Abstract
Background
Methotrexate (MTX) is the cornerstone disease-modifying anti-rheumatic drug in juvenile idiopathic arthritis (JIA). In JIA, it is important to start effective treatment early to avoid long-term sequelae, such as joint damage. To accomplish this goal, it is crucial to know beforehand who is going to respond well to MTX. In addition, MTX adverse effects such as MTX intolerance occur frequently, potentially hindering its efficacy. To avoid inefficacy of an otherwise effective drug, the physician should be timely aware of these adverse events. Consequently, to optimise treatment of JIA patients with MTX, predictors for efficacy and adverse events should be used in daily clinical practice. The aim of this study was to summarise the existing knowledge about such predictors.
Methods
A systematic literature search was performed in PubMed, Embase and The Cochrane Library, and 1,331 articles were identified. These were selected based on their relevance to the topic and critically appraised according to pre-defined criteria. Predictors for MTX efficacy and adverse events were extracted from the literature and tabulated.
Results
Twenty articles were selected. The overall quality of the studies was good. For MTX efficacy, candidate predictors were antinuclear antibody positivity, the childhood health assessment questionnaire score, the myeloid-related protein 8/14 level, long-chain MTX polyglutamates, bilateral wrist involvement and some single nucleotide polymorphisms (SNPs) in the adenosine triphosphate binding cassette and solute carrier transporter gene families. For MTX adverse events, potential predictors were alanine aminotransferase and thrombocyte level and two SNPs in the γ-glutamyl hydrolase and methylenetetrahydrofolate reductase genes. However, validation of most predictors in independent cohorts was still lacking.
Conclusions
Interesting candidate predictors were found, especially for MTX efficacy. However, most of these were not validated. This should be the goal of future efforts. A clinically relevant way to validate the predictors is by means of creating a clinical prediction model.
Electronic supplementary material
The online version of this article (doi:10.1186/1546-0096-12-51) contains supplementary material, which is available to authorized users.
Keywords: Juvenile idiopathic arthritis, Methotrexate, EFficacy, Efficaciousness, Adverse events, MTX intolerance, Prediction
Background
Juvenile Idiopathic Arthritis (JIA) is the most common childhood rheumatologic disorder, with a prevalence of 16–150 per 100,000 children. It is characterised by chronic arthritis of unknown aetiology, lasting at least 6 weeks, with an onset before 16 years of age [1]. JIA is a heterogeneous group of disorders, whose manifestations range from relatively mild inflammation of a single joint, to severe involvement of multiple joints lasting into adulthood and leading to structural joint damage and incapacity. These long-term sequelae should be avoided and it is thought that early and effective therapy in the so-called window of opportunity is crucial in doing so [2–4].
The most widely used disease-modifying anti-rheumatic drug (DMARD) in the treatment of JIA is methotrexate (MTX), which has been used for more than 25 years. It is an inexpensive and safe drug and is beneficial in around 70% of JIA patients [5, 6]. Other treatment options include intra-articular joint injections or the more potent biologicals for MTX or corticosteroid resistant cases. It is still impossible to predict the individual prognosis and hence the treatment requirements at the onset of the disease [7], leading to the current step-up approach of starting MTX and adding a biological if the patient does not respond sufficiently well to MTX monotherapy. However, given the abovementioned goal to start effective treatment immediately in order to prevent joint damage and the fact that MTX monotherapy is completely ineffective in around 30% of patients, it is essential to know beforehand who is going to respond well to MTX and who is not. The latter group may then be prescribed a biological from the outset.
Next to drug effectiveness, its side effects should be taken into account. It has been shown previously that MTX despite being safe frequently causes transient elevation of liver enzymes and potentially also cytopenias, for which periodic evaluation of blood counts and liver function tests are advised [6, 8]. Perhaps more importantly, gastrointestinal side effects and MTX intolerance occur frequently [9–12]. MTX intolerance has been shown to influence the quality of life of patients negatively [13]. Furthermore, these adverse effects potentially cause non-compliance and hence ineffectiveness of an otherwise effective drug [9, 12, 14, 15], interfering with the goal to induce early disease remission. To avoid this problem, the risk of occurrence of these adverse effects should be known early, in order for the physician to intervene timely.
Therefore, to optimise treatment of JIA patients, it is necessary to predict the probability of response as well as the risk of developing adverse events. This systematic literature review aims to find and summarise studies, which assessed factors capable of doing so.
Methods
On 20 April 2014, a systematic literature search was performed in PubMed, Embase and The Cochrane Library, without any publication date or language constraints. Using the algorithm in Table 1 to retrieve all papers regarding JIA and MTX, 1,331 articles were identified (Figure 1). These were then screened for applicability to the research subject, the identification of predictors of MTX efficacy and adverse effects (outcome) in JIA patients (domain). Based on the title and the abstract, 45 articles were selected for full-text screening (Figure 1). To ensure that all relevant articles had been found, references of selected articles were screened to identify any missed papers.
Table 1.
