Abstract
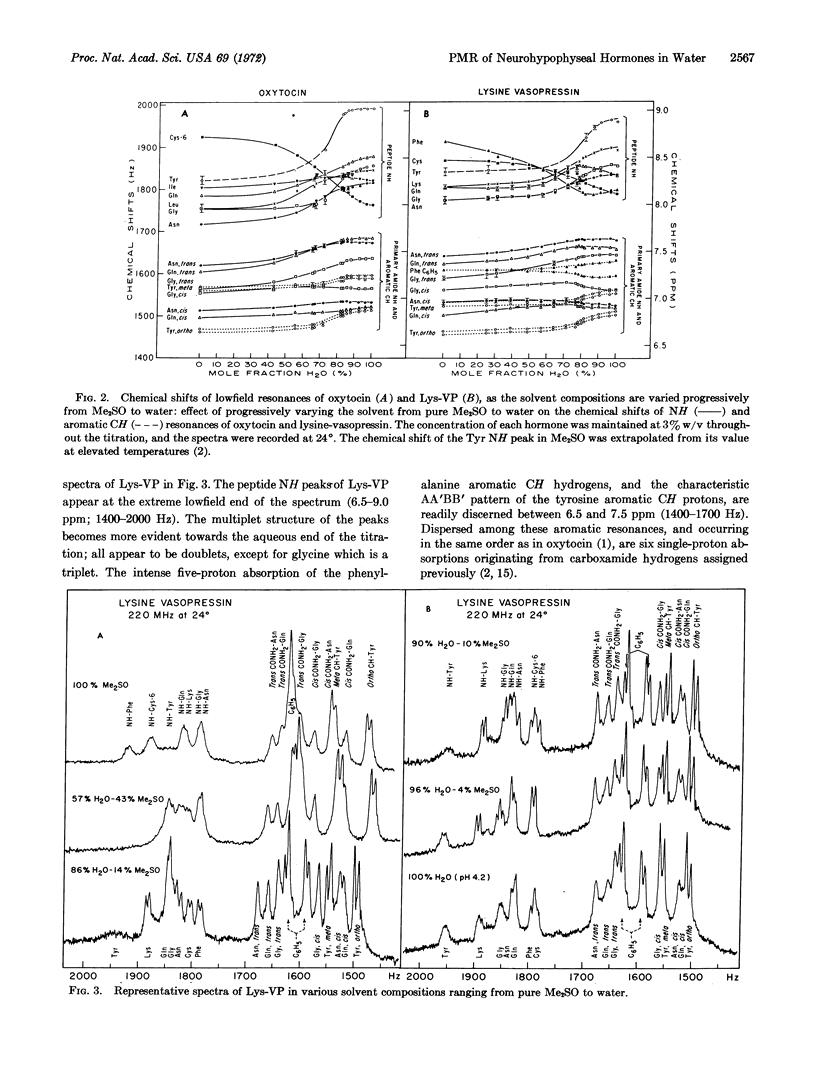
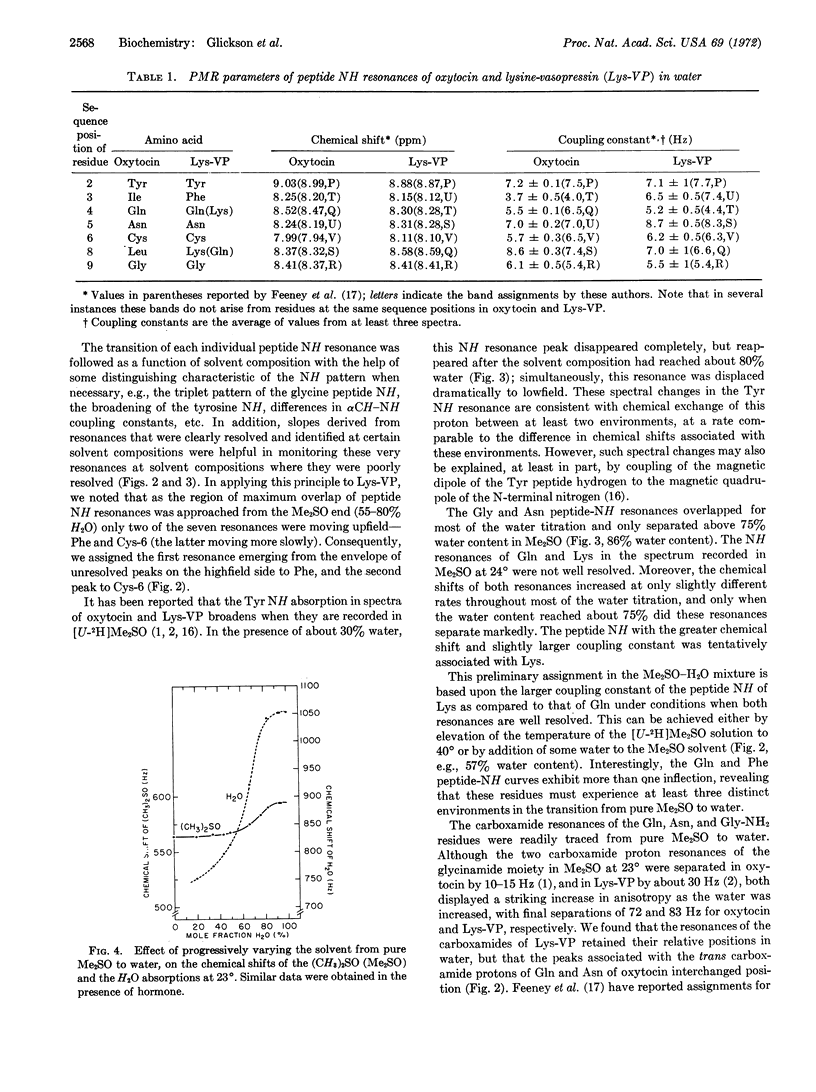
The peptide amide, primary carboxamide, and aromatic proton resonances were assigned to specific hydrogens of oxytocin and lysine vasopressin (Lys-VP) in water at 23° at pH 2.5 and 4.2, respectively. We started with the spectral assignments of oxytocin and Lys-VP determined in deuterated dimethylsulfoxide (Me2SO) and monitored the course of each of these resonances as the proportion of water to Me2SO was gradually increased. Changes in each of the two hormones in chemical shifts and in some coupling constants indicate that conformational alterations occur in both oxytocin and Lys-VP during the solvent transition from Me2SO to water. This study is a specific application of a general method for correlating spectral assignments in different solvents and for monitoring conformational changes accompanying solvent transitions. Application of this technique requires only that the solvent components be miscible over the entire transitional range, that spectral changes of the solute be simple enough to follow, and that the associated structural changes of the solute be “rapid on the proton magnetic resonance time-scale.”
