 1.
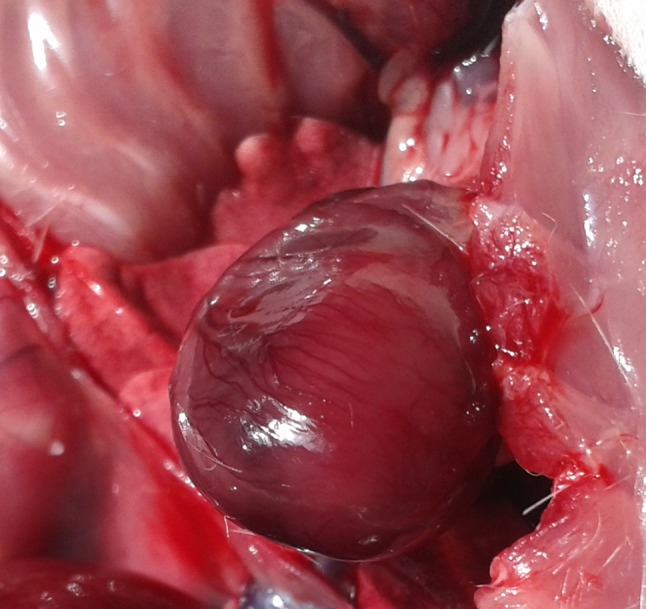
Isoproterenol induced myocardial infarction
Group II: included rats with induced hypercholesterolemia and AMI that received no treatment. They were given 1 ml of dimethyl sulfoxide (DMSO) 1 % orally/day for 7 days prior to induction of AMI and served as a positive untreated control group.
Group III: included hypercholesterolemic rats that received aspirin in a prophylactic dose of 30 mg/kg/day orally for 7 days prior to induction of AMI [11].
Group IV: included hypercholesterolemic rats that received clopidogrel in a prophylactic dose of 10 mg/kg/day orally for 7 days prior to induction of AMI [12].
Group V: included hypercholesterolemic rats with AMI that were pretreated with both aspirin and clopidogrel in the same previously mentioned doses for the same periods before induction of AMI [11, 12].
The calculated doses of either aspirin, clopidogrel or both were dissolved in 1 ml of DMSO 1 % and were given via an oral gavage syringe.
The next day following induction of AMI, rats of all groups were fasted overnight with free access to water. They were anesthetized by ether inhalation (which does not interfere with any of the studied parameters), and blood samples were withdrawn from the abdominal aortae, left to stand for 1 h (to allow proper clot formation and easier separation of enough serum), then centrifuged at 3,000 rpm for 15 min. Sera were separated, divided into aliquots and used for determination of the following parameters:
Serum levels of PTX3 [13], using the Pentraxin 3, Long (PTX3) BioAssay™ ELISA Kit (Rat) from United States Biological. The optical density was read from each well at 450 nm, and the concentrations were calculated by converting the optical density against a standard curve.
Serum levels of TGF-β1 [14], using the Rat Transforming Growth Factor Beta 1 (TGF-B1) ELISA Kit from Kamiya Biomedical Company (Cat. No. KT-30309). The optical density was read from each well at 450 nm, and the concentrations were calculated by converting the optical density against a standard curve.
Serum CK activity [15], using the Creatine Kinase Kit from BioSystems S.A. and kinetic UV method according to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) specifications (COD 11790).
Serum lactate dehydrogenase (LDH) activity [16], using the colorimetric kinetic method and the QuantichromTM Lactate Dehydrogenase Kit (DLDH-100) from BioAssay Systems USA.
Lipid profile, including serum levels of total cholesterol (TC), using the cholesterol oxidase/peroxidase kit from BioSystems S.A. (COD 11505) [17], and triglycerides (TG), using the glycerol phosphate oxidase/peroxidase kit from BioSystems S.A. (COD 11528) [18].
