Abstract
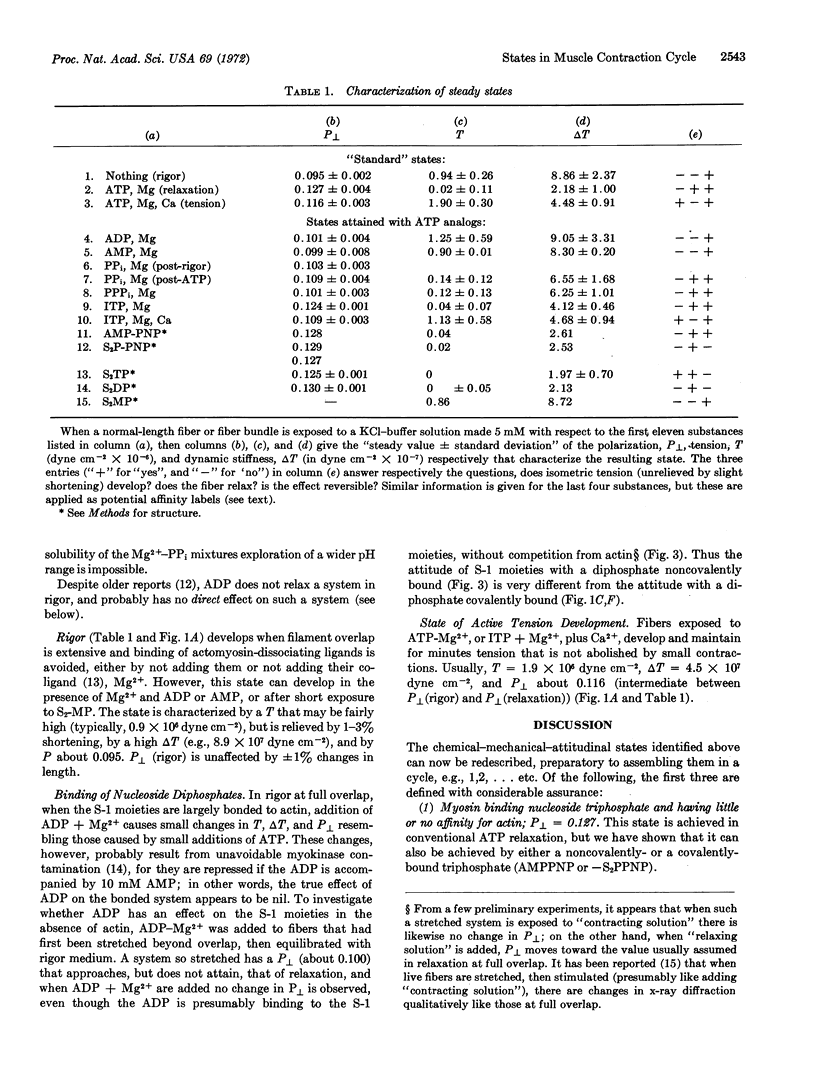
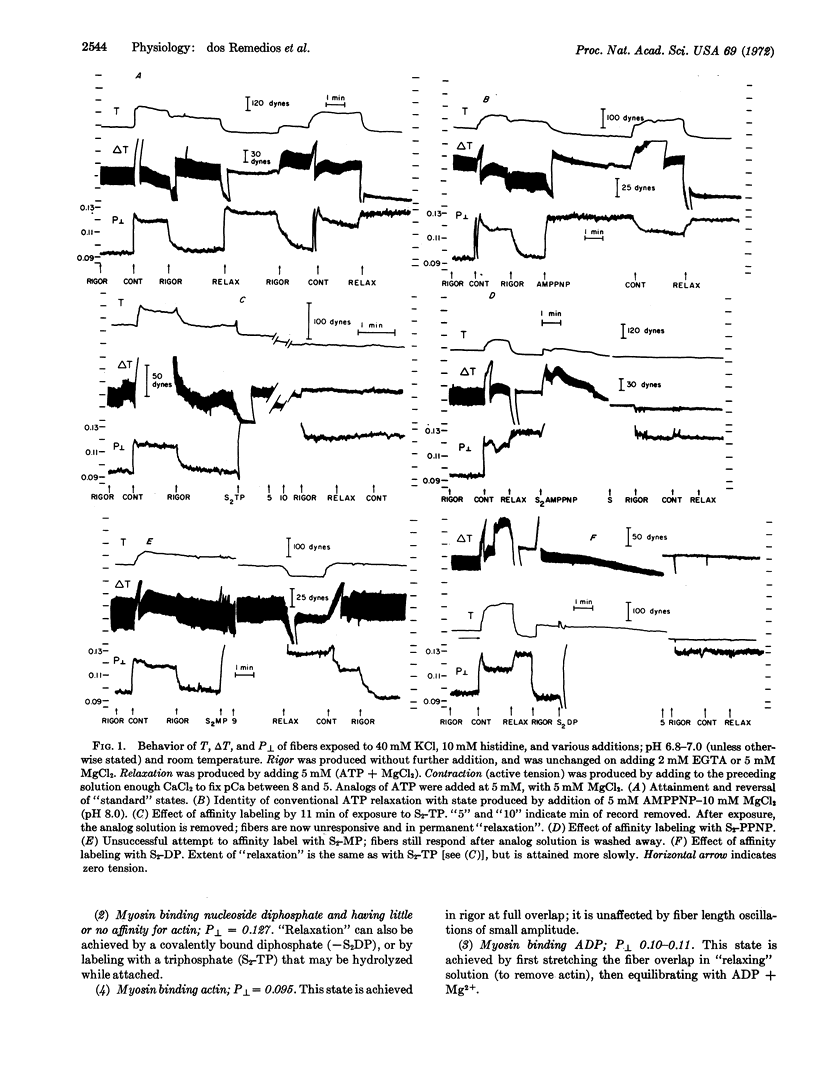
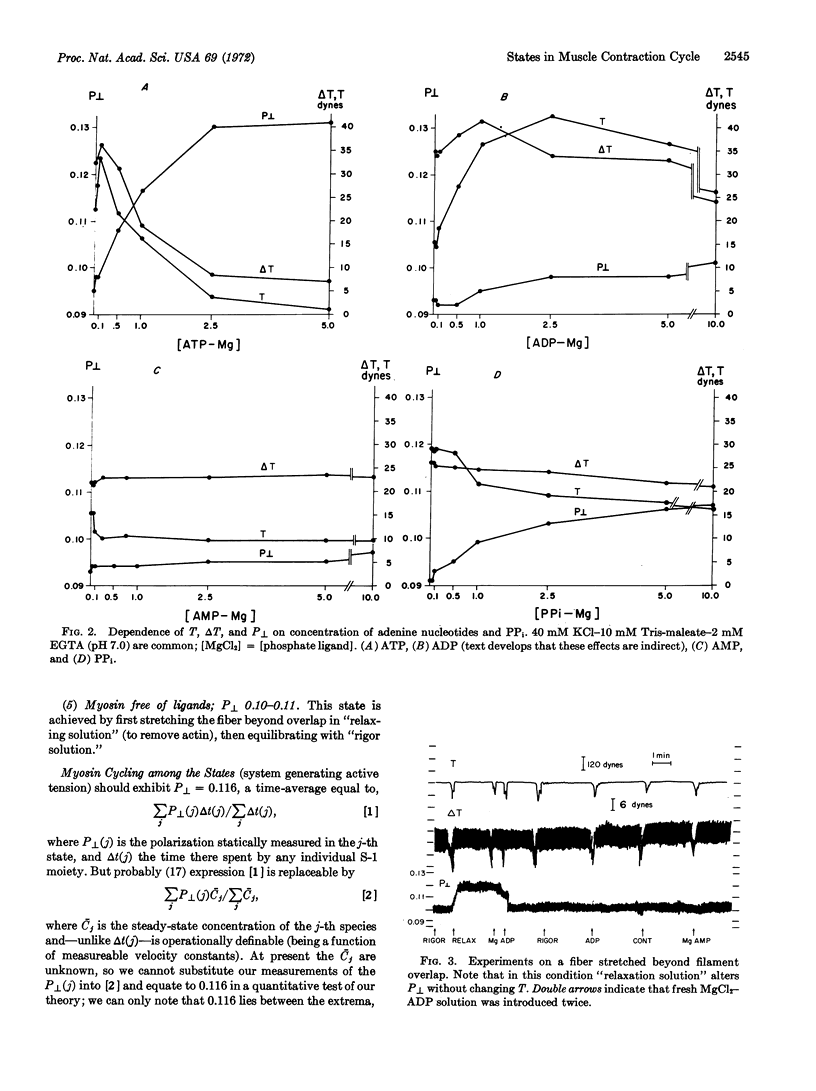
By using appropriate analogs of ATP, isometrically-held glycerol-extracted psoas fibers from rabbits are forced successively into states corresponding to molecular species in the contractile cycle. In each state measurements are made of P[unk], a fluorescence polarization parameter thought to relate to attitude of S-1 moieties of the myosin molecules. Also, the value of P[unk] is measured during active tension development. It is suggested that this value is a time-average of the P[unk] as S-1 moieties move through the various states of the cycle. Proposals are made concerning the sequence of states in the cycle.
