Abstract
Hepatitis C virus (HCV) is a major cause of viral hepatitis and currently infects approximately 170 million people worldwide. An infection by HCV causes high rates of chronic hepatitis (> 75%) and progresses to liver cirrhosis and hepatocellular carcinoma ultimately. HCV can be eliminated by a combination of pegylated α-interferon and the broad-spectrum antiviral drug ribavirin; however, this treatment is still associated with poor efficacy and tolerability and is often accompanied by serious side-effects. While some novel direct-acting antivirals against HCV have been developed recently, high medical costs limit the access to the therapy in cost-sensitive countries. To search for new natural anti-HCV agents, we screened local agricultural products for their suppressive activities against HCV replication using the HCV replicon cell system in vitro. We found a potent inhibitor of HCV RNA expression in the extracts of blueberry leaves and then identified oligomeric proanthocyanidin as the active ingredient. Further investigations into the action mechanism of oligomeric proanthocyanidin suggested that it is an inhibitor of heterogeneous nuclear ribonucleoproteins (hnRNPs) such as hnRNP A2/B1. In this review, we presented an overview of functional foods and ingredients efficient for HCV infection, the chemical structural characteristics of oligomeric proanthocyanidin, and its action mechanism.
Keywords: Hepatitis C virus, Blueberry leaves, Functional foods, Oligomeric proanthocyanidin, Heterogeneous nuclear ribonucleoproteins
Core tip: An infection by hepatitis C virus (HCV) causes chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. While the combination of pegylated α-interferon and ribavirin is used for the elimination of HCV, a new anti-HCV drug is required due to the poor efficacy and serious side-effects associated with this combination therapy. We searched for new anti-HCV agents from natural products and then identified oligomeric proanthocyanidin from blueberry leaves. Further investigations suggested that several heterogeneous nuclear ribonucleoproteins may be the candidate proteins involved in the proanthocyanidin-mediated inhibition of HCV subgenomic expression. Oligomeric proanthocyanidin isolated from blueberry leaves may have potential usefulness as an anti-HCV compound.
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of viral hepatitis and currently infects approximately 170 million people worldwide[1,2]. An infection by HCV causes high rates of chronic hepatitis (> 75%) and progresses to liver cirrhosis and hepatocellular carcinoma ultimately[3]. A total of 27% and 25% of individuals that develop liver cirrhosis and hepatocellular carcinoma worldwide, respectively, arise in HCV-infected people[4]. The World Health Organization reported that between 350000 and 500000 people die from HCV-related diseases each year. However, there is no effective vaccine against HCV infection at present.
Currently, the combination of pegylated α-interferon and a broad spectrum antiviral drug, ribavirin, is used as the standard therapy for chronic HCV infection[2,5,6]. However, its option is unfortunately limited by efficacy, tolerability, and significant side-effects. Therefore, it had been required to establish a new therapeutic modality without serious adverse effects. Recently, direct-acting antivirals (DAAs) that inhibit HCV-specific proteins have be clinically investigated[7,8]. For example, boceprevir and telaprevir are new DAAs that were first approved by the United States Food and Drug Administration (FDA) in 2011[9]. DAAs are expected to provide new promising treatment options in hepatitis C patients; however, at present, they face difficulties to disseminate worldwide due to high costs. Therefore, new anti-HCV agents that are safe, economical, and complementary with present therapies, are still required.
Since the development of HCV-related liver cirrhosis and hepatocellular carcinoma requires a prolonged period (20-30 years), the progression of this disease may be influenced by a diet including dairy products. Interest in functional foods and their ingredients as natural resources for cancer prevention and treatment is increasing[10,11]. Eating habits, foods, nutrients contained in them, and other food constituents play important roles on the development of several types of cancer and 35% of cancer deaths are estimated to be possibly related to dietary factors[12]. Polyphenols derived from various fruits and vegetables have recently been suggested to be effective in the prevention of cancer. The South Kyushu region of Japan, including the prefecture of Miyazaki, has been recognized as a high prevalence area of HCV and it emerges as a social issue. Therefore, attempts were made to identify functional food ingredients having suppressive activities against HCV replication as an industry-academia-government collaboration study[13]. By screening of 1700 samples from 283 agricultural products in Miyazaki prefecture, we found that oligomeric proanthocyanidin, a polyphenolic ingredient abundantly contained in the leaves of the blueberry plant, suppressed the expression of HCV subgenomic RNA in an HCV replicon cell system[13].