Keywords: spectral assignments, neurohypophyseal hormones, solvent effects, amide proton resonances, three-dimensional structure
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAIG L. C., HARFENIST E. J., PALADINI A. C. DIALYSIS STUDIES. 7. THE BEHAVIOR OF ANGIOTENSIN, OXYTOCIN, VASOPRESSIN, AND SOME OF THEIR ANALOGS. Biochemistry. 1964 Jun;3:764–769. doi: 10.1021/bi00894a005. [DOI] [PubMed] [Google Scholar]
- Feeney J., Roberts G. C., Rockey J. H., Burgen A. S. Conformational studies of oxytocin and lysine vasopressin in aqueous solution using high resolution NMR spectroscopy. Nat New Biol. 1971 Jul 28;232(30):108–110. doi: 10.1038/newbio232108a0. [DOI] [PubMed] [Google Scholar]
- Glickson J. D., Mayers D. F., Settine J. M., Urry D. W. Spectroscopic studies on the conformation of gramicidin A'. Proton magnetic resonance assignments, coupling constants, and H-D exchange. Biochemistry. 1972 Feb 15;11(4):477–486. doi: 10.1021/bi00754a001. [DOI] [PubMed] [Google Scholar]
- Ivanov V. T., Miroshnikov A. I., Abdullaev N. D., Senyavina L. B., Arkhipova S. F., Uvarova N. N., Khalilulina K. K., Bystrov V. F., Ovchinnikov Y. A. Conformation of the Na+ complex of antamanide in solution. Biochem Biophys Res Commun. 1971 Feb 19;42(4):654–663. doi: 10.1016/0006-291x(71)90538-9. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Schwartz I. L., Walter R. Oxytocin and neurohypophyseal peptides: spectral assignment and conformational analysis by 220 MHz nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1269–1275. doi: 10.1073/pnas.64.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B. Automated synthesis of peptides. Science. 1965 Oct 8;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- Ohnishi M., Urry D. W. Temperature dependence of amide proton chemical shifts: the secondary structures of gramicidin S and valinomycin. Biochem Biophys Res Commun. 1969 Jul 23;36(2):194–202. doi: 10.1016/0006-291x(69)90314-3. [DOI] [PubMed] [Google Scholar]
- Prestegard J. H., Chan S. I. Proton magnetic resonance studies of the cation-binding properties of nonactin. II. Comparison of the sodium ion, potassium ion, and cesium ion complexes. J Am Chem Soc. 1970 Jul 15;92(14):4440–4446. doi: 10.1021/ja00717a049. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Chandrasekaran R., Kopple K. D. Variation of the NH-C alpha-H coupling constant with dihedral angle in the NMR spectra of peptides. Biopolymers. 1971 Nov;10(11):2113–2131. doi: 10.1002/bip.360101108. [DOI] [PubMed] [Google Scholar]
- Stern A., Gibbons W. A., Craig L. C. A conformational analysis of gramicidin S-A by nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1968 Oct;61(2):734–741. doi: 10.1073/pnas.61.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Ohnishi M., Walter R. Secondary structure of the cyclic moiety of the peptide hormone oxytocin and its deamino analog. Proc Natl Acad Sci U S A. 1970 May;66(1):111–116. doi: 10.1073/pnas.66.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Walter R. Proposed conformation of oxytocin in solution. Proc Natl Acad Sci U S A. 1971 May;68(5):956–958. doi: 10.1073/pnas.68.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T. A., Hruska F. E., Hikichi K., Danyluk S. S., Bell C. L. Nuclear magnetic resonance study of the structure and interactions of actinomycin D: temperature and solvent effects on the N--H and NH2 groups. Nature. 1969 Jul 19;223(5203):302–303. doi: 10.1038/223302a0. [DOI] [PubMed] [Google Scholar]
- Von Dreele P. H., Brewster A. I., Scheraga H. A., Ferger M. F., Du Vigneaud V. Nuclear magnetic resonance spectrum of lysine-vasopressin and its structural implications. Proc Natl Acad Sci U S A. 1971 May;68(5):1028–1031. doi: 10.1073/pnas.68.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R., Glickson J. D., Schwartz I. L., Havran R. T., Meienhofer J., Urry D. W. Conformation of lysine vasopressin: a comparison with oxytocin. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1920–1924. doi: 10.1073/pnas.69.7.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R., Rudinger J., Schwartz I. L. Chemistry and structure-activity relations of the antidiuretic hormones. Am J Med. 1967 May;42(5):653–677. doi: 10.1016/0002-9343(67)90087-3. [DOI] [PubMed] [Google Scholar]