Search strategy a
| Search algorithm | PubMed b | Embase b | Cochrane c | |
|---|---|---|---|---|
| #1 | “Juvenile idiopathic arthritis” OR “juvenile chronic arthritis” OR “juvenile rheumatoid arthritis” OR “juvenile rheumatic arthritis” OR “childhood arthritis” OR “juvenile arthritis” OR JIA OR JCA OR JRA | 7,844 | 10,906 | 296 |
| #2 | Methotrexate OR MTX OR “disease-modifying antirheumatic drug” OR “disease-modifying antirheumatic drugs” OR “disease-modifying anti rheumatic drug” OR “disease-modifying anti rheumatic drugs” OR DMARD OR DMARDs | 34,919 | 50,157 | 5,251 |
| #3 | #1 AND #2 | 662 | 1,229 | 64 |
aSearch performed on 20 April 2014.
bIn PubMed and Embase terms were searched in title and abstract only.
cIn The Cochrane Library terms were searched in title, abstract and keywords only.
Figure 1.
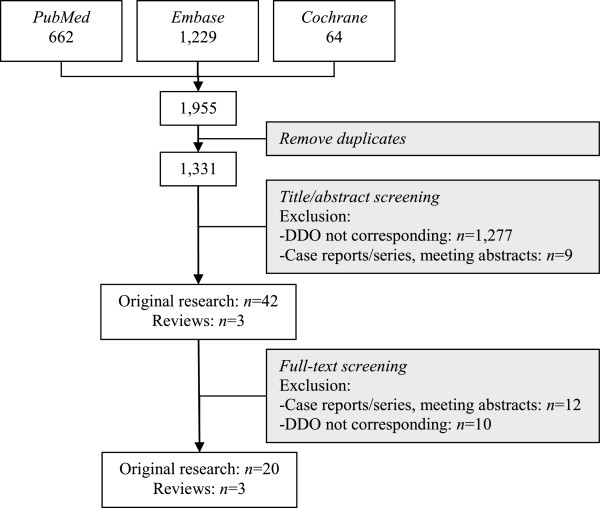
Flow chart. Flow chart of the article selection procedure. Abbreviations: DDO, domain, determinant and outcome.
Selected articles were critically appraised, using predefined criteria (Table 2). Studies that were selected, aimed to find predictors for MTX efficacy or side effects within 6 months after the start of therapy in JIA patients, using standardised outcome criteria.
Table 2.
Critical appraisal
| Reference | Design | Relevance | Validity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dom | Det | Out | Blind | Rec | SoC | Loss | Mis | ||
| Outcome: MTX efficacy | |||||||||
| [16] | Retrospective (validation prospective) | + | + | +/− | + | + | + | + | + |
| [17] | Mixed retrospective and prospective | + | + | +/− | + | + | + | + | + |
| [18] | Prospective | + | + | +/− | + | + | + | + | ? |
| [19] | Prospective | + | + | +/− | +/− | + | ? | ? | + |
| [20] | Cross-sectional | + | + | +/− | +/− | + | ? | ? | + |
| [10] | Retrospective | + | + | +/− | ? | + | ? | + | + |
| [21] | Retrospective | + | + | +/− | ? | + | ? | ? | + |
| [22] | Mixed retrospective and prospective | + | +/− | +/− | ? | + | ? | + | + |
| [23] | Prospective (validation unknown) | + | +/− | +/− | ? | + | ? | ? | ? |
| [24] | Prospective (validation unknown) | + | +/− | +/− | ? | + | ? | ? | ? |
| [25] a | Retrospective | +/− | +/− | + | ? | + | ? | +/− | + |
| [26] | Prospective | +/− | + | +/− | ? | + | ? | + | + |
| [27] | Prospective | +/− | + | +/− | ? | + | ? | + | +/− |
| [28] | Prospective | +/− | + | +/− | ? | + | ? | + | ? |
| [29] | Retrospective | +/− | +/− | + | ? | + | ? | +/− | + |
| [25] b | Retrospective | +/− | +/− | +/− | ? | + | ? | +/− | + |
| [30] | Retrospective | + | + | - | ? | + | ? | + | + |
| [31] | Cross-sectional | + | + | - | +/− | + | ? | ? | ? |
| [32] | Retrospective | + | +/− | - | +/− | + | ? | ? | ? |
| [33] | Retrospective | +/− | + | - | ? | + | ? | ? | + |
| Outcome: MTX adverse effects | |||||||||
| [34] | Prospective | + | + | +/− | + | + | + | + | + |
| [18] | Prospective | + | + | +/− | + | + | + | ? | ? |
| [10] | Retrospective | + | + | +/− | ? | + | ? | + | + |
| [21] | Retrospective | + | + | +/− | ? | + | ? | ? | + |
| [20] | Cross-sectional | + | + | +/− | +/− | + | ? | ? | + |
| [31] | Cross-sectional | + | + | +/− | +/− | + | ? | ? | ? |
| [32] | Retrospective | + | +/− | +/− | +/− | + | ? | ? | ? |
| [35] | Cross-sectional | - | + | + | ? | - | ? | + | + |
Abbreviations: Blind blinding, Det determinant, Dom domain, Loss loss to follow up, Mis missing predictors, Out outcome, Rec recruitment, SoC standardization of care.
Bold articles were excluded for analysis.
aPredictors after 6 months for outcome after 5 years; bPredictors at baseline for outcome after 6 months.
