Abstract
As a result of donor shortage and high postoperative morbidity and mortality after liver transplantation, hepatectomy is the most widely applicable and reliable option for curative treatment of hepatocellular carcinoma (HCC). Because intrahepatic tumor recurrence is frequent after loco-regional therapy, repeated treatments are advocated provided background liver function is maintained. Among treatments including local ablation and transarterial chemoembolization, hepatectomy provides the best long-term outcomes, but studies comparing hepatectomy with other nonsurgical treatments require careful review for selection bias. In patients with initially unresectable HCC, transarterial chemo-or radio-embolization, and/or systemic chemotherapy can down-stage the tumor and conversion to resectable HCC is achieved in approximately 20% of patients. However, complete response is rare, and salvage hepatectomy is essential to help prolong patients’ survival. To counter the short recurrence-free survival, excellent overall survival is obtained by combining and repeating different treatments. It is important to recognize hepatectomy as a complement, rather than a contraindication, to other nonsurgical treatments in a multidisciplinary approach for patients with HCC, including recurrent or unresectable tumors.
Keywords: Hepatocellular carcinoma, Hepatectomy, Repeat hepatectomy, Conversion therapy, Multidisciplinary treatment
Core tip: Previous studies comparing hepatectomy with other nonsurgical treatments for hepatocellular carcinoma (HCC) evaluated which provided superior survival benefit. However, considering the high recurrence rate after curative loco-regional treatment, and limited indications for hepatectomy because of background liver damage, it is important to recognize hepatectomy as a complement to other nonsurgical treatment, rather than a contraindication. A multidisciplinary approach combining and repeating different treatments prolongs patients’ survival with HCC, including those with recurrent or initially unresectable tumors.
INTRODUCTION
Liver transplantation is the most promising strategy for radical treatment for hepatocellular carcinoma (HCC) because it eradicates both the tumors and the background damaged liver; hepatectomy is second. However, high perioperative morbidity and mortality, and a shortage of donors limit application of liver transplantation. Poon et al[1,2] reported that although the risk of postoperative tumor recurrence was low after transplantation, the long-term prognosis after transplantation was comparable to patients who underwent hepatectomy among patients with Child-Pugh class A background liver disease. Therefore, hepatectomy remains a reliable and widely applicable surgical treatment; however, the main limitation is that it is not indicated in patients with impaired liver function resulting from cirrhosis irrespective of the etiology of the liver disease. Multimodal therapy combining nonsurgical treatments including local ablation and transarterial chemoembolization (TACE) with hepatectomy and/or liver transplantation have been advocated for recurrent HCC, multinodular HCC, or initially unresectable HCC. This review was aimed to evaluate the role of hepatectomy among the various treatments for recurrent or advanced HCC.
Hepatectomy for recurrent HCC following local treatment
Because HCC usually develops in the injured liver, tumors frequently recur even after curative local treatment. The incidence of intrahepatic recurrence within 2 years after primary hepatic resection is 70%[3]. However, because recurrences occur most commonly in the remnant liver, comprising 85%-90% of initial recurrence sites[3], repeat hepatectomy or other local treatment is indicated. In general, treatments are selected based on the same criteria as the primary HCC. Several studies compared the results of repeat hepatectomy with nonsurgical treatment and showed that repeat hepatectomy was associated with a better prognosis[4-6]. However, these studies were retrospective analyses and may have included the selection bias that the repeat hepatectomy group usually included patients with better background liver function and less multinodular tumors. Repeat hepatic resection is indicated for only a limited proportion of patients (6%-53%) and the 5-year overall survival after second hepatectomy is reported as 22%-78%[4-19]. The repeat resection rate, 5-year recurrence-free survival rate, and overall survival rate after second hepatectomy in these studies are summarized in Table 1. The difference in the survival rate would probably have been influenced by the difference in the background liver damage, types of recurrence, and tumoral factors such as size, number, and vascular invasions, but precise assessment was difficult due to the insufficient data. A small number of studies reported the outcomes after a third or fourth hepatectomy[15,19]. In the two series evaluating the outcomes of 1117 and 791 patients who underwent primary hepatectomy for HCC, a second, third, and fourth hepatectomy was performed in 23% (149/641) and 53% (163/308), 37% (35/96), and 65% (36/55), and 27% (8/30) and 69% (9/13) of the patients with recurrence, respectively. Five-year overall survival after a second and third hepatectomy was 56% and 59% in Wu et al’s[15] series and 60% and 43% in Yamashita et al’s[19] series, respectively. Factors related to both primary and recurrent tumors such as tumor size, number, and vascular invasion and also the degree of background liver damage as assessed by Child-Pugh class, indocyanine green retention rate, or platelet counts were reported as prognostic predictors. Recurrence-free interval and/or type of recurrence, multicentric occurrence or intrahepatic metastases[17,20], were also commonly reported to be prognostic predictors in several studies. Intrahepatic metastases usually occur via the portal vein, and are therefore associated with portal vein invasion. Distinction of them is important because intrahepatic recurrence is associated with malignant behavior compared to multicentric occurrence. Differentiation is possible by histopathological examination as defined by the Liver Cancer Study Group of Japan (Table 2)[21] but there is no established method to differentiate intrahepatic metastases vs multicentric occurrence preoperatively, an issue requiring further research.