Statistical Analysis
Data are presented as mean ± SD. Data analysis was performed and figures were obtained using SPSS version 20 computer software. ANOVA (F) test followed by least significant difference (LSD) was performed to compare variables between different studied groups. Significance was set at P < 0.05.
Results
Serum Levels of Pentraxin 3 and Transforming Growth Factor-β1 in the Different Studied Groups
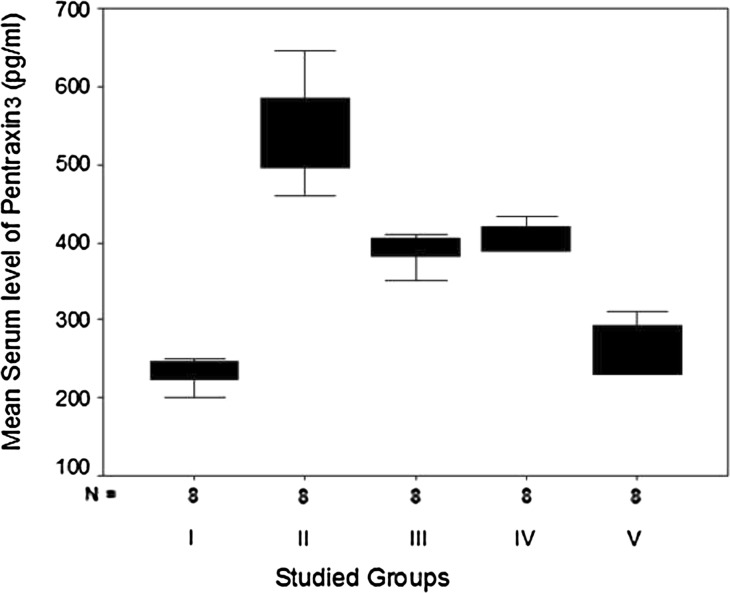
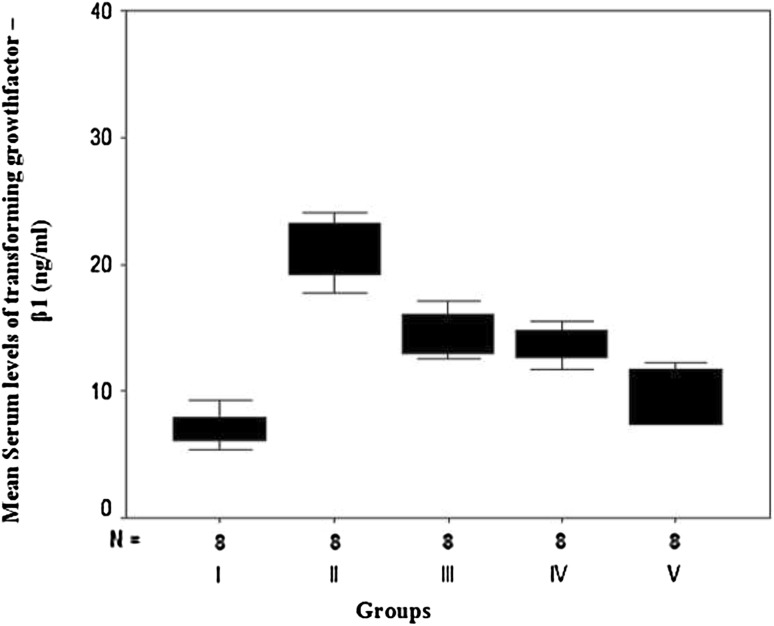
The current work showed that serum levels of both PTX3 and TGF-β1 were significantly elevated in hypercholesterolemic rats with isoproterenol-induced AMI in groups II, III, IV and V as compared with the normal healthy control rats of group I (Table 1; Figs. 2 and 3). Rats that were pretreated with aspirin alone (group III), clopidogrel alone (group IV) or a combination (group V) showed a statistically significant reduction in their levels as compared with the non-treated rats of group II. Moreover, pre-treatment of rats with a combination of aspirin and clopidogrel led to a more statistically significant reduction in PTX3 and TGF-β1 levels than pretreated with each drug separately. However, no statistically significant difference was detected between rats pretreated with aspirin alone (group III) and those pretreated with clopidogrel alone (group IV) (F = 88.50, P = 0.0016 and F = 36.87, P = 0.0012, respectively).