Keywords: psoas, rabbit, ATP analogs, myosin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson J. F., Morales M. F. Polarization of tryptophan fluorescence in muscle. Biochemistry. 1969 Nov;8(11):4517–4522. doi: 10.1021/bi00839a044. [DOI] [PubMed] [Google Scholar]
- BENDALL J. R. Further observations on a factor (The `Marsh' factor) effecting relaxation of ATP-shortened muscle-fibre models and the effect of Ca and Mg ions upon it. J Physiol. 1953 Aug;121(2):232–254. doi: 10.1113/jphysiol.1953.sp004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios C. G., Millikan R. G., Morales M. F. Polarization of tryptophan fluorescence from single striated muscle fibers. A molecular probe of contractile state. J Gen Physiol. 1972 Jan;59(1):103–120. doi: 10.1085/jgp.59.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely B., Martonosi A. The binding of ADP to myosin. Biochim Biophys Acta. 1969 Jan 14;172(1):158–170. doi: 10.1016/0005-2728(69)90101-7. [DOI] [PubMed] [Google Scholar]
- Murphy A. J., Duke J. A., Stowring L. Synthesis of 6-mercapto-9-beta-D-ribofuranosylpurine 5'-triphosphate, a sulfhydryl analog of ATP. Arch Biochem Biophys. 1970 Mar;137(1):297–298. doi: 10.1016/0003-9861(70)90441-8. [DOI] [PubMed] [Google Scholar]
- Murphy A. J., Morales M. F. Number and location of adenosine triphosphatase sites of myosin. Biochemistry. 1970 Mar 31;9(7):1528–1532. doi: 10.1021/bi00809a008. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Gergely J. The conformation of myosin during the steady state of ATP hydrolysis: studies with myosin spin labeled at the S 1 thiol groups. Biochem Biophys Res Commun. 1971 Aug 20;44(4):826–830. doi: 10.1016/0006-291x(71)90785-6. [DOI] [PubMed] [Google Scholar]
- Tokiwa T. EPR spectral observations on the binding of ATP and F-actin to spin-labeled myosin. Biochem Biophys Res Commun. 1971 Jul 16;44(2):471–476. doi: 10.1016/0006-291x(71)90625-5. [DOI] [PubMed] [Google Scholar]
- Tokiwa T., Morales M. F. Independent and cooperative reactions of myosin heads with F-actin in the presence of adenosine triphosphate. Biochemistry. 1971 Apr 27;10(9):1722–1727. doi: 10.1021/bi00785a033. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich M., Rüegg J. C. Stretch induced formation of ATP-32P in glycerinated fibres of insect flight muscle. Experientia. 1971 Jan 15;27(1):45–46. doi: 10.1007/BF02137732. [DOI] [PubMed] [Google Scholar]
- WATANABE S., SARGEANT T., ANGLETON M. ROLE OF MAGNESIUM IN CONTRACTION OF GLYCERINATED MUSCLE FIBERS. Am J Physiol. 1964 Oct;207:800–808. doi: 10.1152/ajplegacy.1964.207.4.800. [DOI] [PubMed] [Google Scholar]
- White D. C. Rigor contraction and the effect of various phosphate compounds on glycerinated insect flight and vertebrate muscle. J Physiol. 1970 Jul;208(3):583–605. doi: 10.1113/jphysiol.1970.sp009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]