Table 1.
Repeat resection rate, 5-year recurrence-free survival rate, and overall survival rate after repeat hepatectomy in previous studies
| Ref. | Year | Number of primary hepatectomy | Number of second hepatectomy/HCC recurrence after primary hepatectomy | 5-yr recurrence free survival after repeat hepatectomy | 5-yr overall survival after repeat hepatectomy |
| Poon et al[4] | 1999 | 244 | 11/105 (10%) | NA | 69% |
| Nakajima et al[7] | 2001 | 94 | 12/57 (21%) | Not reached | 52% |
| Sugimachi et al[8] | 2001 | 474 | 78/300 (26%) | NA | 47.50% |
| Minagawa et al[9] | 2003 | 334 | 56/183 (31%) | 17% | 56% |
| Chen et al[5] | 2004 | 627 | 34/286 (12%) | NA | 56.80% |
| Taura et al[10] | 2006 | 610 | 55/465 (12%) | NA | NA |
| Itamoto et al[11] | 2007 | 483 | 70/279 (25%) | 10% | 50% |
| Shimada et al[12] | 2007 | 319 | 13/211 (6%) | NA | 25% |
| Tralhão et al[6] | 2007 | 190 | 16/97 (19%) | NA | 31% |
| Liang et al[13] | 2008 | NA | 73/853 (9%) | 10.50% | 27.60% |
| Choi et al[14] | 2008 | 353 | 9/97 (9%) | NA | 78% |
| Wu et al[15] | 2009 | 1177 | 149/641(23%) | 31.80% | 56.40% |
| Kishi et al[16] | 2011 | 221 | 8/134 (6%) | NA | 37.50% |
| Huang et al[17] | 2012 | NA | 82/NA | 8.20% | 22.40% |
| Tsujita et al[18] | 2012 | NA | 112/NA | NA | 67.30% |
| Yamashita et al[19] | 2013 | 791 | 163/308 (53%) | 29% | 60% |
HCC: Hepatocellular carcinoma; NA: Not assessed.
Table 2.
Three types of definition of intrahepatic metastases by the Liver Cancer Study Group of Japan[21]
| Definition | |
| 1 | Tumors clearly growing from portal vein tumor thrombi |
| 2 | Tumors surrounding a large main tumor with multiple satellite nodules |
| 3 | A small solitary tumor that is near the main tumor and histologically similar to or less differentiated than the main tumor |
It is important that hepatectomy and other local treatments be considered complementary and not exclusive. The dissociation between low recurrence-free survival and rather high overall survival shown in Table 1 reflects the slow progression of the disease and the importance of repeating treatment, usually TACE. Repeating locoregional treatment such as ethanol injection (PEI), radiofrequency ablation (RFA), or TACE, for intrahepatic recurrence prolongs patient survival[10,22-25], and provides a comparable prognosis after RFA compared with repeat hepatectomy[7,12,13,24]. Taura et al[10] compared the long-term outcomes of 610 patients with HCC who underwent hepatectomy before 1990 and after 1991. There was no change in the disease-free survival (early vs late period, 28% vs 26%, respectively, at 5 years), but survival after tumor recurrence increased significantly in the later period (12% vs 22% at 5 years) and overall survival also improved (39% vs 58% at 5 years). The authors concluded that increased application of RFA to solitary intrahepatic recurrence, which was the most common type of recurrence, contributed to the improved prognosis[10]. Kishi et al[16] reported that the number rather than the type of treatment for tumor recurrence was associated with prolonged survival.