Criteria: Domain: + Children with confirmed JIA, according to currently valid ILAR criteria, starting MTX +/− Children with JCA/JRA according to previously valid criteria, or children with JIA and additional criteria (e.g. hospitalized, specific categories only), starting MTX - Children without JIA/JCA/JRA, or no MTX; Determinant: + Prediction model or single predictors corrected for confounding in multivariable analysis +/− Single predictors in univariate analysis - No predictors; Outcome: + Efficacy: Any standardized outcome measurement, follow up >1 year. Adverse effects: Any outcome measurement, follow up >1 year +/− follow-up <1 year - Efficacy: No use of standardized outcome criteria; Blinding: + Both patient and physician blinded (or not applicable in case of objective measurements) +/− Patient or physician not blinded - Not blinded; Recruitment: + Predictors determined at time of start of MTX or <6 months (or time of determination does not matter as in genetic evaluations, gender, age at onset, etc.) +/− Predictors determined more than 6 months after start of MTX, but <1 year - Predictors determined after 1 year, or completely at random; Standardization of care: + All participants treated according to standards of care - No standardized care; Loss to follow up (missing outcome): + <20% and unselective loss to follow up; or >20%, unselective and solved with a statistically valid method (imputation) +/− >20% (not imputed) but unselective loss to follow up - Selective loss to follow up; Missing predictors: + <20% and unselective; or >20%, unselective and solved with a statistically valid method (imputation) +/− >20% (not imputed) but unselective - Selective missing predictors.
The assessed predictors in the selected studies were summarised in tabular form. Because of the high number of studies and predictors, it was decided to show the direction of the effect of each predictor only, instead of providing an odds ratio and 95% confidence interval. A cut point of P <0.05 was defined to denote significance. Even though some of the assessed studies aimed at constructing a prediction model, for which this cut point is not important, we report significant predictors (P <0.05) only.
Results
After full-text screening, 20 original research papers and 3 reviews were selected (Figure 1), of which the former were critically appraised (Table 2). The overall relevance and validity of the selected papers was good, leading to only a few papers being excluded from the analysis. No study described whether all patients were eligible to receive the same treatment, but we knew this was the case in our own studies and assumed it was the case in all other studies. Hardly any article described if the physician and researchers were blinded for the outcome at the time of predictor determinations. Follow up was only short term (<1 year) in almost all studies.
MTX efficacy
Fifteen studies assessed MTX efficacy, of which some used a derivation cohort and an independent replication cohort. Since these were independent cohorts, results obtained in these cohorts were reported as if obtained in separate studies. In most of the studies, MTX was started within a median time of 1.5 years after disease onset. The most often used outcome criteria were the American College of Rheumatology (ACR) response criteria. Follow up ranged from 6 months to 1 year, but was as much as 7.3 years in one study (Table 3).
Table 3.
Characteristics of included studies
| Reference | Design | Country of origin | N | Inclusion criteria | Outcome a | Follow up |
|---|---|---|---|---|---|---|
| [18] b | Prospective | The Netherlands | 113 | JIA, starting MTX | 1k, 2a, 2d, 2f | 1 y |
| [19] | Prospective | UK | 87 | JIA, starting MTX | 1b, 1j | 6 mo |
| [17] c | Retrospective and prospective | The Netherlands | 287 | JIA, starting MTX | 1c | 1 y |
| [10] | Retrospective | Germany | 411 | JIA, starting MTX | 1a, 1b, 1c, 2i | 1 y |
| [16] (deriv) | Retrospective | The Netherlands | 183 | JIA, starting MTX | 1e | 1 y |
| [16] (rep) | Prospective | The Netherlands | 104 | JIA, starting MTX | 1e | 1 y |
| [23] (deriv)d | Prospective | UK | 197 | JIA, starting MTX | 1d | 6 mo |
| [23] (rep)d | Unknown | USA | 210 | JIA, starting MTX | 1g | 6 mo |
| [31] | Cross-sectional | Japan | 92 | JIA, at least 3 mo MTX | 2e | Mean 58.2 moe |
| [24] (deriv)d | Prospective | UK | 197 | JIA, starting MTX | 1d | 6 mo |
| [24] (rep)d | Unknown | USA | 210 | JIA, starting MTX | 1g | 6 mo |
| [20] | Cross-sectional | Czech Republic | 69 | JIA, at least 3 mo MTX | 1i, 2d, 2f, 2g, 2h | Median 1.3-1.4 ye |
| [28] f | Prospective | Multinational (PRINTO) | 563 | RF negative polyarticular course JIA, starting MTX | 1a, 1c | 6 mo |
| [26] | Prospective | Italy | 60 | JIA, ≥2 active joints in oligo persistent, ≥5 active joints in other categories | 1b | 1 y |
| [27] f | Prospective | Multinational (PRINTO) | 521 | RF negative polyarticular course JIA, starting MTX | 1l | 6 mo |
| [25] | Retrospective | Italy | 125 | Polyarticular JIA, starting MTX | 1f, 1i | 6 mo, 5 y |
| [32] | Retrospective | Germany | 58 | JIA, at least 3 mo MTX | 2d, 2i | Mean 48 months |
| [21] | Retrospective | Italy | 80 | JIA, at least 6 mo MTX | 1a, 2c, 2g | Efficacy: 6 mo |
| Toxicity: median 6–9 mo | ||||||
| [29] | Retrospective | USA | 49 | JRA, starting MTX | 1h | Mean 2.6 y (range 1.0-7.3 y) |
| [34] b | Prospective | The Netherlands | 152 | JIA, starting MTX | 2b | 1 y |
| [22] | Retrospective and prospective | Czech Republic, UK, The Netherlands | 694 | JIA, starting MTX | 1f | 6 mo |
Abbreviations: ACR30/50/70 American College of Rheumatology pediatric 30, 50 or 70 response criteria, respectively, AE adverse event, ALT alanine aminotransferase, AST aspartate aminotransferase, CHQ child health questionnaire, deriv derivation cohort, GI gastrointestinal, HRQOL health-related quality of life, JADAS juvenile arthritis disease activity score, JIA juvenile idiopathic arthritis, min minutes, MISS methotrexate intolerance severity score, mo months, MTX methotrexate, NR non-response, PhS physical component summary score, PsS psychosocial component summary score, RA rheumatoid arthritis, rep replication cohort, RF rheumatoid factor, ULN upper limit of normal, y years.