Table 1.
Comparison of serum levels of pentraxin 3 (PTX3) and transforming growth factor-β1 (TGF-β1) in the different studied groups
| Parameter | Group I (normal controls) (n = 8) | Group II (non-treated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | Group III (aspirin-pretreated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | Group IV (clopidogrel-pretreated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | Group V (combined aspirin and clopidogrel pretreated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | F and P |
|---|---|---|---|---|---|---|
| PTX3 (pg/ml) | ||||||
| Min.–max. | 200–250 | 460–645 | 350–410 | 388–433 | 230–311 | 88.50 and 0.0016** |
| Mean ± SD | 233.62a ± 16.94 | 537.75b ± 68.19 | 390.12c ± 19.59 | 405.75c ± 17.26 | 260.50d ± 34.57 | |
| TGF-β (ng/ml) | ||||||
| Min.–max. | 5.47–9.35 | 17.74–33.13 | 12.49–17.20 | 11.71–18.69 | 7.40–12.31 | 36.87 and 0.0012** |
| Mean ± SD | 7.09a ± 1.26 | 22.40b ± 4.80 | 14.53c ± 1.81 | 14.01c ± 2.20 | 9.53d ± 2.10 | |
n number of rats
** High statistical significance
a, b, c, d Letters indicate statistically significant difference
Fig. 2.
Box plots for comparison of mean values of serum pentraxin3 (PTX3) (pg/ml) in the studied groups
Fig. 3.
Box plots for comparison of mean values of serum transforming growth factor-beta1 (TGF-β) (ng/ml) in the studied groups
Serum Levels of Creatine Kinase, Lactate Dehydrogenase, Total Cholesterol and Triglycerides in the Different Studied Groups
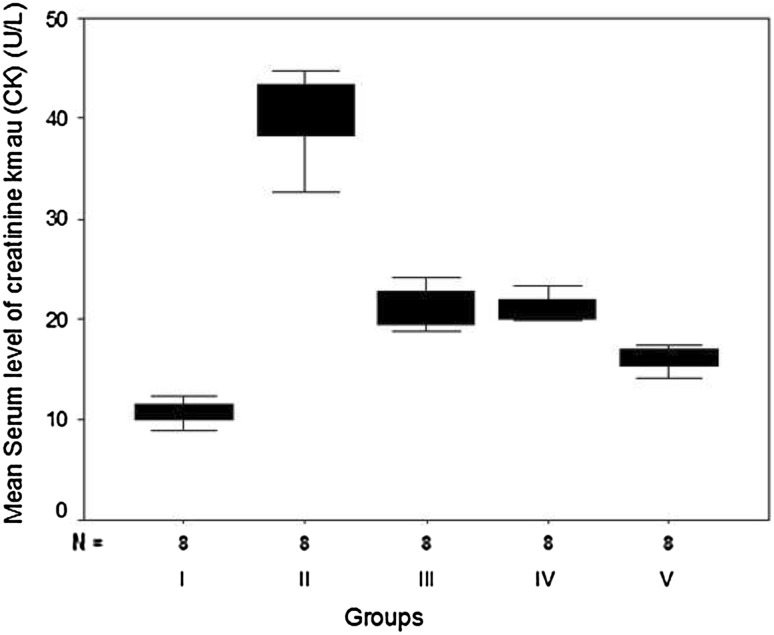
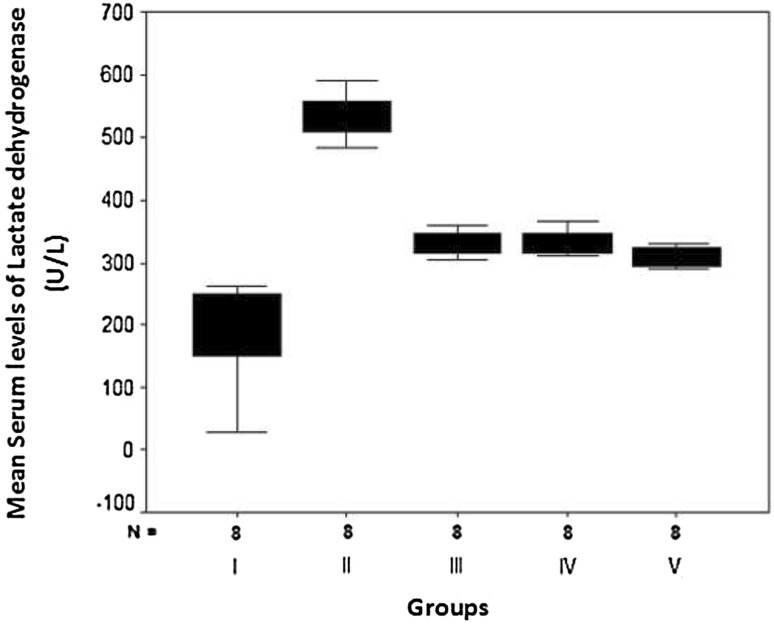
The present study revealed that 1 week of pretreatment with aspirin (group III), clopidogrel (group IV) or both (group V) resulted in a statistically significant reduction of elevated serum levels of both CK and LDH in hypercholesterolemic rats with isoproterenol-induced AMI in comparison with non-treated rats (group II) (Table 2; Figs. 4 and 5). However, their levels were