Abstract
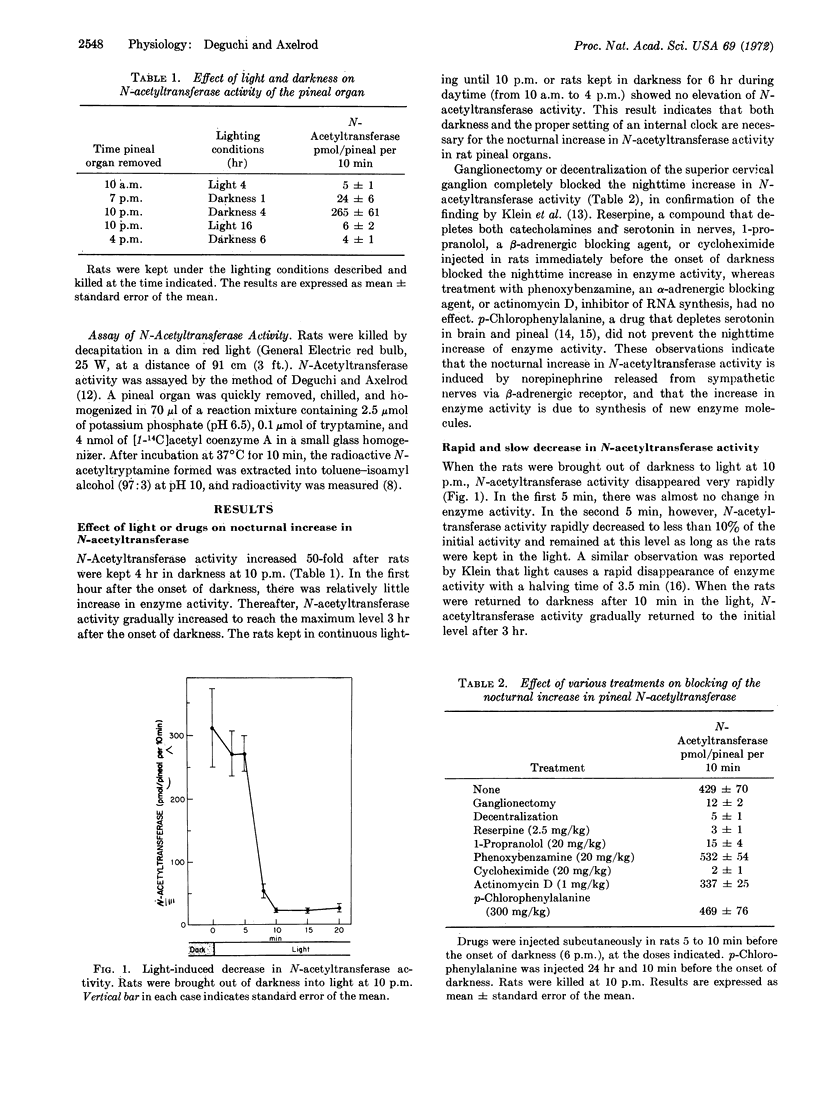
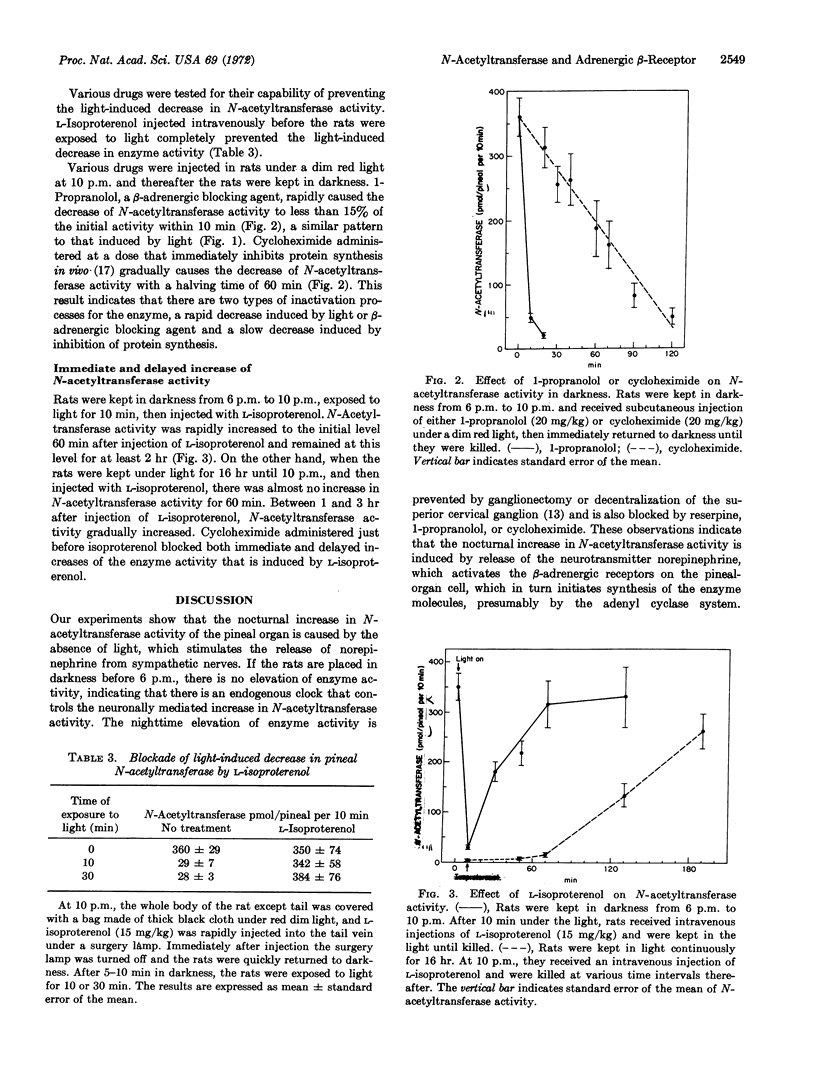
Serotonin N-acetyltransferase (EC 2.3.1.5) activity in the rat pineal organ is enhanced 50-fold at night. Rats exposed to light at night or kept in darkness during the daytime do not show any elevation of enzyme activity. Treatment with reserpine, a compound that depletes norepinephrine from nerves, 1-propranolol, a β-adrenergic blocking agent, or cycloheximide, an inhibitor of protein synthesis, abolishes the nocturnal increase in serotonin N-acetyltransferase activity, indicating that the enzyme activity is modulated by neural release of norepinephrine from sympathetic nerves via β-adrenergic receptors, and that the increase in enzyme activity is due to synthesis of new enzyme molecules. When rats are exposed to light at night or injected with 1-propranolol, there is a precipitous fall in serotonin N-acetyltransferase activity (half-life 5 min). Cycloheximide administered at night results in a slow fall in enzyme activity (half-life 60 min). When rats are kept in darkness and then exposed to light for 10 min, L-isoproterenol rapidly initiates the elevation of serotonin N-acetyltransferase activity to the initial level in 60 min. On the other hand, when the rats are kept in continuous light, L-isoproterenol initiates an increase in serotonin N-acetyltransferase activity after a lag phase of 60 min. The results indicate that there are two types of changes in serotonin N-acetyltransferase activity; a rapid increase and decrease mediated by the β-adrenergic receptor, and a slow increase and decrease in enzyme activity that appears to represent the turnover of the enzyme.
Keywords: diurnal, neural regulation, norepinephrine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., WURTMAN R. J., SNYDER S. H. CONTROL OF HYDROXYINDOLE O-METHYLTRANSFERASE ACTIVITY IN THE RAT PINEAL GLAND BY ENVIRONMENTAL LIGHTING. J Biol Chem. 1965 Feb;240:949–954. [PubMed] [Google Scholar]
- Axelrod J., Shein H. M., Wurtman R. J. Stimulation of C14-melatonin synthesis from C14-tryptophan by noradrenaline in rat pineal in organ culture. Proc Natl Acad Sci U S A. 1969 Feb;62(2):544–549. doi: 10.1073/pnas.62.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Induction and superinduction of serotonin N-acetyltransferase by adrenergic drugs and denervation in rat pineal organ. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2208–2211. doi: 10.1073/pnas.69.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M. S., Weiss B., Costa E. Adenosine 3'-5'-monophosphate in rat pineal gland: increase induced by light. Science. 1970 Oct 9;170(3954):188–190. doi: 10.1126/science.170.3954.188. [DOI] [PubMed] [Google Scholar]