As was referred in the beginning of the introduction, liver transplantation is the most promising, and salvage liver transplantation for recurrent HCC, which have been reported with 5-year survival rate of 54%-61% could be a choice of treatment because these figures were comparable with that after primary liver transplantation for HCC that was 59%-72%[26-29]. However, shortage of donor organ, expensive medical costs, and contraindication for elderly patients preclude popularization of this strategy. Indication for salvage transplantation have not been established, but various factors including recurrence free survival, microvascular involvement, satellite nodules, as well as tumor number and size at the time of primary hepatectomy and/or transplantation should be considered. Further, intention-to-treat analyses comparing patients who underwent hepatectomy with liver cirrhosis of potentially eligible for transplantation and patients listed for primary liver transplantation showed comparable overall (5-year survival; hepatectomy vs listed for transplantation; 66% vs 58%; P = NS) and disease-free (41% vs 54%: P = NS) survival mainly due to the influence of waiting period[30]. Another intention-to treat analysis also showed the limited value of salvage transplantation with only 28% of transplantability rate and comparable prognosis with the patients with liver resection[31].
Salvage hepatectomy for refractory HCC after other local treatment
Here, the term “refractory HCC” is defined as HCC recognized as remnant, unresponsive, or locally recurred tumor at the site treated with locoregional treatment such as ablation or TACE. The indications for hepatectomy are dictated by the degree of background liver damage, while the indications for RFA are limited less by the degree of liver damage and more by tumor size and location, especially with respect to major vascular structures. We occasionally experience difficult complete resection after local recurrence or remnant HCC after RFA because of unclear tumor borders (Figure 1). Several studies have shown that locally recurrent HCCs after RFA were more invasive because of lower tumor differentiation grade, capsule invasion, and vascular invasion, resulting in the need for extensive liver resection with increased operation time and blood loss[32-36]. In such cases, repeat RFA is rarely indicated and salvage hepatectomy should be the first-choice treatment. The mechanism of aggressive tumor behavior is not clear. Increased intratumoral pressure by RFA may favor intravascular tumor spread[37,38]. Difficulty in early diagnosis of recurrence because of blended necrotic and active areas without a clear delineation may also be a factor[34]. Although recurrence after salvage hepatectomy for these recurrent tumors is frequent, with 5-year recurrence-free survival of 0%-33%, 5-year overall survival is reported as 43%-67%[34-36]. Whether surgical resection or RFA should be selected for HCC that are amenable to both treatments is a controversial issue[39,40], and which is better is still in debate. In a randomized controlled trial by Huang et al[41] comparing surgical resection and RFA in patients with HCC meeting the Milan criteria[42], 115 patients were enrolled in each group and both recurrence-free and overall survival was better in the resection group (resection vs RFA: 5-year recurrence-free survival, 51.3% vs 28.7%, P = 0.017; 5-year overall survival, 75.7% vs 54.8%, P = 0.001)[41]. Hasegawa et al[43] reported the results of a Japanese nationwide survey comparing the results of surgical resection, RFA, and PEI in patients with no more than three HCC tumors and with none over 3 cm. A total of 12968 patients with 5361, 5548, and 2059 patients undergoing surgical resection, RFA, and PEI, respectively, were analyzed, and the 5-year recurrence was 63.8%, 71.7%, and 76.9%, respectively (surgical resection vs RFA, P = 0.0001; RFA vs PEI, P = 0.0001) and the 5-year overall survival was 71.1%, 61.1%, and 56.3%, respectively (surgical resection vs RFA, P = 0.0001; RFA vs PEI, P = 0.005). Although these were the outcomes for the treatment of primary HCC and there have been no established evidence suggesting which of the hepatectomy or ablation is better first choice for recurrent HCC, these results suggest that surgical resection should be selected as a first-line treatment for HCC that is amenable to either surgical resection or ablation, and curative resection should be attempted for local recurrence after ablation for as long as possible.
Figure 1.
