Abstract
Fidaxomicin is approved for the treatment of adults with Clostridium difficile–associated diarrhea, many of whom have difficulty swallowing an intact tablet. The study objective was to evaluate the stability and recovery of crushed DIFICID® (fidaxomicin) 200-mg tablets dispersed in water, applesauce, or Ensure® brand liquid nutritional supplement, and to determine the recovery of fidaxomicin from the administration of an aqueous dispersion of a crushed DIFICID® tablet through a nasogastric (NG) tube. DIFICID® tablets were crushed and dispersed in water, applesauce, or Ensure®. The stability and recovery of fidaxomicin were evaluated over 24 h in these vehicles. In a separate experiment, the ability to recover a full dose of fidaxomicin when administering as an aqueous dispersion through an NG tube was assessed. When DIFICID® tablets were crushed and dispersed in water, the active ingredient, fidaxomicin, was stable for up to 2 h at room temperature. Additionally, it was stable for up to 24 h in dispersions with applesauce or Ensure®. Recovery of fidaxomicin after crushing and dispersing in any of the three vehicles studied ranged from 95 to 108 %, which is within the normal range of individual tablet variability. When crushed, dispersed in water, and administered through an NG tube, the average recovery of fidaxomicin was 96 %. Stability and recovery of fidaxomicin were confirmed when DIFICID® tablets were crushed and dispersed in water, applesauce, or Ensure®. In addition, administration of an aqueous dispersion of a crushed tablet through an NG tube is supported by acceptable recovery of fidaxomicin.
Key Points
| Crushed DIFICID® (fidaxomicin) 200-mg tablets are stable and recoverable for at least 2 h after being dispersed in water, applesauce, or Ensure® at room temperature. |
| The ability to administer aqueous dispersions of crushed DIFICID® tablets through a nasogastric tube with acceptable recovery was demonstrated. |
Introduction
Clostridium difficile infection (CDI) is the most common cause of healthcare facility–associated diarrhea and accounts for 15–25 % of such infections [1]. The most common symptom of CDI is C. difficile–associated diarrhea (CDAD). The rate of recurrent CDI is high, approximately 15–30 % [2–5], and can exceed 60 % in patients with multiple episodes [6]. According to a recent report, C. difficile may be exceeding methicillin-resistant Staphylococcus aureus as the most prevalent healthcare facility–acquired pathogen in the USA [7]. In 2009, there were 336,600 hospitalizations that involved CDI, accounting for 0.9 % of all hospital stays [8]; the annual incidence could be hypothesized to exceed 1 million if other locations of infection such as long-term care and outpatient settings are considered.
Fidaxomicin, the active ingredient in DIFICID®/DIFICLIR™, is a macrocyclic antibiotic recently approved in several countries, including the USA, for the treatment of adults with CDAD [9]. It is nearly fully delivered to the colon as a result of minimal systemic absorption [10]. The efficacy of fidaxomicin was demonstrated in two large, multicenter, randomized, double-blind, parallel-group, noninferiority, phase III clinical trials [2, 3]. A total of 1,105 patients ages 18–94 years with either no prior CDAD episode or one prior episode within the prior 3 months were treated orally with 200 mg of fidaxomicin two times daily or 125 mg of vancomycin four times daily for 10 days. In both studies, fidaxomicin was noninferior to vancomycin for initial clinical response, whereas fidaxomicin was superior to vancomycin in reducing recurrence and increasing sustained clinical response, the latter defined as achieving clinical response at the end of treatment and without proven or suspected CDAD recurrence through the posttreatment observation period of 28 days.
Whether because of increased age, critical illness, or various other reasons, certain patients have difficulty swallowing an intact tablet. It is, therefore, common practice to crush tablets and disperse them in liquids or soft foods. The study presented here assesses the impact of such practice on the stability and dose recovery of fidaxomicin from DIFICID® 200-mg tablets when crushed and dispersed in water, applesauce, or Ensure®. In a separate experiment, the recovery of a full dose of an aqueous dispersion of a crushed DIFICID® tablet after being passed through a nasogastric (NG) tube was examined. While a published case report describes the administration of fidaxomicin through an NG tube [11], the present study provides, to our knowledge, the only rigorous study relevant to the stability and recovery of crushed DIFICID® tablets.
