Abstract
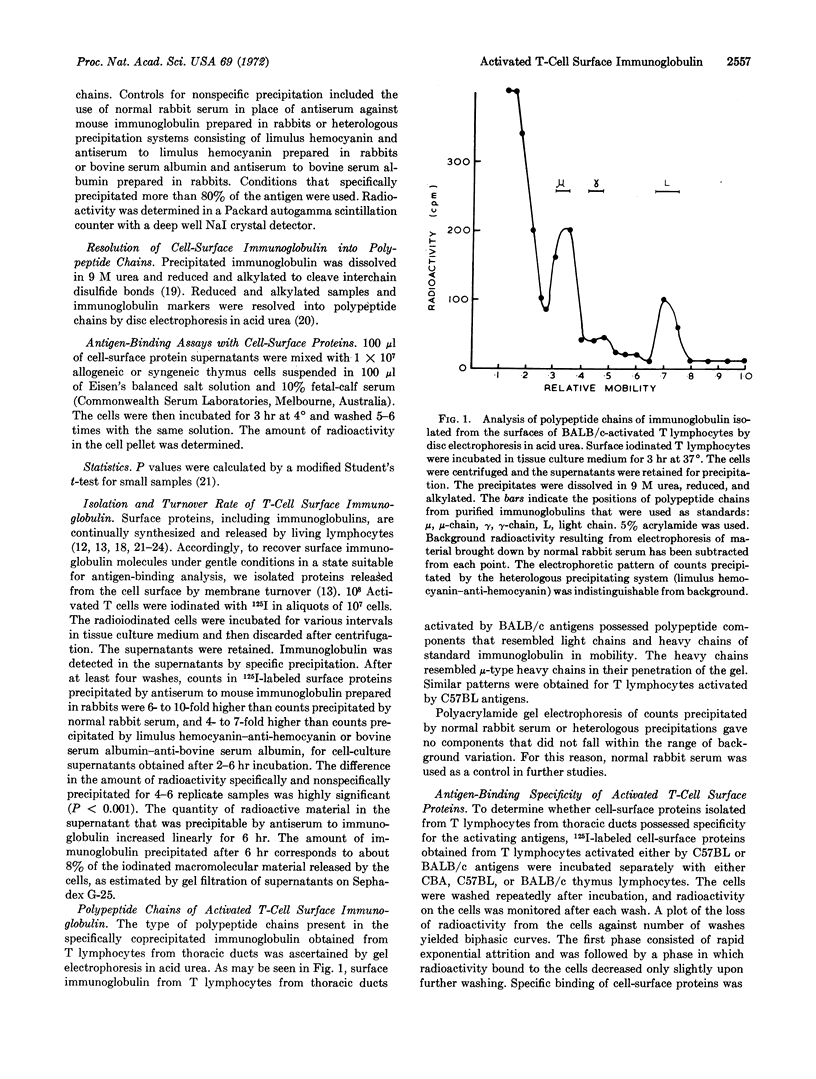
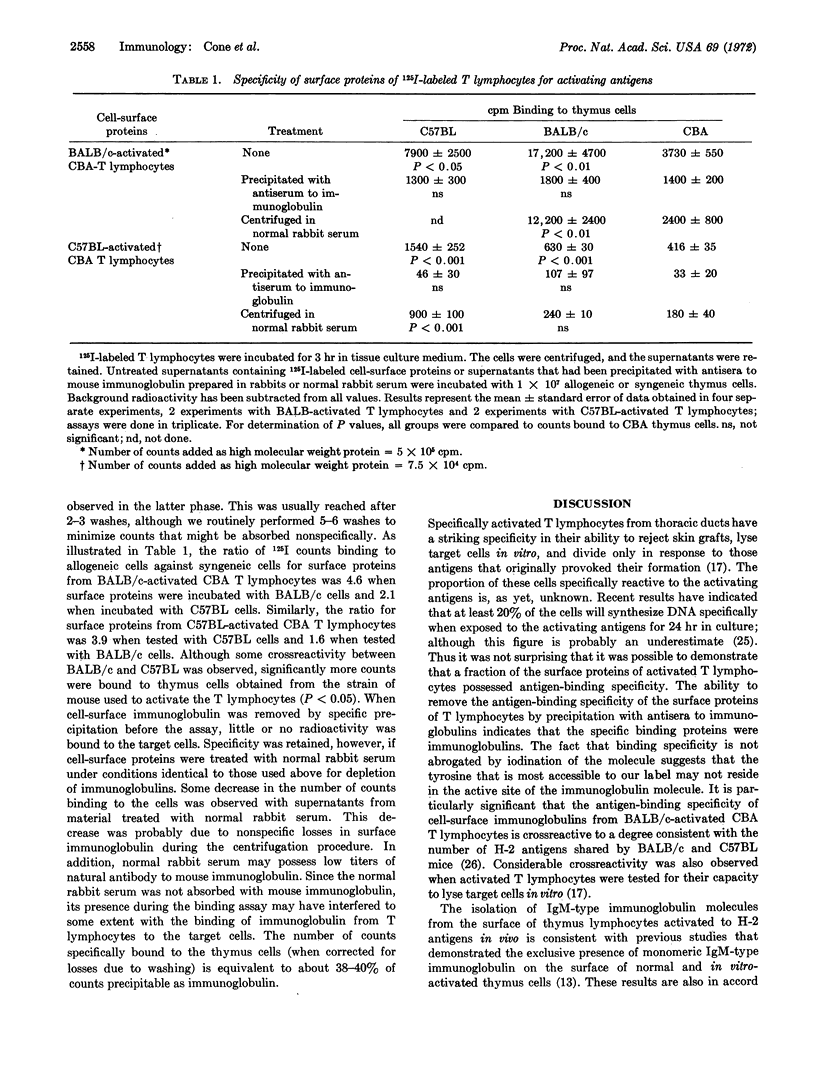
Lactoperoxidase-catalyzed radioiodination of cell-surface proteins was used in the isolation of cell-surface immunoglobulin from thymus-derived thoracic duct lymphocytes activated to histocompatibility-2 antigens. Immunoglobulin was identified by specific precipitation with antiserum to mouse immunoglobulin. Polyacrylamide gel electrophoresis of reduced and alkylated precipitates showed that the immunoglobulin molecules possessed μ-type heavy chains and light chains. Cell-surface immunoglobulin isolated from thymus-derived cells activated to histocompatibility-2 antigens possessed binding specificity for the activating antigens.