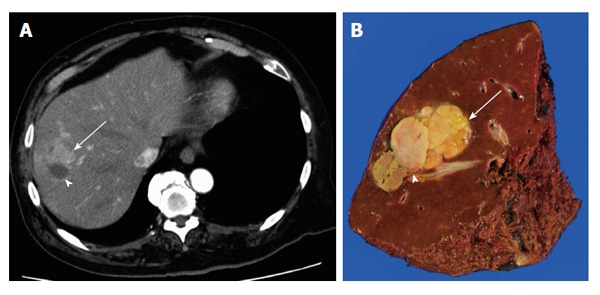
Recurrent hepatocellular carcinoma adjacent to a radiofrequency ablation scar. A: Computed tomography showing the tumor with unclear borders with arterial enhancement (arrow) adjacent to the scar (arrowhead); B: Cut surface of the resected specimen showing the recurrent tumor (arrow) and radiofrequency ablation scar (arrowhead).
In the treatment of multinodular HCC, surgical resection can be complementary with other nonsurgical therapies to obtain good long-term prognosis even though TACE is usually indicated for multinodular HCC, rather than surgical resection. The guidelines for HCC treatment from the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver[44,45], based on the Barcelona Clinic Liver Cancer criteria[46] recommend hepatic resection only for patients with solitary tumor without portal hypertension. In the Japanese guidelines, surgical resection is indicated for patients with up to three tumors. For four or more tumors, TACE or transarterial infusion is indicated as the first-choice treatment[47]. We occasionally experience multinodular HCCs treated with repeated TACE showing complete necrosis of a large proportion of the tumors with a small number of remnant viable tumors (Figure 2). It is still unclear whether salvage hepatic resection of the remaining viable tumors is beneficial. A small number of studies have shown benefits with a multimodal approach by combining hepatic resection with simultaneous ablation[48] or reduction surgery followed by ablation and adjuvant TACE or arterial infusion therapy[49]. However, these were retrospective studies with a small number of patients and the details of the exact number of tumors were not provided. Furthermore, differentiation between intrahepatic metastasis and multicentric occurrence is important, as discussed earlier, and criteria as to the number of nodules indicated for hepatectomy remains unclear.
Figure 2.
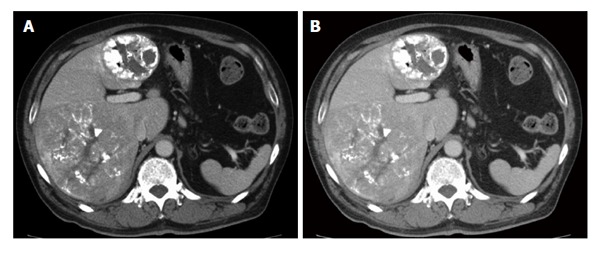
Multinodular hepatocellular carcinomas. Transarterial chemoembolization achieved complete response in one tumor in segment IV with accumulation of lipiodol showing no arterial enhancement in contrast to the other tumor in segments VI and VII that was enhanced in the arterial phase (A) and washed out in the portal phase (B).
Hepatectomy for down-staged HCC for initially unresectable tumors
In contrast to colorectal liver metastases, in which systemic chemotherapy and/or hepatic artery infusion chemotherapy can convert the unresectable tumor to resectable in > 40% of patients[50-52], HCC conversion therapy has not been established.
Yao et al[53] proposed the University of California, San Francisco down-staging protocol inclusion criteria for liver transplantation as: (1) one lesion > 5 cm and up to 8 cm; (2) two to three lesions with at least one lesion > 3 cm and not exceeding 5 cm, with a total tumor diameter up to 8 cm; or (3) four to five lesions with none > 3 cm, with a total tumor diameter up to 8 cm. The authors reported that down-staging was successful in 43/61 patients (71%) and 35 patients underwent liver transplantation with a 4-year survival after transplantation of 92%[53]. Lei et al[54] applied the criteria to hepatectomy and reported the outcomes of 66 of 102 patients (59%) with successful down-staging by TACE and/or RFA. Of the 66 patients, 31 and 35 patients underwent liver transplantation and hepatectomy, respectively, and both recurrence-free (68% and 60% at 5 years, respectively) and overall survival (77% and 69% at 5 years, respectively) were comparable[54]. TACE and/or hepatic artery infusion therapy is usually used as the down-staging treatment. The conversion rate from unresectable to resectable HCC by these modalities was reported as 13%-18%, with a 5-year survival of 49%-56%[55,56].