a 1a: Achievement of ACR30; 1b: Achievement of ACR50; 1c: Achievement of ACR70; 1d: Achievement of ACR70 vs. non-achievement of ACR30; 1e: Achievement of ACR70 in 2/3 visits; 1f: NR vs. ACR30 vs. ACR50 vs. ACR70; 1g: >70% improvement in joint count vs. <30%; 1h: Adapted ACR criteria for RA: morning stiffness <15 min, no fatigue, no joint swelling, no joint pain for 2 consecutive months; 1i: Clinical inactive disease on MTX monotherapy according to Wallace criteria; 1j: JADAS-10; 1k: JADAS-27; 1l: HRQOL: CHQ PhS ≥30 and PsS ≥30; 2a: MISS: intolerant (score >6); 2b: MISS: intolerant (score >6) after 6 and/or 12 months; 2c: ALT/AST > ULN; 2d: ALT/AST >2 ULN; 2e: ALT >5 ULN; 2f: Bone marrow suppression (any cytopenia); 2g: GI toxicity; 2h: Other (alopecia, headaches, behavioural changes, nodulosis); 2i: Any AE; bThis is the same cohort as the replication cohort of [16], but different outcome and/or predictors; cThis cohort is the derivation and replication cohort of [16] together, but uses a slightly different outcome and different predictors; dThese are the same cohorts, but they use different predictors; eTime after start of MTX; fThese are the same cohorts, but they use different outcome measurements.
The results of these studies are shown in Additional file 1: Table S1. Demographics, as well as JIA categories, were analysed extensively and were not predictive in almost all studies. Disease activity parameters showed inconsistent results in general, but the childhood health assessment questionnaire (CHAQ) score was a potential predictor. The same held true for the physician’s global assessment (PGA), although less convincingly so. The involvement of individual joints was assessed in too few studies to be conclusive, but bilateral wrist involvement was a potential predictor. Among laboratory data, positive antinuclear antibody (ANA) was a predictor of better response in three studies. Other interesting predictors could be long-chain MTX polyglutamates (PGs), the myeloid-related protein (MRP) 8/14 (also known as S100A8/A9), the pro-inflammatory molecule osteopontin, or even the haemoglobin level, although these were assessed in only one study each (Additional file 1: Table S1).
Next to these predictors, many single-nucleotide polymorphisms (SNPs) were analysed. These were SNPs in genes involved in the MTX metabolic pathway and in genes with altered post-treatment gene expression. Moreover, recently a genome-wide analysis study (GWAS) was published [22]. Of the latter study, only gene regions showing association with MTX response could be reported in this review.
Overall, no unequivocal predictive SNP has been found yet, because many were assessed in only one study, or were predictive in one study and showed no effect in others. However, some SNPs (rs1045642, rs35592 and rs4793665) in the B1, C1 and C3 members of the adenosine triphosphate binding cassette (ABC) transporter family, and others (rs3763980 and rs1051266) in the 16A7 and 19A1 members of the solute carrier (SLC) transporter family were interesting. Furthermore, the gene regions associated in the GWAS study were promising predictors (Additional file 1: Table S1).
In all, many potential predictors for MTX efficacy were assessed, yielding some interesting candidates, which, however, were analysed in too few studies to draw a firm conclusion yet.
MTX adverse events
Seven of the selected studies assessed MTX adverse events (Table 3). The assessed outcome varied from overall adverse events to single adverse events such as liver toxicity, gastrointestinal complaints or MTX intolerance measured with the Methotrexate Intolerance Severity Score (MISS) [9]. None of the articles focused on (serious) infections. Since the outcome MTX adverse events is a composite of all these outcomes, all studies were included. Follow up ranged from 6 months to a mean of 58.2 months.
Many predictors were evaluated in only one or two studies, making the results inconclusive. However, interesting predictors were the alanine aminotransferase (ALT) and thrombocyte level, as well as a SNP (rs1800909) in the γ-glutamyl hydrolase (GGH) gene, involved in the breakdown of MTX PGs, and another SNP (rs1801133) in the methylenetetrahydrofolate reductase (MTHFR) gene, involved in the folate metabolism. Finally, the polyarticular categories could potentially pose a risk to develop MTX side effects (Additional file 2: Table S2).
Discussion
This systematic literature review aimed to find predictors for MTX efficacy and adverse events in JIA patients. For MTX efficacy, many candidate predictors were investigated, and some interesting results were found, such as ANA positivity, the CHAQ score, the MRP 8/14 (S100A8/A9) level, long-chain MTX-PGs, bilateral wrist involvement, osteopontin level, haemoglobin, some SNPs in the ABC and SLC transporter gene families and several gene regions elucidated in the recently published GWAS. Most of these variables have not yet been validated in independent cohorts. Therefore, future efforts should be directed at validating these candidate predictors. A clinically relevant way to do so consists in combining these predictors into a prediction model and assessing the prognostic accuracy of the model. Thus far, only one prediction model for MTX efficacy in JIA patients has been developed, containing the erythrocyte sedimentation rate and four SNPs in genes involved in the MTX metabolic pathway [16]. This model could be improved using the abovementioned candidate predictors. The advantage of this method is twofold: statistically, the selection of predictors for the model in the independent cohort will be literature-driven, instead of data-driven, leading to a reduction in the so-called optimism [35]. Clinically, physicians will have an easy-to-use tool at hand to determine the probability of MTX efficacy in individual patients, allowing them to start MTX in patients with a high probability of responding and to initiate biologicals in those with a low probability of responding. The clinical benefit of this approach should ideally be estimated in a randomized clinical trial.