still statistically higher than those of the normal controls of group I. Rats that were pretreated with both aspirin and clopidogrel in combination showed a statistically significant reduction than those which received aspirin or clopidogrel alone (F = 213.66, P = 0.0081 and F = 22.93, P = 0.005, respectively).
Table 2.
Comparison of serum levels of creatine kinase (CK), lactate dehydrogenase (LDH), total cholesterol (TC) and triglycerides (TG) in the different studied groups
| Parameter | Group I (normal controls) (n = 8) | Group II (non-treated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | Group III (aspirin-pretreated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | Group IV (clopidogrel-pretreated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | Group V (combined aspirin and clopidogrel pretreated hypercholesterolemic rats with acute myocardial infarction) (n = 8) | F and P |
|---|---|---|---|---|---|---|
| CK (U/l) | ||||||
| Min.–max. | 8.90–12.30 | 32.70–44.80 | 18.90–24.20 | 19.8–23.30 | 14.10–17.50 | 213.66 and 0.0081** |
| Mean ± SD | 10.63a ± 1.11 | 40.56b ± 3.99 | 21.23c ± 1.94 | 21.11c ± 1.20 | 16.07d ± 1.19 | |
| LDH (U/l) | ||||||
| Min.–max. | 225.70–263.30 | 483.90–590.30 | 305.40–360.20 | 310.50–365.30 | 285.40–330.10 | 22.93 and 0.0051** |
| Mean ± SD | 196.37a ± 90.47 | 535.38b ± 35.90 | 329.95c ± 19.30 | 332.95c ± 19.80 | 271.80d ± 98.11 | |
| TC (mg/dl) | ||||||
| Min.–max. | 82–110 | 130–170 | 128–165 | 132–158 | 129–163 | 18.11 and 0.0013** |
| Mean ± SD | 87.62a ± 30.26 | 151.25b ± 14.07 | 148.13b ± 12.58 | 147.14b ± 9.77 | 140.63b ± 14.52 | |
| TG (mg/dl) | ||||||
| Min.–max. | 45–81 | 90–151 | 82–148 | 84–143 | 87–146 | 16.14 and 0.0046** |
| Mean ± SD | 59.12a ± 25.68 | 133.75b ± 20.22 | 127.88b ± 25.73 | 116.88b ± 21.97 | 121.00b ± 21.86 | |
n number of rats
** High statistical significance
a, b, c, d Letters indicate statistically significant difference
Fig. 4.