In contrast to colorectal liver metastases, in which pathologic response is correlated with the prognosis after curative hepatectomy[57], such correlation was not necessarily confirmed in patients with HCC. Of note, Ravaioli et al[58] reported that incomplete necrosis by TACE was an independent predictor of poor recurrence-free survival after liver transplantation. Furthermore, several studies showed that preoperative TACE was associated with an increased risk of extrahepatic metastases[59-61]. This might be explained by Adachi et al’s[62] hypothesis that viable HCC cells are less firmly attached and likely to spill into the bloodstream during intraoperative manipulation after incomplete response to TACE. Because complete necrosis is rarely obtained, especially for large tumors, the routine application of preoperative TACE for resectable HCC is not recommended. However, based on results showing that a proportion of patients can undergo curative resection following down-staging by TACE and obtain long-term survival, aggressive loco-regional treatment to attempt curative resection should be adopted in patients with initially unresectable HCCs.
The development of other treatment strategies for unresectable HCC such as radioembolization by yttrium-90[63] or systemic treatment combining cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF)[64] may increase the rate of conversion. Lau et al[65] reported that 49 of 285 patients (17%) underwent salvage surgery following down-staging by intra-arterial yttrium-90 microspheres or PIAF for initially unresectable HCC and obtained a 5-year survival rate of 57%. Notably, 8 of the 49 patients had extrahepatic metastases initially and these patients also obtained long-term survival with a 5-year survival rate > 40% and neither the extension of the disease nor the degree of tumor pathologic response was associated with the prognosis. Although relatively high response rates are obtained with PIAF, frequent adverse events such as neutropenia and thrombocytopenia preclude wide application, especially in patients with cirrhosis[64,66]. In a recent study by Kaseb et al[67], an independent predictor of an objective response to PIAF was the use of five or more cycles. The authors suggested that patient selection is important because only responding patients will have an improved prognosis with curative hepatectomy.
To discuss the issue of conversion, it should be noted that the definition of “unresectable” cannot be unanimous and differ according to extension of the tumor, background liver function, and surgeons’ judgments. It is also important to recognize that “technically” and “oncologically optimally” resectable are not necessarily the same. It is, however, certain that conversion rate for HCC is still unsatisfactory and the all reports referred above are retrospective studies with small number of patients. Further development of effective treatment for downstaging is expected.
CONCLUSION
Although hepatectomy is indicated for only a small proportion of patients with recurrent or down-staged HCC after primary treatment, an excellent prognosis is obtained if curative resection is achieved, especially for tumors with a multicentric occurrence pattern, rather than intrahepatic metastases. Preoperative differentiation of the two patterns is a future research issue. Even in initially unresectable HCCs, hepatectomy plays a key role in a multidisciplinary approach.
Footnotes
Supported by Grant-in-aid from the 106th Annual Congress of the JSS Memorial Surgical Research Fund, Tokyo, Japan
P- Reviewer: Kang KJ, Takeda S S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
References