Methods and Materials
The stability and recovery of DIFICID® (fidaxomicin) 200-mg tablets were evaluated after crushing and dispersing single tablets in one of three vehicles: water, applesauce (Mott’s® Original Apple Sauce), or Ensure® (Ensure® Nutrition Shake, Homemade Vanilla flavor, Abbott Nutrition/Abbott Laboratories). Once dispersed, the various presentations were assessed over a 24-h period for stability and recovery of the expected dose of fidaxomicin (200 mg). Stability was evaluated by the validated and registered commercial product method for chromatographic purity using high-performance liquid chromatography with ultraviolet detection (HPLC-UV). Chromatographic purity values were calculated and reported as the percentage active compared with a reference standard. The growth of new or existing impurities was compared with product specifications to determine acceptable stability. Recovery was measured using the validated and registered commercial product method for assay by HPLC-UV, with the potency of fidaxomicin calculated against a reference standard of known concentration, and reported as percentage recovered of the labeled tablet strength (200 mg).
For each of the three vehicles, two methods were used to crush the DIFICID® tablet. In the first method (Method 1), the DIFICID® tablet was placed in a polycarbonate pouch and crushed using the Silent Knight tablet crushing system (No. NONSK0100, Medline, Mundelein, IL, USA) (ADR-11-027, Optimer Pharmaceuticals, 2011 October 10). In Method 2, the DIFICID® tablet was crushed between two pieces of weighing paper using a pestle (ADR-12-003, Optimer Pharmaceuticals, 2012 March 2). Crushed material was then transferred, mixed with the test vehicle, and stored at room temperature. Stability and recovery were assessed over a 24-h period. For each time point, an individually prepared sample of the dispersion was analyzed for potency and chromatographic purity.
Water Sample Preparation
For each sample, one DIFICID® (fidaxomicin) 200-mg tablet was crushed and transferred to a flask, to which 40 mL of water was then added, and the contents were mixed thoroughly by inversion. Flasks were stored at room temperature, and at scheduled time points, samples were prepared for HPLC-UV analysis by diluting with organic solvent and centrifuging. A separate flask/preparation was used for each time point.
Applesauce Sample Preparation
For each sample, one DIFICID® (fidaxomicin) 200-mg tablet was crushed and transferred to a flask containing approximately 40 mL of applesauce. The contents were then mixed by shaking and rolling the flask. Flasks were stored at room temperature, and at scheduled time points, samples were prepared for HPLC-UV analysis by diluting with organic solvent and centrifuging. A separate flask/preparation was used for each time point.
Ensure® Sample Preparation
For each sample, one DIFICID® (fidaxomicin) 200-mg tablet was crushed and transferred to a flask, to which 60 mL of Ensure® was then added, and the contents were mixed by shaking the flask. Flasks were stored at room temperature, and at scheduled time points, 100 mL of organic solvent was added and the flask contents were mixed by swirling gently. In order to achieve organic/water layer separation, sodium chloride was added to the mixture. An aliquot of the organic layer was centrifuged, and a portion of the resulting supernatant was further diluted for HPLC-UV analysis. A separate flask/preparation was used for each time point.
Passage Through a Nasogastric (NG) Tube
For each sample, one DIFICID® (fidaxomicin) 200-mg tablet was crushed using Method 2 and transferred to a 100-mL volumetric flask. Approximately 40 mL of distilled water was then added to the flask; contents were mixed thoroughly by hand, and then shaken on an orbital shaker for 15 min. Contents were verified by visual inspection to be completely disintegrated, and the flask was brought up to the volume by addition of distilled water. The aqueous dispersion was then passed through a size 10 Fr (3.3 mm) × 45-inch NG enteral feeding tube with twin-port and stylet (ADR-11-0002, Optimer Pharmaceuticals, 2011 November 15) and subsequently collected for analysis. To achieve this, each NG tube was attached to a 50-mL syringe with the plunger removed and a 200-mL volumetric flask was placed at the other end of the NG tube for collection. The aqueous dispersion was slowly poured into the syringe, allowing it to drain through the NG tube under gravity into the collection flask. The original flask containing the aqueous fidaxomicin dispersion was then rinsed twice with 15 mL of water. Each of these rinses was poured into the syringe and allowed to drain through the NG tube into the collecting flask. The plunger was then attached to the syringe and depressed to expel any remaining dispersion from the syringe and the NG tube. The receiving flask was brought up to volume and samples were prepared for HPLC-UV analysis by diluting with organic solvent and centrifuging. Three separate samples were prepared in this fashion.