Keywords: mouse T cells, 125I, precipitation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhurst A. D., Warner N. L., Sprent J. Surface immunoglobulins on thymus and thymus-derived lymphoid cells. J Exp Med. 1971 Oct 1;134(4):1005–1015. doi: 10.1084/jem.134.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Baur S., Vitetta E. S., Sherr C. J., Schenkein I., Uhr J. W. Isolation of heavy and light chains of immunoglobulin from the surfaces of lymphoid cells. J Immunol. 1971 Apr;106(4):1133–1135. [PubMed] [Google Scholar]
- Cone R. E., Marchalonis J. J., Rolley R. T. Lymphocyte membrane dynamics. Metabolic release of cell surface proteins. J Exp Med. 1971 Dec 1;134(6):1373–1384. doi: 10.1084/jem.134.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Rosenthal A. S., Paul W. E. Receptors on immunocompetent cells. 3. Specificity and nature of receptors on dinitrophenylated guinea pig albumin- 125 I-binding cells of immunized guinea pigs. J Exp Med. 1971 Aug 1;134(2):517–531. doi: 10.1084/jem.134.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Warner N. L., Mackay I. R. Specificity and nature of the antigen-combining sites on fetal and mature thymus lymphocytes. J Immunol. 1972 May;108(5):1439–1446. [PubMed] [Google Scholar]
- Edelman G. M., Millette C. F. Molecular probes of spermatozoan structures. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2436–2440. doi: 10.1073/pnas.68.10.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Diener E. Reversible blocking effect of anti-mouse immunoglobulin serum on the induction of immunity and tolerance in vitro. Nat New Biol. 1971 Jun 9;231(23):183–184. doi: 10.1038/newbio231183a0. [DOI] [PubMed] [Google Scholar]
- Herd Z. L., Ada G. L. The retention of 125I-immunoglobulins, IgG subunits and antigen-antibody complexes in rat footpads and draining lymph nodes. Aust J Exp Biol Med Sci. 1969 Feb;47(1):63–72. doi: 10.1038/icb.1969.5. [DOI] [PubMed] [Google Scholar]
- Hogg N. M., Greaves M. F. Antigen-binding thymus-derived lymphocytes. II. Nature of the immunoglobulin determinants. Immunology. 1972 Jun;22(6):967–980. [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Rajewsky K. Evidence for surface-associated immunoglobulin on T and B lymphocytes. Eur J Immunol. 1971 Dec;1(6):447–452. doi: 10.1002/eji.1830010608. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., McConahey P. J., Jansen I., Dixon F. J. Synthesis of plasma membrane-associated and secretory immunoglobulin in diploid lymphocytes. J Exp Med. 1972 Jan;135(1):136–149. doi: 10.1084/jem.135.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J. F., Kettman J. R., Dutton R. W. Immunoglobulins on the surface of thymus-derived cells engaged in the initiation of a humoral immune response. J Exp Med. 1971 Sep 1;134(3 Pt 1):618–629. doi: 10.1084/jem.134.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Atwell J. L., Cone R. E. Isolation of surface immunoglobulin from lymphocytes from human and murine thymus. Nat New Biol. 1972 Feb 23;235(60):240–242. doi: 10.1038/newbio235240a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Atwell J. L. Isolation and partial characterization of lymphocyte surface immunoglobulins. J Exp Med. 1972 Apr 1;135(4):956–971. doi: 10.1084/jem.135.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason S., Warner N. L. The immunoglobulin nature of the antigen recognition site on cells mediating transplantation immunity and delayed hypersentivity. J Immunol. 1970 Mar;104(3):762–765. [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R., Marchalonis J. J. A simple and rapid acrylamide gel method for estimating the molecular weights of proteins and protein subunits. Anal Biochem. 1970 Apr;34(2):436–450. doi: 10.1016/0003-2697(70)90128-4. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma V. R., Silverton E. W., Davies D. R., Terry W. D. The three-dimensional structure at 6 A resolution of a human gamma Gl immunoglobulin molecule. J Biol Chem. 1971 Jun 10;246(11):3753–3759. [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Activation of thymus cells by histocompatibility antigens. Nat New Biol. 1971 Sep 15;234(50):195–198. doi: 10.1038/newbio234195a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Release of cell surface immunoglobulin by mouse splenic lymphocytes. J Immunol. 1972 Feb;108(2):577–579. [PubMed] [Google Scholar]
- Warner N. L., Byrt P., Ada G. L. Blocking of the lymphocyte antigen receptor site with anti-immunoglobulin sera in vitro. Nature. 1970 Jun 6;226(5249):942–943. doi: 10.1038/226942a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J., Lewis H. Metabolic characteristics of lymphocyte surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):225–232. doi: 10.1002/eji.1830020306. [DOI] [PubMed] [Google Scholar]