- 1.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245:51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arii S, Teramoto K, Kawamura T, Okamoto H, Kaido T, Mori A, Imamura M. Characteristics of recurrent hepatocellular carcinoma in Japan and our surgical experience. J Hepatobiliary Pancreat Surg. 2001;8:397–403. doi: 10.1007/s005340100000. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen WT, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Wu CW. Recurrent hepatocellular carcinoma after hepatic resection: prognostic factors and long-term outcome. Eur J Surg Oncol. 2004;30:414–420. doi: 10.1016/j.ejso.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Tralhão JG, Dagher I, Lino T, Roudié J, Franco D. Treatment of tumour recurrence after resection of hepatocellular carcinoma. Analysis of 97 consecutive patients. Eur J Surg Oncol. 2007;33:746–751. doi: 10.1016/j.ejso.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima Y, Ko S, Kanamura T, Nagao M, Kanehiro H, Hisanaga M, Aomatsu Y, Ikeda N, Nakano H. Repeat liver resection for hepatocellular carcinoma. J Am Coll Surg. 2001;192:339–344. doi: 10.1016/s1072-7515(00)00789-4. [DOI] [PubMed] [Google Scholar]
- 8.Sugimachi K, Maehara S, Tanaka S, Shimada M, Sugimachi K. Repeat hepatectomy is the most useful treatment for recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2001;8:410–416. doi: 10.1007/s005340100002. [DOI] [PubMed] [Google Scholar]
- 9.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–710. doi: 10.1097/01.sla.0000094549.11754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg. 2006;244:265–273. doi: 10.1097/01.sla.0000217921.28563.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itamoto T, Nakahara H, Amano H, Kohashi T, Ohdan H, Tashiro H, Asahara T. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2007;141:589–597. doi: 10.1016/j.surg.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Sakamoto Y, Esaki M, Kosuge T, Morizane C, Ikeda M, Ueno H, Okusaka T, Arai Y, Takayasu K. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2007;14:2337–2347. doi: 10.1245/s10434-007-9415-7. [DOI] [PubMed] [Google Scholar]
- 13.Liang HH, Chen MS, Peng ZW, Zhang YJ, Zhang YQ, Li JQ, Lau WY. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15:3484–3493. doi: 10.1245/s10434-008-0076-y. [DOI] [PubMed] [Google Scholar]
- 14.Choi GH, Kim DH, Kang CM, Kim KS, Choi JS, Lee WJ, Kim BR. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:618–629. doi: 10.1245/s10434-007-9671-6. [DOI] [PubMed] [Google Scholar]
- 15.Wu CC, Cheng SB, Yeh DC, Wang J, P’eng FK. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg. 2009;96:1049–1057. doi: 10.1002/bjs.6690. [DOI] [PubMed] [Google Scholar]
- 16.Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, Yoshioka R, Kokudo N, Yamaguchi T. Repeat treatment for recurrent hepatocellular carcinoma: is it validated? Langenbecks Arch Surg. 2011;396:1093–1100. doi: 10.1007/s00423-011-0837-0. [DOI] [PubMed] [Google Scholar]
- 17.Huang ZY, Liang BY, Xiong M, Zhan DQ, Wei S, Wang GP, Chen YF, Chen XP. Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-center experience in China. Ann Surg Oncol. 2012;19:2515–2525. doi: 10.1245/s10434-012-2269-7. [DOI] [PubMed] [Google Scholar]
- 18.Tsujita E, Yamashita Y, Takeishi K, Matsuyama A, Tsutsui S, Matsuda H, Toshima T, Taketomi A, Shirabe K, Ishida T, et al. Poor prognostic factors after repeat hepatectomy for recurrent hepatocellular carcinoma in the modern era. Am Surg. 2012;78:419–425. [PubMed] [Google Scholar]
- 19.Yamashita Y, Shirabe K, Tsuijita E, Takeishi K, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Utsunomiya T, Maehara Y. Third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2013;154:1038–1045. doi: 10.1016/j.surg.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, Fujii H, Kono H, Matsumoto Y. Surgical treatment of recurrent hepatocellular carcinoma based on the mode of recurrence: repeat hepatic resection or ablation are good choices for patients with recurrent multicentric cancer. J Hepatobiliary Pancreat Surg. 2001;8:353–359. doi: 10.1007/s005340170008. [DOI] [PubMed] [Google Scholar]
- 21.Japan LCSGo. General rules for the clinical and pathological study of primary liver cancer. 2nd ed. Tokyo: Kenehara; 2003. [Google Scholar]
- 22.Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Chen MH, Yin SS, Yan K, Gao W, Wang YB, Huo L, Zhang XP, Xing BC. Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early- and late-phase recurrence. AJR Am J Roentgenol. 2006;186:S275–S283. doi: 10.2214/AJR.04.1573. [DOI] [PubMed] [Google Scholar]
- 24.Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, Joh JW, Park CK. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319–2329. doi: 10.1245/s10434-006-9220-8. [DOI] [PubMed] [Google Scholar]