Regarding MTX adverse events, the results were less clear. Although some interesting candidate predictors were found, such as ALT and thrombocyte level and two SNPs in the GGH and MTHFR genes, here too, validation of these was lacking. Furthermore, it seemed questionable if these predictors would be sufficient to predict the individual patients’ risk of developing MTX adverse effects. Consequently, future efforts should be directed both at validating existing candidate predictors and at finding new predictors. These too should be combined in a clinical prediction model (an example has been submitted: Van Dijkhuizen EHP, Bulatovic Calasan M, Pluijm SMF, De Rotte MCFJ, Vastert SJ, Kamphuis S, De Jonge R, Wulffraat NM: Prediction of Methotrexate Intolerance in Juvenile Idiopathic Arthritis, submitted). Such a tool could be used to monitor high-risk patients more closely, intervening as soon as adverse events occur, for example by lowering the dose, stopping MTX temporarily or prescribing other drugs. On the other hand, low-risk patients could be saved the burden of frequent checks.
Recently, a review was published about genetic predictors of MTX efficacy and toxicity in rheumatoid arthritis [36]. SNPs investigated in five or more independent studies were considered (n = 4). Only ATIC rs2372536 showed a potential association of the minor allele with toxicity. This SNP did not show an association with adverse events in our review. Conversely, whereas we found an association of SLC19A1 rs1051266 with efficacy (though in a single study), results about this SNP were inconsistent in the adult review. Thus, the results of the adult and paediatric review were quite different. This might be due to incomparability of children and adults in this respect, maybe because of a different metabolization of MTX [37]. On the other hand, it shows there is still much to do in the field of MTX prediction.
Most SNPs investigated in this review were located in genes involved in the MTX metabolic pathway. In short, MTX enters the cell via the members of the SLC protein family. It becomes polyglutamated by FPGS, causing its cellular retention. Depolyglutamation is brought about by GGH. MTX is pumped out of the cell by members of the ABC transporter family. Intracellular MTX-PGs exert a range of actions. First, they inhibit DHFR and influence MTHFR, enzymes in the folate pathway involved in polyamine synthesis. Secondly, they inhibit TYMS, an enzyme in the pyrimidine synthesis pathway. Finally, by blocking ATIC, MTX-PGs stimulate the production of adenosine, an anti-inflammatory agent. Other important enzymes in this pathway are ITPA and AMPD1, which themselves are not influenced by MTX [17, 36]. It can be hypothesized that SNPs in any of these genes cause increased or decreased sensibility to the actions of MTX-PGs and hence lead to altered MTX efficacy.
Other potential predictors included the MRP8/14 (S100A8/A9) level, a danger signal and activator of toll-like receptor 4, which in turn plays a role in the innate immune response in inflammatory conditions. MRP8/14 was earlier shown to predict disease flare or continuation of remission after withdrawal of MTX in children who were in remission [5]. Another candidate predictor, osteopontin, is expressed by natural killer cells and activated T cells, and plays a role in the production of pro-inflammatory cytokines. It is overexpressed in synovial T cells in patients with rheumatoid arthritis, demonstrating its role in inflammatory arthritis [26]. The theoretical background of these candidate predictors may increase the likelihood that they really affect MTX efficacy, however, it should be kept in mind that the found associations do not prove a causal effect.
The most frequently used outcome criteria with respect to MTX efficacy were the American College of Rheumatology (ACR) response criteria [38]. These criteria, though validated, have their limitations in practical use. For example, a patient with 69% improvement in all core set criteria will not be an ACR70 responder, whereas someone with 70% improvement in three core set criteria and worsening up to 29% in the remainder will be an ACR70 responder, despite the fact that the former obviously responds better than the latter. Hence, if one takes the achievement of ACR70 response status as an outcome measurement, considerable misclassification may occur. Alternatively, the percentage of change of the juvenile arthritis disease activity score (JADAS) could be used as an outcome measurement to assess MTX efficacy [39, 40]. Because this is a continuous and composite outcome, the risk of misclassification may be reduced. Recently, the ACR criteria and the JADAS were compared, showing excellent ability of the JADAS to classify the ACR response categories [41]. To answer the question which outcome measurement should be preferred, both measurements should be compared to a reference standard, such as a panel of experts.
The studies that were analysed in this review were generally of good quality. They recruited patients at the start of MTX and followed them prospectively. However, many articles did not describe whether the studies were blinded, an absence of which could lead to biases. On a review level, due to our extensive search strategy, it is likely that we found all pertinent papers. However, some negative results might not have been published, leading to reporting and publication bias. The results found in different studies were often quite variable. This may be due to heterogeneity of patient groups, differences in sample size and the absence or presence of linkage disequilibrium between the tested SNP and the actual polymorphism which causes the altered MTX response [36]. Furthermore, other patient factors may confound the observed relationship, reason why it is important to perform a multivariate analysis. This was not done in all studies. Finally, the authors of this review were co-authors of some of the included papers, causing them to know more about the study design and potentially biasing them to be more lenient towards their own studies.