Results
Water Vehicle
The stability and recovery of fidaxomicin in water over 24 h are shown in Table 1A. The recovery of fidaxomicin after aqueous dispersion was acceptable for both crushing methods, with Method 1 yielding recoveries of 95–104 % and Method 2 yielding recoveries of 102–104 %. All results were within the normal variability for individual DIFICID® tablets as defined in the product specifications.
Table 1.
Stability and recovery of fidaxomicin dispersed in water, applesauce, or Ensure®
| Time (h) | |||||
|---|---|---|---|---|---|
| 0 | 2 | 6 | 12 | 24 | |
| A. Water | |||||
| Method 1 | |||||
| % Recovery, mean (SD) (n = 3) | 95.3 (2.32) | nt | 104.4 (3.07) | nt | 100.6 (2.23) |
| % Impurity Aa | <LOQ | nt | 0.19 | nt | 0.65 |
| Method 2 | |||||
| % Recovery, mean (SD) (n = 6) | 103.8 (1.28) | 102.9 (1.57) | 102.2 (1.49) | 104.1 (1.13) | nt |
| % Impurity Aa | <LOQ | 0.11 | 0.25 | 0.38 | nt |
| B. Applesauce | |||||
| Method 1 | |||||
| % Recovery, mean (SD) (n = 3) | 105.0 (1.37) | nt | 108.2 (4.29) | nt | 104.6 (2.22) |
| % Impurity Aa | <LOQ | nt | <LOQ | nt | <LOQ |
| Method 2 | |||||
| % Recovery, mean (SD) (n = 6) | 104.8 (1.20) | nt | 104.5 (1.05) | 101.7 (1.96) | 104.6 (0.80) |
| % Impurity Aa | <LOQ | nt | <LOQ | <LOQ | <LOQ |
| C. Ensure® | |||||
| Method 1 | |||||
| % Recovery, mean (SD) (n = 3) | 95.5 (3.41) | nt | 99.1 (5.92) | nt | 99.0 (3.87) |
| % Impurity Aa | <LOQ | nt | <LOQ | nt | 0.08 |
| Method 2 | |||||
| % Recovery, mean (SD) (n = 6) | 105.6 (1.03) | nt | 107.7 (1.35) | 105.4 (1.48) | 107.8 (1.71) |
| % Impurity Aa | <LOQ | nt | 0.06 | 0.06 | 0.14 |
h hour, LOQ limit of quantification (0.05 %), nt not tested, SD standard deviation
aLimit in drug product is not more than 0.2 %
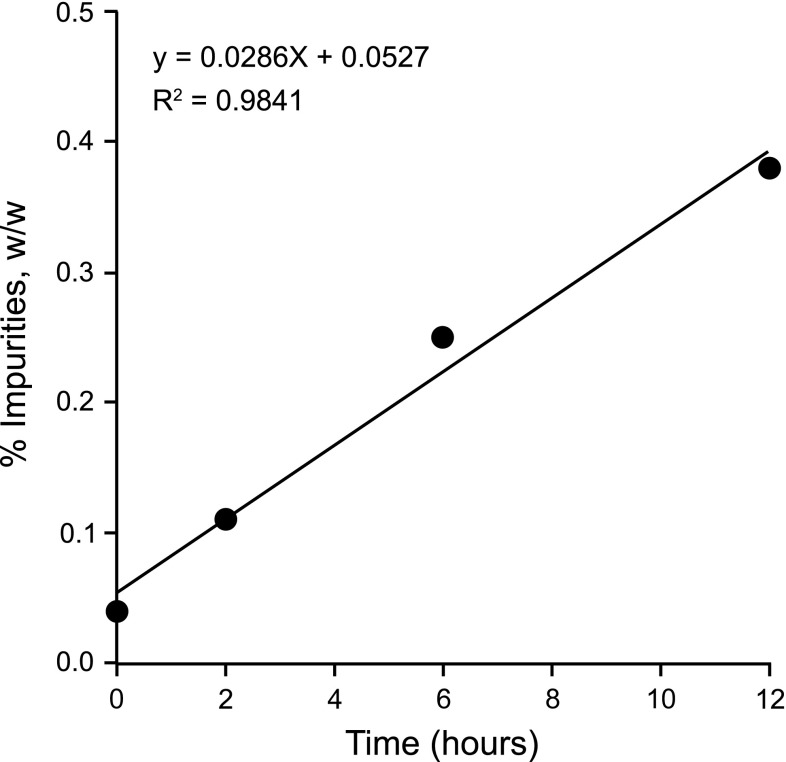
Multiple degradation products were observed in the chromatographic purity assessment of crushed DIFICID® tablets dispersed in water. However, only one impurity, designated Impurity A, was observed to exceed the specification limit of 0.2 % during the course of the 24-h study using either crushing method. Growth of this impurity was used to define the acceptable stability of crushed DIFICID® tablets dispersed in water. The amount of Impurity A exceeded the limit at 6 h (0.25 %) in the Method 2 study; thus the stability of crushed DIFICID® tablets dispersed in water was assigned to be 2 h at room temperature. Figure 1 shows the linear increase in Impurity A from 0 to 12 h from the Method 2 study data.
Fig. 1.
Linear regression plot of Impurity A in a water matrix (Method 2)
Applesauce Vehicle
The recovery of fidaxomicin ranged from 105 to 108 % for Method 1 and 102–105 % for Method 2 over the 24-h period (Table 1B). All results were within the normal variability for individual DIFICID® tablets, as defined in the product specifications.