- 25.Okuwaki Y, Nakazawa T, Kokubu S, Hidaka H, Tanaka Y, Takada J, Watanabe M, Shibuya A, Minamino T, Saigenji K. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am J Gastroenterol. 2009;104:2747–2753. doi: 10.1038/ajg.2009.414. [DOI] [PubMed] [Google Scholar]
- 26.Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885–892; discussion 892-893. doi: 10.1097/01.sla.0000098621.74851.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang S, Lee SG, Moon DB, Ahn CS, Kim KH, Lee YJ, Ha TY, Song GW. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transpl. 2007;13:741–746. doi: 10.1002/lt.21157. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Wei Y, Wang W, Chen K, Yan L, Wen T, Zhao J, Xu M, Li B. Salvage liver transplantation for recurrent hepatocellular carcinoma within UCSF criteria after liver resection. PLoS One. 2012;7:e48932. doi: 10.1371/journal.pone.0048932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Hyuck David Kwon C, Man Kim J, Joh JW, Woon Paik S, Kim BW, Wang HJ, Lee KW, Suh KS, Lee SK. Time of hepatocellular carcinoma recurrence after liver resection and alpha-fetoprotein are important prognostic factors for salvage liver transplantation. Liver Transpl. 2014;20:1057–1063. doi: 10.1002/lt.23919. [DOI] [PubMed] [Google Scholar]
- 30.Del Gaudio M, Ercolani G, Ravaioli M, Cescon M, Lauro A, Vivarelli M, Zanello M, Cucchetti A, Vetrone G, Tuci F, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. 2008;8:1177–1185. doi: 10.1111/j.1600-6143.2008.02229.x. [DOI] [PubMed] [Google Scholar]
- 31.Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. 2012;55:132–140. doi: 10.1002/hep.24680. [DOI] [PubMed] [Google Scholar]
- 32.Torzilli G, Del Fabbro D, Palmisano A, Marconi M, Makuuchi M, Montorsi M. Salvage hepatic resection after incomplete interstitial therapy for primary and secondary liver tumours. Br J Surg. 2007;94:208–213. doi: 10.1002/bjs.5603. [DOI] [PubMed] [Google Scholar]
- 33.Masuda T, Beppu T, Ishiko T, Horino K, Baba Y, Mizumoto T, Hayashi H, Okabe H, Horlad H, Doi K, et al. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg. 2008;15:589–595. doi: 10.1007/s00534-007-1288-4. [DOI] [PubMed] [Google Scholar]
- 34.Portolani N, Baiocchi GL, Coniglio A, Grazioli L, Frassi E, Gheza F, Giulini SM. Sequential multidisciplinary treatment of hepatocellular carcinoma: the role of surgery as rescue therapy for failure of percutaneous ablation therapies. J Surg Oncol. 2009;100:580–584. doi: 10.1002/jso.21375. [DOI] [PubMed] [Google Scholar]
- 35.Sugo H, Ishizaki Y, Yoshimoto J, Imamura H, Kawasaki S. Salvage hepatectomy for local recurrent hepatocellular carcinoma after ablation therapy. Ann Surg Oncol. 2012;19:2238–2245. doi: 10.1245/s10434-012-2220-y. [DOI] [PubMed] [Google Scholar]
- 36.Imai K, Beppu T, Chikamoto A, Mima K, Okabe H, Hayashi H, Nitta H, Ishiko T, Baba H. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol Res. 2014:Epub ahead of print. doi: 10.1111/hepr.12313. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Yamanaka N, Oriyama T, Furukawa K, Okamoto E. Factors regulating tumor pressure in hepatocellular carcinoma and implications for tumor spread. Hepatology. 1997;26:283–287. doi: 10.1002/hep.510260205. [DOI] [PubMed] [Google Scholar]
- 38.Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137–1140. doi: 10.3748/wjg.v10.i8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?: surgeon’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:422–424. doi: 10.1007/s00534-009-0239-7. [DOI] [PubMed] [Google Scholar]
- 40.Kudo M, Chung H. Single HCC between 2 and 5 cm: the grey zone: hepatologist’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:434–437. doi: 10.1007/s00534-009-0242-z. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 42.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724–729. doi: 10.1016/j.jhep.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 45.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 46.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 47.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 48.Itoh S, Morita K, Ueda S, Sugimachi K, Yamashita Y, Gion T, Fukuzawa K, Wakasugi K, Taketomi A, Maehara Y. Long-term results of hepatic resection combined with intraoperative local ablation therapy for patients with multinodular hepatocellular carcinomas. Ann Surg Oncol. 2009;16:3299–3307. doi: 10.1245/s10434-009-0721-0. [DOI] [PubMed] [Google Scholar]