Conclusion
In conclusion, this systematic review of the literature with respect to predictors for MTX efficacy and adverse events in JIA patients shows that a number of interesting candidates were found. However, validation of these potential predictors is still lacking in many cases. Therefore, future efforts should be directed at validating these candidate predictors, potentially by means of a clinical prediction model. For the outcome adverse events, next to validating existing candidates, more candidate predictors should be investigated.
Electronic supplementary material
Additional file 1: Table S1: Results for outcome MTX efficacya. (DOCX 436 KB)
Additional file 2: Table S2: Results for outcome absence of MTX adverse eventsa. (DOCX 116 KB)
Funding
This project has received funding from the 7th Framework programme of the EU, SP3-People, support for training and career development for researchers (Marie Curie), Network for Initial Training (ITN), FP7-PEOPLE-2011-ITN, under the Marie Skłodowska-Curie grant agreement No 289903 [grant to EHPvD] and SHARE project, EAHC grant number 2011 1202 [grant to NMW]. The sponsors had no role in the study planning, design, management or data analysis.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PvD performed the literature search and drafted the manuscript. NW conceived of the study and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
EH Pieter van Dijkhuizen, Email: E.H.P.Dijkhuizen@umcutrecht.nl.
Nico M Wulffraat, Email: N.Wulffraat@umcutrecht.nl.
References
- 1.Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138–2149. doi: 10.1016/S0140-6736(11)60244-4. [DOI] [PubMed] [Google Scholar]
- 2.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48:1771–1774. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- 3.Tynjala P, Vahasalo P, Tarkiainen M, Kroger L, Aalto K, Malin M, Putto-Laurila A, Honkanen V, Lahdenne P. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis. 2011;70:1605–1612. doi: 10.1136/ard.2010.143347. [DOI] [PubMed] [Google Scholar]
- 4.Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, Szer IS, Ringold S, Brunner HI, Schanberg LE, Sundel RP, Milojevic D, Punaro MG, Chira P, Gottlieb BS, Higgins GC, Ilowite NT, Kimura Y, Hamilton S, Johnson A, Huang B, Lovell DJ. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64:2012–2021. doi: 10.1002/art.34343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foell D, Wulffraat N, Wedderburn LR, Wittkowski H, Frosch M, Gerss J, Stanevicha V, Mihaylova D, Ferriani V, Tsakalidou FK, Foeldvari I, Cuttica R, Gonzalez B, Ravelli A, Khubchandani R, Oliveira S, Armbrust W, Garay S, Vojinovic J, Norambuena X, Gamir ML, Garcia-Consuegra J, Lepore L, Susic G, Corona F, Dolezalova P, Pistorio A, Martini A, Ruperto N, Roth J. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA. 2010;303:1266–1273. doi: 10.1001/jama.2010.375. [DOI] [PubMed] [Google Scholar]
- 6.Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SK, Falcini F, Dolezalova P, Alessio M, Burgos-Vargas R, Corona F, Vesely R, Foster H, Davidson J, Zulian F, Asplin L, Baildam E, Consuegra JG, Ozdogan H, Saurenmann R, Joos R, Pistorio A, Woo P, Martini A. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004;50:2191–2201. doi: 10.1002/art.20288. [DOI] [PubMed] [Google Scholar]
- 7.Van Dijkhuizen EHP, Wulffraat NM. Early predictors of prognosis in juvenile idiopathic arthritis: a systematic literature review. Annals Rheumatic. 2014;24:6. doi: 10.1136/annrheumdis-2014-205265. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz-Alvarez O, Morishita K, Avery G, Green J, Petty RE, Tucker LB, Malleson PN, Cabral DA. Guidelines for blood test monitoring of methotrexate toxicity in juvenile idiopathic arthritis. J Rheumatol. 2004;31:2501–2506. [PubMed] [Google Scholar]
- 9.Bulatovic M, Heijstek MW, Verkaaik M, Van Dijkhuizen EHP, Armbrust W, Hoppenreijs EP, Kamphuis S, Kuis W, Egberts TC, Sinnema G, Rademaker CM, Wulffraat NM. High prevalence of methotrexate intolerance in juvenile idiopathic arthritis: development and validation of a methotrexate intolerance severity score. Arthritis Rheum. 2011;63:2007–2013. doi: 10.1002/art.30367. [DOI] [PubMed] [Google Scholar]
- 10.Klein A, Kaul I, Foeldvari I, Ganser G, Urban A, Horneff G. Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: an observational study with patients from the German Methotrexate Registry. Arthritis Care Res (Hoboken) 2012;64:1349–1356. doi: 10.1002/acr.21697. [DOI] [PubMed] [Google Scholar]
- 11.Murray KJ, Lovell DJ. Advanced therapy for juvenile arthritis. Best Pract Res Clin Rheumatol. 2002;16:361–378. doi: 10.1016/S1521-6942(02)90234-2. [DOI] [PubMed] [Google Scholar]