After 24 h at room temperature, all impurities were below drug product specification limits (Table 1B). This supports the stability of fidaxomicin for up to 24 h when dispersed in applesauce and stored at ambient room temperature.
Ensure® Vehicle
The recovery of fidaxomicin ranged from 96 to 99 % in Method 1 and 105–108 % in Method 2 over the 24-h period (Table 1C). All results were within the normal variability for individual DIFICID® tablets, as defined in the product specifications.
After 24 h at room temperature, all impurities were below drug product specification limits (Table 1C). This supports the stability of fidaxomicin for up to 24 h when dispersed in Ensure®.
Note on Crushing
It should be noted that some loss of tablet powder to the pouch used in crushing Method 1 was visually observed. In a separate weight-based experiment, the average loss was determined to be about 6 %. Though the measured recovery values are acceptable, when using a pouch-based system, care should be exercised to minimize material loss.
Recovery from an NG Tube
There was no visible tablet material or film-coating residue detected after administration of the aqueous fidaxomicin dispersion and rinsing with water as described. Visual inspection for assessment of clumping and particle adsorption to the interior of the NG tubing revealed no deficiencies. The percent recovery of fidaxomicin was ≥93 % for all three runs, with an average recovery of 96 % (Table 2).
Table 2.
Recovery of fidaxomicin after passage through a nasogastric tube
| Fidaxomicin recovery | |||
|---|---|---|---|
| Run 1 (%) | Run 2 (%) | Run 3 (%) | Mean (SD) (%) |
| 98.4 | 93.0 | 97.4 | 96.3 (2.9) |
SD standard deviation
Discussion
Prior to this study, no information had been available regarding (1) the stability and recovery of DIFICID® (fidaxomicin) 200-mg tablets when crushed and dispersed in assorted oral delivery vehicles, including water, applesauce, and Ensure®; and (2) the recovery of aqueous fidaxomicin dispersions after being passed through an NG tube. Drug impurities and degradation products remained within specifications for 2 h when dispersed in water and for at least 24 h when dispersed in applesauce or Ensure® under ambient room temperature conditions. This demonstrates that crushed and dispersed DIFICID® tablets are stable under such conditions. Recovery of fidaxomicin from crushed DIFICID® tablets was within normal tablet variability for all conditions and vehicles studied, indicating that the expected amount of fidaxomicin can be delivered after crushing and dispersing DIFICID® tablets in water, applesauce, or Ensure®. Additionally, acceptable recovery of fidaxomicin has been demonstrated after passing aqueous dispersions of crushed DIFICID® tablets through a representative NG tube.