- 49.Wakabayashi H, Ushiyama T, Ishimura K, Izuishi K, Karasawa Y, Masaki T, Watanabe S, Kuriyama S, Maeta H. Significance of reduction surgery in multidisciplinary treatment of advanced hepatocellular carcinoma with multiple intrahepatic lesions. J Surg Oncol. 2003;82:98–103. doi: 10.1002/jso.10203. [DOI] [PubMed] [Google Scholar]
- 50.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 51.Beppu T, Miyamoto Y, Sakamoto Y, Imai K, Nitta H, Hayashi H, Chikamoto A, Watanabe M, Ishiko T, Baba H. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol. 2014;21 Suppl 3:S405–S413. doi: 10.1245/s10434-014-3577-x. [DOI] [PubMed] [Google Scholar]
- 52.D’angelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF, Capanu M, Kingham TP, Fong Y, Dematteo RP, et al. Phase II Trial of Hepatic Artery Infusional and Systemic Chemotherapy for Patients With Unresectable Hepatic Metastases From Colorectal Cancer: Conversion to Resection and Long-term Outcomes. Ann Surg. 2014:Epub ahead of print. doi: 10.1097/SLA.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei JY, Yan LN, Wang WT. Transplantation vs resection for hepatocellular carcinoma with compensated liver function after downstaging therapy. World J Gastroenterol. 2013;19:4400–4408. doi: 10.3748/wjg.v19.i27.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, Zhou J, Qiu SJ, Lu JZ. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674–678. doi: 10.1159/000018676. [DOI] [PubMed] [Google Scholar]
- 56.Tang ZY, Zhou XD, Ma ZC, Wu ZQ, Fan J, Qin LX, Yu Y. Downstaging followed by resection plays a role in improving prognosis of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:495–498. [PubMed] [Google Scholar]
- 57.Blazer DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 58.Ravaioli M, Grazi GL, Ercolani G, Fiorentino M, Cescon M, Golfieri R, Trevisani F, Grigioni WF, Bolondi L, Pinna AD. Partial necrosis on hepatocellular carcinoma nodules facilitates tumor recurrence after liver transplantation. Transplantation. 2004;78:1780–1786. doi: 10.1097/01.tp.0000145892.97114.ee. [DOI] [PubMed] [Google Scholar]
- 59.Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–126. doi: 10.1002/bjs.1800820141. [DOI] [PubMed] [Google Scholar]
- 60.Chen XP, Hu DY, Zhang ZW, Zhang BX, Chen YF, Zhang WG, Qiu FZ. Role of mesohepatectomy with or without transcatheter arterial chemoembolization for large centrally located hepatocellular carcinoma. Dig Surg. 2007;24:208–213. doi: 10.1159/000102901. [DOI] [PubMed] [Google Scholar]
- 61.Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, Yoshioka R, Kokudo N, Yamaguchi T. Preoperative transarterial chemoembolization for hepatocellular carcinoma. Hepatogastroenterology. 2012;59:2295–2299. doi: 10.5754/hge10730. [DOI] [PubMed] [Google Scholar]
- 62.Adachi E, Matsumata T, Nishizaki T, Hashimoto H, Tsuneyoshi M, Sugimachi K. Effects of preoperative transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma. The relationship between postoperative course and tumor necrosis. Cancer. 1993;72:3593–3598. doi: 10.1002/1097-0142(19931215)72:12<3593::aid-cncr2820721208>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 63.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 64.Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, Mok TS, Yeo W, Liew CT, Leung NW, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–1681. [PubMed] [Google Scholar]
- 65.Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299–305. doi: 10.1097/01.sla.0000133123.11932.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 67.Kaseb AO, Shindoh J, Patt YZ, Roses RE, Zimmitti G, Lozano RD, Hassan MM, Hassabo HM, Curley SA, Aloia TA, et al. Modified cisplatin/interferon α-2b/doxorubicin/5-fluorouracil (PIAF) chemotherapy in patients with no hepatitis or cirrhosis is associated with improved response rate, resectability, and survival of initially unresectable hepatocellular carcinoma. Cancer. 2013;119:3334–3342. doi: 10.1002/cncr.28209. [DOI] [PMC free article] [PubMed] [Google Scholar]