Box plots for Comparison of mean values of serum creatine kinase (CK) (U/L) in the studied groups
Fig. 5.
Box plots for comparison of mean values of serum lactate dehydrogenase (LDH) (U/L) in the studied groups
Regarding serum levels of TC and TG, this study revealed that their serum levels were significantly elevated in hypercholesterolemic rats with AMI (group II, III, IV and V) as compared with the normal controls of group I (F = 18.11, P = 0.0013 and F = 16.14, P = 0.0046, respectively).
In addition, aspirin and/or clopidogrel pretreatment did not lead to a statistically significant reduction in their levels.
Discussion
There is an intense interest in the relationship between inflammation, thrombosis, platelet aggregation and hyperlipidemia in patients with coronary artery diseases. A number of inflammatory markers have been identified which can be used for risk stratification [8]. PTX3 is a novel inflammatory marker produced by the heart and vasculature in response to primary inflammatory stimuli, and it could be a sensitive and specific diagnostic and prognostic indicator in AMI [19].
Fundamentally, acute coronary syndromes are platelet-centric diseases, resulting from platelet-rich thrombi that develop at the site of vessel wall injury. In addition to aggregation, platelets modulate a plethora of other important pathophysiological processes, including inflammation and coagulation [6]. Therefore, the goal of the present study was to investigate and analyze the prophylactic effect of two antiplatelet regimens (aspirin and clopidogrel) on diagnostic and prognostic markers of isoproterenol-induced AMI in hypercholesterolemic rats.
Isoproterenol is a synthetic catecholamine (β-adrenergic agonist) that, in a large dose, generates highly cytotoxic free radicals, causing cardiac dysfunction, increased lipid peroxidation, and altered activity of cardiac enzymes and antioxidants, leading to infarct-like necrosis of the myocardium [10].
Findings of the current study revealed that isoproterenol-induced AMI was associated with a statistically significant increase in serum levels of PTX3. The elevated serum levels of PTX3 confirmed previous reports that suggested that AMI was associated with induction of PTX3 mRNA and protein expression in the ischemic areas by endothelial cells, macrophages and neutrophils, and PTX3 plasma concentrations increased significantly, starting 8 h after infarction, peaking at 24 h, and remaining up to 72 h [20, 21]. It was reported that PTX3 could be a sensitive and specific marker for acute coronary syndromes diagnosis when compared with cardiac markers in patients admitted to the emergency department within the first hours of onset of chest pain [22].
Moreover, Barbati et al. [23] reported that PTX3 plasma concentration is an independent predictor of mortality in patients with AMI. Similarly, pre-percutaneous coronary intervention (pre-PCI) high PTX3 levels were associated with high-risk plaque components and impaired post-PCI myocardial perfusion [24]. In contrast, Eggers et al. [25] found that the prognostic information provided by PTX3 levels is limited and inferior to CRP in patients with non-ST-elevation acute coronary syndrome.
An expanding body of evidence supports the existence of a close relationship between platelets, inflammation, atherosclerosis and atherothrombosis. Platelet activation is followed by the release of a range of proinflammatory mediators. Inflammatory biomarkers have shown promising predictive power for the assessment of atherothrombotic risk, raising the intriguing possibility of clinical benefit from drugs that reduce these biomarkers [26].
In the present study, the administration of aspirin and clopidogrel either singly or in combination 7 days before induction of AMI led to a statistically significant reduction of serum levels of PTX3 and TGF-β1.
Regarding PTX3, it was reported that combined antiplatelet therapy with clopidogrel and aspirin is commonly used for the prevention of cardiovascular events, and when it is used for an appropriate indication and duration, its benefits clearly outweigh its risks [27]. Also, in a recent study done by Yamasaki et al. [28], it was found that low doses of both aspirin and clopidogrel led to reduction of PTX3 and high-sensitivity CRP levels at the very early onset of myocardial infarction (MI) with ST-segment elevation, and the investigators stated that PTX3 could more directly reflect vascular inflammatory status than high-sensitivity CRP. In addition, the findings of the present study are supported by a study performed on patients with AMI and stable angina pectoris undergoing PCI that revealed higher circulating PTX3 levels in AMI than in angina pectoris, while aspirin and clopidogrel administration resulted in a reduction of PTX3 levels [29].