- Grossman A., Boctor A. Evidence for reversible inactivation of induced tyrosine aminotransferase in rat liver in vivo. Proc Natl Acad Sci U S A. 1972 May;69(5):1161–1164. doi: 10.1073/pnas.69.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. C., Berg G. R., Weller J. Melatonin synthesis: adenosine 3',5'-monophosphate and norepinephrine stimulate N-acetyltransferase. Science. 1970 May 22;168(3934):979–980. doi: 10.1126/science.168.3934.979. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science. 1970 Sep 11;169(3950):1093–1095. doi: 10.1126/science.169.3950.1093. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L., Moore R. Y. Melatonin metabolism: neural regulation of pineal serotonin: acetyl coenzyme A N-acetyltransferase activity. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3107–3110. doi: 10.1073/pnas.68.12.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe B. K., Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966 Dec;154(3):499–516. [PubMed] [Google Scholar]
- Lynch H. J. Diurnal oscillations in pineal melatonin content. Life Sci I. 1971 Jul 15;10(14):791–795. doi: 10.1016/0024-3205(71)90033-6. [DOI] [PubMed] [Google Scholar]
- Neff N. H., Barrett R. E., Costa E. Kinetic and fluorescent histochemical analysis of the serotonin compartments in rat pineal gland. Eur J Pharmacol. 1969 Mar;5(4):348–356. doi: 10.1016/0014-2999(69)90112-5. [DOI] [PubMed] [Google Scholar]
- QUAY W. B. CIRCADIAN RHYTHM IN RAT PINEAL SEROTONIN AND ITS MODIFICATIONS BY ESTROUS CYCLE AND PHOTOPERIOD. Gen Comp Endocrinol. 1963 Oct;3:473–479. doi: 10.1016/0016-6480(63)90079-0. [DOI] [PubMed] [Google Scholar]
- Volkman P. H., Heller A. Pineal N-acetyltransferase activity: effect of sympathetic stimulation. Science. 1971 Aug 27;173(3999):839–840. doi: 10.1126/science.173.3999.839. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J., Sedvall G., Moore R. Y. Photic and neural control of the 24-hour norepinephrine rhythm in the rat pineal gland. J Pharmacol Exp Ther. 1967 Sep;157(3):487–492. [PubMed] [Google Scholar]
- Wurtman R. J., Shein H. M., Larin F. Mediation by -adrenergic receptors of effect of norepinephrine on pineal synthesis of ( 14 C)serotonin and ( 14 C)melatonin. J Neurochem. 1971 Sep;18(9):1683–1687. doi: 10.1111/j.1471-4159.1971.tb03741.x. [DOI] [PubMed] [Google Scholar]