- 12.van der Meer A, Wulffraat NM, Prakken BJ, Gijsbers B, Rademaker CM, Sinnema G. Psychological side effects of MTX treatment in juvenile idiopathic arthritis: a pilot study. Clin Exp Rheumatol. 2007;25:480–485. [PubMed] [Google Scholar]
- 13.Brunner HI, Johnson AL, Barron AC, Passo MH, Griffin TA, Graham TB, Lovell DJ. Gastrointestinal symptoms and their association with health-related quality of life of children with juvenile rheumatoid arthritis: validation of a gastrointestinal symptom questionnaire. J Clin Rheumatol. 2005;11:194–204. doi: 10.1097/01.rhu.0000173616.81928.44. [DOI] [PubMed] [Google Scholar]
- 14.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Martini A, Rabinovich CE, Ruperto N. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–482. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horneff G. Update on biologicals for treatment of juvenile idiopathic arthritis. Expert Opin Biol Ther. 2013;13:361–376. doi: 10.1517/14712598.2013.735657. [DOI] [PubMed] [Google Scholar]
- 16.Bulatovic M, Heijstek MW, Van Dijkhuizen EHP, Wulffraat NM, Pluijm SM, de Jonge R. Prediction of clinical non-response to methotrexate treatment in juvenile idiopathic arthritis. Ann Rheum Dis. 2012;71:1484–1489. doi: 10.1136/annrheumdis-2011-200942. [DOI] [PubMed] [Google Scholar]
- 17.de Rotte MC, Bulatovic M, Heijstek MW, Jansen G, Heil SG, van Schaik RH, Wulffraat NM, de Jonge R. ABCB1 and ABCC3 gene polymorphisms are associated with first-year response to methotrexate in juvenile idiopathic arthritis. J Rheumatol. 2012;39:2032–2040. doi: 10.3899/jrheum.111593. [DOI] [PubMed] [Google Scholar]
- 18.Bulatovic Calasan M, den Boer E, de Rotte MC, Vastert SJ, Kamphuis S, de Jonge R, Wulffraat NM. Ann Rheum Dis. 2013. Methotrexate polyglutamates in erythrocytes are associated with lower disease activity in juvenile idiopathic arthritis patients. [DOI] [PubMed] [Google Scholar]
- 19.Moncrieffe H, Ursu S, Holzinger D, Patrick F, Kassoumeri L, Wade A, Roth J, Wedderburn LR. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 2013;52:1467–1476. doi: 10.1093/rheumatology/ket152. [DOI] [PubMed] [Google Scholar]
- 20.Tukova J, Chladek J, Hroch M, Nemcova D, Hoza J, Dolezalova P. 677TT genotype is associated with elevated risk of methotrexate (MTX) toxicity in juvenile idiopathic arthritis: treatment outcome, erythrocyte concentrations of MTX and folates, and MTHFR polymorphisms. J Rheumatol. 2010;37:2180–2186. doi: 10.3899/jrheum.091427. [DOI] [PubMed] [Google Scholar]
- 21.Ravelli A, Viola S, Migliavacca D, Ruperto N, Pistorio A, Martini A. The extended oligoarticular subtype is the best predictor of methotrexate efficacy in juvenile idiopathic arthritis. J Pediatr. 1999;135:316–320. doi: 10.1016/S0022-3476(99)70127-7. [DOI] [PubMed] [Google Scholar]
- 22.Cobb J, Cule E, Moncrieffe H, Hinks A, Ursu S, Patrick F, Kassoumeri L, Flynn E, Bulatovic M, Wulffraat N, van Zelst B, de Jonge R, Bohm M, Dolezalova P, Hirani S, Newman S, Whitworth P, Southwood TR, De Iorio M, Wedderburn LR, Thomson W. Genome-wide data reveal novel genes for methotrexate response in a large cohort of juvenile idiopathic arthritis cases. Pharmacogenomics J. 2014;14:356–364. doi: 10.1038/tpj.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinks A, Moncrieffe H, Martin P, Ursu S, Lal S, Kassoumeri L, Weiler T, Glass DN, Thompson SD, Wedderburn LR, Thomson W. Association of the 5-aminoimidazole-4-carboxamide ribonucleotide transformylase gene with response to methotrexate in juvenile idiopathic arthritis. Ann Rheum Dis. 2011;70:1395–1400. doi: 10.1136/ard.2010.146191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moncrieffe H, Hinks A, Ursu S, Kassoumeri L, Etheridge A, Hubank M, Martin P, Weiler T, Glass DN, Thompson SD, Wedderburn LR. Generation of novel pharmacogenomic candidates in response to methotrexate in juvenile idiopathic arthritis: correlation between gene expression and genotype. Pharmacogenet Genomics. 2010;20:665–676. doi: 10.1097/FPC.0b013e32833f2cd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartoli M, Taro M, Magni-Manzoni S, Pistorio A, Traverso F, Viola S, Magnani A, Gasparini C, Martini A, Ravelli A. The magnitude of early response to methotrexate therapy predicts long-term outcome of patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2008;67:370–374. doi: 10.1136/ard.2007.073445. [DOI] [PubMed] [Google Scholar]
- 26.Masi L, Ricci L, Zulian F, Del Monte F, Simonini G, Capannini S, De Martino M, Brandi ML, Falcini F. Serum osteopontin as a predictive marker of responsiveness to methotrexate in juvenile idiopathic arthritis. J Rheumatol. 2009;36:2308–2313. doi: 10.3899/jrheum.081156. [DOI] [PubMed] [Google Scholar]