Considering TGF-β1, similar to the findings of the current work, Herder et al. [30] reported that there was a high TGF-β1 content in atherosclerotic plaques as well as high serum levels. Moreover, in a study performed on Iranian patients, it was reported that there is a close association between the TGF-β1 polymorphisms and the risk of AMI and there is also a significant relationship between serum TGF-β1 and occurrence of AMI [31]. Similarly, Chen et al. [32] found significantly higher serum levels of TGF-β1 in AMI than in both stable and unstable angina pectoris, and they stated that serum levels of TGF-β1 may become a useful biomarker for diagnosis and risk stratification of coronary artery diseases. Consistent with our findings, it was found that dual antiplatelet therapy using aspirin and clopidogrel had a vasculoprotective and anti-inflammatory effect on patients undergoing PCI, by reducing the concentrations of TGF-β1 [33].
Findings of the present study revealed that isoproterenol-induced MI was associated with increased levels of myocardial necrosis markers vice CK and LDH, reflecting structural and functional changes in the cardiac muscle, leading to myocardial necrosis [34].
These cardiac enzymes are released in the circulation because of a deficiency of oxygen supply which results in a disturbance of cell membrane integrity, as well as because of isoproterenol-induced oxidative stress, which directly injures the cell membrane [35].
Aspirin and/or clopidogrel pretreatment for hypercholesterolemic rats before induction of AMI led to a significant reduction of these cardiac enzymes, reflecting their cardioprotective effect by reducing platelet aggregation and decreasing the vascular inflammatory process. This result is in accordance with a study done by Wang et al. [36], which reported that antiplatelet therapy could have an important effect in improving the outcomes of patients with AMI undergoing primary PCI. Also, it was found that antiplatelet agents such as aspirin and clopidogrel remain the cornerstone treatment in patients with cardiovascular diseases, as they decrease the mortality as well as the recurrence of cardiovascular events [37].
Conclusion
The emergence of diverse novel biomarkers of coronary artery diseases has provided insight into the varied pathophysiology of these diseases and could have tremendous potential in aiding prediction and prognostic strategy. Also, it could be concluded that prophylactic combination of both aspirin and clopidogrel should be used as an important therapeutic option for hypercholesterolemic patients, after further clinical studies have been conducted, to attenuate the complex vascular inflammatory process which is a key step in the setting of AMI, with subsequent decrease in myocardial dysfunction and mortality.
Conflicts of interest
None.