On the basis of these data, crushing a DIFICID® (fidaxomicin) 200-mg tablet and dispersing it in three common vehicles (water, applesauce, or Ensure®) does not negatively impact the key quality parameters of purity and potency of the administered drug product. The active ingredient, fidaxomicin, is stable and recoverable under these conditions.
Conclusion
It is a common practice to crush solid oral formulations, such as tablets, and disperse them in various vehicles to facilitate administration, including NG tube administration in water, to patients who have difficulty swallowing. This study has demonstrated that the stability and ability to deliver the expected dose of fidaxomicin is not negatively impacted by such practices. Crushed DIFICID® (fidaxomicin) 200-mg tablets are stable and recoverable for at least 2 h after being dispersed in water, applesauce, or Ensure® under ambient room temperature conditions. This window of stability should provide sufficient time for the preparation and administration of crushed DIFICID® tablets for this alternative means of dosing. The ability to administer aqueous dispersions of crushed DIFICID® tablets through an NG tube with acceptable recovery was also demonstrated. Future work could potentially focus on formulating a stable suspension or solution.
The manuscript does not contain clinical studies or patient data.
Funding
The study was funded by Optimer (now Cubist) Pharmaceuticals.
Conflicts of interest
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the opinion, policy, or position of their current affiliations. AT, MR, TH, MH, and SC were employed by Optimer Pharmaceuticals at the time the research was conducted. JDJ was an employee of Cubist Pharmaceuticals at the time of manuscript authoring. CAD is an employee of Cubist Pharmaceuticals and holds stock with the company. AT, MR, and TH held stock in Optimer Pharmaceuticals at the time the work was performed. SC remains a shareholder of Cubist Pharmaceuticals.
Contributor Information
Anna Tousseeva, Email: anna.tousseeva@gmail.com.
J. Derek Jackson, Email: j.derek.jackson@gmail.com.
Mark Redell, Email: mark.redell@themedco.com.
Teresa Henry, Email: trlamm@hotmail.com.
Michael Hui, Email: mh10255@yahoo.com.
Shelley Capurso, Email: scapurso@rocketmail.com.
C. Andrew DeRyke, Phone: 781.860.8203, Email: andrew.deryke@cubist.com.
References
- 1.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 2.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Eng J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 3.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 4.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(Suppl 6):21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 7.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 8.Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville: US Agency for Healthcare Research and Quality; 2012. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. Accessed 14 Apr 2014. [PubMed]
- 9.Sears P, Ichikawa Y, Ruiz N, Gorbach S. Advances in the treatment of Clostridium difficile with fidaxomicin: a narrow spectrum antibiotic. Ann NY Acad Sci. 2013;1291:33–41. doi: 10.1111/nyas.12135. [DOI] [PubMed] [Google Scholar]
- 10.Sears P, Crook DW, Louie TJ, Miller MA, Weiss K. Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with Clostridium difficile infection. Clin Infect Dis. 2012;55(Suppl 2):S116–S120. doi: 10.1093/cid/cis337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maseda E, Hernandez-Gancedo C, Lopez-Tofiño A, Suarez-de-la Rica A, Garcia-Bujalance S, Gilsanz F. Use of fidaxomicin through a nasogastric tube for the treatment of septic shock caused by Clostridium difficile infection in a patient with oral cancer admitted to the Surgical Critical Care Unit. Rev Esp Quimioter. 2013;26(4):375–377. [PubMed] [Google Scholar]