- 27.Cespedes-Cruz A, Gutierrez-Suarez R, Pistorio A, Ravelli A, Loy A, Murray KJ, Gerloni V, Wulffraat N, Oliveira S, Walsh J, Penades IC, Alpigiani MG, Lahdenne P, Saad-Magalhaes C, Cortis E, Lepore L, Kimura Y, Wouters C, Martini A, Ruperto N. Methotrexate improves the health-related quality of life of children with juvenile idiopathic arthritis. Ann Rheum Dis. 2008;67:309–314. doi: 10.1136/ard.2007.075895. [DOI] [PubMed] [Google Scholar]
- 28.Vilca I, Munitis PG, Pistorio A, Ravelli A, Buoncompagni A, Bica B, Campos L, Hafner R, Hofer M, Ozen S, Huemer C, Bae SC, Sztajnbok F, Arguedas O, Foeldvari I, Huppertz HI, Gamir ML, Magnusson B, Dressler F, Uziel Y, Van Rossum MA, Hollingworth P, Cawkwell G, Martini A, Ruperto N. Predictors of poor response to methotrexate in polyarticular-course juvenile idiopathic arthritis: analysis of the PRINTO methotrexate trial. Ann Rheum Dis. 2010;69:1479–1483. doi: 10.1136/ard.2009.120840. [DOI] [PubMed] [Google Scholar]
- 29.Wallace CA, Sherry DD, Mellins ED, Aiken RP. Predicting remission in juvenile rheumatoid arthritis with methotrexate treatment. J Rheumatol. 1993;20:118–122. [PubMed] [Google Scholar]
- 30.Albers HM, Wessels JA, van der Straaten RJ, Brinkman DM, Suijlekom-Smit LW, Kamphuis SS, Girschick HJ, Wouters C, Schilham MW, le Cessie S, Huizinga TW, ten Cate R, Guchelaar HJ. Time to treatment as an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:46–51. doi: 10.1002/art.24087. [DOI] [PubMed] [Google Scholar]
- 31.Yanagimachi M, Naruto T, Hara T, Kikuchi M, Hara R, Miyamae T, Imagawa T, Mori M, Kaneko T, Morita S, Goto H, Yokota S. Influence of polymorphisms within the methotrexate pathway genes on the toxicity and efficacy of methotrexate in patients with juvenile idiopathic arthritis. Br J Clin Pharmacol. 2011;71:237–243. doi: 10.1111/j.1365-2125.2010.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmeling H, Biber D, Heins S, Horneff G. Influence of methylenetetrahydrofolate reductase polymorphisms on efficacy and toxicity of methotrexate in patients with juvenile idiopathic arthritis. J Rheumatol. 2005;32:1832–1836. [PubMed] [Google Scholar]
- 33.Ravelli A, Ramenghi B, Di Fuccia G, Ruperto N, Zonta L, Martini A. Factors associated with response to methotrexate in systemic-onset juvenile chronic arthritis. Acta Paediatr. 1994;83:428–432. doi: 10.1111/j.1651-2227.1994.tb18135.x. [DOI] [PubMed] [Google Scholar]
- 34.Patil P, Rawcliffe C, Olaleye A, Moore S, Fox A, Sen D, Ioannou Y. Comparison of methotrexate induced nausea and vomiting between adolescent and adult patients with inflammatory arthritis. Rheumatology. 2012;51:iii107–iii108. [Google Scholar]
- 35.Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York: Springer; 2009. [Google Scholar]
- 36.Malik F, Ranganathan P. Methotrexate pharmacogenetics in rheumatoid arthritis: a status report. Pharmacogenomics. 2013;14:305–314. doi: 10.2217/pgs.12.214. [DOI] [PubMed] [Google Scholar]
- 37.Albertioni F, Flato B, Seideman P, Beck O, Vinje O, Peterson C, Eksborg S. Methotrexate in juvenile rheumatoid arthritis. Evidence of age dependent pharmacokinetics. Eur J Clin Pharmacol. 1995;47:507–511. doi: 10.1007/BF00193703. [DOI] [PubMed] [Google Scholar]
- 38.Ruperto N, Ravelli A, Falcini F, Lepore L, De SR, Zulian F, Buoncompagni A, Sardella ML, Strano C, Alessio M, Viola S, Martini A. Performance of the preliminary definition of improvement in juvenile chronic arthritis patients treated with methotrexate. Italian Pediatric Rheumatology Study Group. Ann Rheum Dis. 1998;57:38–41. doi: 10.1136/ard.57.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, Malattia C, Viola S, Martini A, Ravelli A. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:658–666. doi: 10.1002/art.24516. [DOI] [PubMed] [Google Scholar]
- 40.Horneff G, Becker I. Definition of improvement in juvenile idiopathic arthritis using the Juvenile Arthritis Disease Activity Score. Rheumatology (Oxford) 2014;53:1229–1234. doi: 10.1093/rheumatology/ket470. [DOI] [PubMed] [Google Scholar]
- 41.Ringold S, Bittner R, Neogi T, Wallace CA, Singer NG. Performance of rheumatoid arthritis disease activity measures and juvenile arthritis disease activity scores in polyarticular-course juvenile idiopathic arthritis: analysis of their ability to classify the American College of Rheumatology pediatric measures of response and the preliminary criteria for flare and inactive disease. Arthritis Care Res (Hoboken) 2010;62:1095–1102. doi: 10.1002/acr.20205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: Results for outcome MTX efficacya. (DOCX 436 KB)
Additional file 2: Table S2: Results for outcome absence of MTX adverse eventsa. (DOCX 116 KB)