Contributor Information
Adham R. Mohamed, Email: dr.adham_rashed@yahoo.com
Wessam F. El-Hadidy, Phone: (002)01224547007, Email: dr_wessam2002@yahoo.com
Hazem F. Mannaa, Email: hazem.farag2003@yahoo.com
References
- 1.Roffi M, Brindle M, Robbins M, Mukherjee D. Current perspectives on coronary revascularization in diabetic patients. Indian Heart J. 2007;59:124–136. [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Kume N, Mitsuoka H, Hayashida K, Tanaka M. Pentraxin 3 as a biomarker for acute coronary syndrome: comparison with biomarkers for cardiac damage. J Cardiol. 2011;58(1):38–45. doi: 10.1016/j.jjcc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20(2):35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Maugeri N, Rovere P, Slavich M, et al. Early and transient release of leukocyte pentraxin 3 during acute myocardial infarction. J Immunol. 2011;187(2):970–979. doi: 10.4049/jimmunol.1100261. [DOI] [PubMed] [Google Scholar]
- 6.Gurbel PA, Tantry US. The rationale for and comparisons of different antiplatelet treatment in acute coronary syndrome. J Interv Cardiol. 2008;21(Suppl (1)):S10–S17. doi: 10.1111/j.1540-8183.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 7.Garlanda C, Bottazi B, Bastone A. Pentraxins at the cross roads between innate immunity, inflammation, matrix deposition. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 8.Muhlestein JB. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb Haemost. 2010;103(1):71–82. doi: 10.1160/TH09-03-0177. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan K, Viswanad B, Lydia A, Kaul CL, Romarao P. Combination of high-fat diet-fed and low-dose streptozotocin—treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Zaitone SA, Abo-Gresha NM. Rosuvastatin promotes angiogenesis and reverse isoproterenol- induced acute myocardial infarction in rats: role of iNOS and VEGF. Eur J Pharmacol. 2012;691(1–3):134–142. doi: 10.1016/j.ejphar.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Wang Z, Chen WL, Moore PK, Zhu YZ. Cardioprotective effects of nitric-oxide aspirin in myocardial ischemia—reperfused rats. Am J Physiol Heart Circ Physiol. 2007;293(3):545–552. doi: 10.1152/ajpheart.00064.2007. [DOI] [PubMed] [Google Scholar]
- 12.Dhanjal TS, Medina RA, Leem J, Clark JE, South Worth R, Curtis MJ. Trapped platelets activated in ischemia initiate ventricular fibrillation. Circ Arrhythm Electrophysiol. 2013;6(5):995–1001. doi: 10.1161/CIRCEP.113.000591. [DOI] [PubMed] [Google Scholar]
- 13.Okutani D, Han B, Mura M, Waddell TK, Keshavjee S, Liu M. High-volume ventilation induces pentraxin 3 expression in multiple acute lung injury models in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L144–L153. doi: 10.1152/ajplung.00002.2006. [DOI] [PubMed] [Google Scholar]
- 14.Varedi M, Jeschke MG, Englander EW, Herndon DN, Barrow RE. Serum TGF-beta in thermally injured rats. Shock. 2001;6(5):380–382. doi: 10.1097/00024382-200116050-00010. [DOI] [PubMed] [Google Scholar]
- 15.Bishop C, Chu TM, Shihabi ZK. Single stable reagent for creatine kinase assay. Clin Chem. 1971;17(6):548–550. [PubMed] [Google Scholar]
- 16.Babson AL, Babson SR. Kinetic calorimetric measurement of serum lactate dehydrogenase activity. Clin Chem. 1973;19(7):766–769. [PubMed] [Google Scholar]
- 17.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 18.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19(5):476–482. [PubMed] [Google Scholar]
- 19.Suzuki S, Takeishi Y, Niizeki T, et al. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J. 2008;155(1):75–81. doi: 10.1016/j.ahj.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Salio M, Chimenti S, De Angelis N, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117(8):1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 21.Kunes P, Holubcova Z, Kolackova M, Krejisek J. Pentraxin 3 (PTX3): an endogenous modulator of the inflammatory response. Mediators Inflamm. 2012;2012:920517. doi: 10.1155/2012/920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustundag M, Orak M, Gulog C, Sayhan MB, Alyano O, Kale E. Comparative diagnostic accuracy of serum levels of neutrophil activating peptide-2 and pentraxin 3 versus troponin-I in acute coronary syndrome. Anadolu Kardiyol Derg. 2011;11(7):588–594. doi: 10.5152/akd.2011.160. [DOI] [PubMed] [Google Scholar]
- 23.Barbati E, Specchia C, Villella M, et al. Influence of pentraxin3 (PTX3) genetic variants on myocardial infarction risk and PTX3 plasma levels. Plos One. 2012;7(12):e 53030. doi: 10.1371/journal.pone.0053030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura S, Inagaki H, Haraguchi G, et al. Relationships of elevated systemic pentraxin 3 levels with high risk coronary plaque components and impaired myocardial perfusion after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. Circ J. 2013;78(1):159–169. doi: 10.1253/circj.cj-13-0329. [DOI] [PubMed] [Google Scholar]
- 25.Eggers KM, Armstrong PW, Califf RM, et al. Clinical and prognostic implications of circulating pentraxin 3 levels in non-ST-elevation acute coronary syndrome. Clin Biochem. 2013;46(16–17):1655–1659. doi: 10.1016/j.clinbiochem.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Lüscher TF. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc Med. 2007;12(2):113–122. doi: 10.1177/1358863X07077462. [DOI] [PubMed] [Google Scholar]
- 27.Terpening C. An appraisal of dual antiplatelet therapy with clopidogrel and aspirin for prevention of cardiovascular events. J Am Board Fam Med. 2009;22:51–56. doi: 10.3122/jabfm.2009.01.070282. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki T, Koizumi T, Tamaki T, et al. Differing behavior of plasma pentraxin 3 and high-sensitive CRP at the very onset of myocardial infarction with ST-segment elevation. Angiol. 2013;1(1):1–5. doi: 10.4172/2329-9495.1000108. [DOI] [Google Scholar]
- 29.Helseth R, Solheim S, Opstad T, Hoffmann P, Arnesen H, Seljeflot I. The time profile of pentraxin 3 in patients with acute ST-elevation myocardial infarction and stable angina pectoris undergoing percutaneous coronary intervention. Mediators Inflamm. 2014;2014:608414. doi: 10.1155/2014/608414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herder C, Peeters W, Zierer A, de Kleijn DP, Moll FL, Roden M. TGF-β1 content in atherosclerotic plaques, TGF-β1 serum concentrations and incident coronary events. Eur J Clin Invest. 2012;42(3):329–337. doi: 10.1111/j.1365-2362.2011.02587.x. [DOI] [PubMed] [Google Scholar]
- 31.Najar RA, Ghaderian SM, Panah AS. Association of transforming growth factor–β1 gene polymorphism with genetic susceptibility to acute myocardial infarction. Am J Med Sci. 2011;342(5):365–370. doi: 10.1097/MAJ.0b013e318215908a. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Lei W, Chen W, et al. Serum TGF-β1 and SMAD3 levels are closely associated with coronary artery disease. BMC Cardiovas Disord. 2014;14:18. doi: 10.1186/1471-2261-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudzik B, Szkodzinski J, Danikiewicz K, et al. Effect of omeperazole on the concentration of interleukin-6 and transforming growth factor–β1 in patients receiving dual antiplatelet therapy after percutaneous coronary intervention. Eur Cytokine Netw. 2010;21(4):257–263. doi: 10.1684/ecn.2010.0213. [DOI] [PubMed] [Google Scholar]
- 34.Lobo HG, Fereira NL, Sousa RB, Carvalho ER, Lobo PL. Experimental model of myocardial infarction induced by isoproterenol in rats. Rev Bras Circ Cardiovasc. 2011;26(3):469–476. doi: 10.5935/1678-9741.20110024. [DOI] [PubMed] [Google Scholar]
- 35.Yue-tao L, Hong-mei J, Xing-chang C, Hong-wu Z, Zhong-mei Z. The metabolic disturbances of isoproterenol induced myocardial infarction in rats based on tissue targeted metabonomics. Mol Biosyst. 2013;9:2823–2834. doi: 10.1039/c3mb70222g. [DOI] [PubMed] [Google Scholar]
- 36.Wang CH, Yang J, Shen ZJ, Fang Q, Zhang SY, Fan ZJ. Effect of duration of clopidogrel use on clinical follow-up outcomes in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Zhonghua Yi Xue Za Zhi. 2010;90(24):1682–1685. [PubMed] [Google Scholar]
- 37.Acharji S, Lakshmanodoss U, Rudzinski W, Stapleton D, Kaluski E. Use of antiplatelet agents in patients with atherosclerotic diseases. Postgrad Med. 2013;125(5):19–30. doi: 10.3810/pgm.2013.09.2694. [DOI] [PubMed] [Google Scholar]