In this review, we presented an overview of functional foods and ingredients efficient for HCV infection, the chemical structural characteristics of oligomeric proanthocyanidin, and its action mechanism.
HCV LIFE CYCLE AND ANALYTICAL TOOL
HCV belongs to Hepacivirus genus of the Flaviviridae family and has a positive-sense single stranded RNA of 9.6 kb wrapped with enveloped membrane[14]. After their adsorption on the surface of host cells, HCV particles are internalized into endocytic compartments and viral genomic RNA is then released into the cytoplasm by fusion of the viral envelope and cellular membrane. Genomic RNA serves as mRNA for viral proteins and is translated into a single polyprotein (3011 amino acids), resulting in 4 structural proteins (Core, E1, E2, and p7) and 6 non-structural (NS) proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) by post-translational processing (Figure 1A). It also serves as a template for viral genome replication. Non-translated regions (NTRs), 5’NTR and 3’NTR, are connected with the HCV polyprotein-coding region, and modulate viral protein synthesis and genome replication. The assembly of these viral components occurs on the endoplasmic reticulum (ER) membrane. Viral proteins and genomic RNA assemble on the cytoplasmic side of the membrane and then progeny virions bud into the ER lumen, followed by their release to the extracellular space. In the life cycle of HCV, each viral protein functions as described below[14]. Core is a highly basic protein that encapsidates HCV genomic RNA. E1 and E2 are glycoproteins integrated into the viral envelope. p7 functions as an ion channel and an antiviral drug, amantadine, is the p7 ion channel blocker[15]. Importantly, several steps of HCV infectious process are coordinated by NS proteins. NS2 and NS3 are a cysteine protease and serine protease, respectively, that play roles in the post-translational processing of viral proteins. NS3 serine protease activity requires NS4A as a cofactor. NS4B and NS5A have been suggested to serve in viral assembly on the ER membrane and NS5B is an RNA-dependent RNA polymerase. Many studies to date have reported that these viral proteins are associated not only with viral replication, but also pathogenicity via interactions with various host proteins. The identification of host proteins associated with the HCV life cycle is very important for anti-HCV drugs, and the HCV replicon cell system has contributed significantly to the development of these drugs[16,17]. This system consists of the human hepatocellular carcinoma line Huh-7 in which the transfected luciferase gene connected with HCV subgenomic RNA including the downstream coding regions of NS3 and the expression of HCV subgenomic RNA can be quantified by luciferase activity (Figure 1B). It provides a useful tool for HCV drug development and the elucidation of mechanisms underlying HCV genome replication[17]. We have used this HCV replicon system to screen functional foods with anti-HCV activity.
Figure 1.

Structure of the hepatitis C virus genome and cell system for anti-hepatitis C virus drug discovery. A: HCV genomic RNA and viral proteins. HCV genomic RNA encodes a single polyprotein of 3011 amino acids. After being translated, the polyprotein is processed into 4 structural proteins (Core, E1, E2, and p7) and 6 non-structural (NS) proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B). The polyprotein-coding region is flanked by 5’ and 3’NTRs. Viral RNA also serves as a template for viral genome replication and both NTRs modulate viral protein synthesis and genome replication; B: The HCV replicon cell system. Huh-7 cells were transfected with the luciferase gene connected with HCV subgenomic RNA including the downstream coding regions of NS3. The expression of HCV subgenomic RNA could be quantified by luciferase activity. HCV: Hepatitis C virus; NTRs: Non-translated regions.
THERAPEUTIC OPTIONS FOR CHRONIC HCV INFECTION
Currently, the combination of pegylated α-interferon and a broad spectrum antiviral drug, ribavirin, is used as the standard therapy for chronic HCV infection[2,5,6]. However, the HCV genotype is an important determinant of its efficacy and tolerability. Whereas the virological response to this combination therapy is more than 70% for genotypes 2 and 3, it is less than 50% for genotype 1[18-20]. Furthermore, this therapy causes significant side-effects such as thrombocytopenia, flu-like symptoms, fever, rash, anorexia, and thyroid dysfunction. Depression and irritability that are expressed as neuropsychological disorders during therapy impair quality of life universally. Therefore, it had been required to establish a new therapeutic modality without serious adverse effects.
Recently, DAAs that inhibit HCV-specific proteins have been clinically investigated[7,8]. Two DAAs, boceprevir and telaprevir first came to the HCV drug market and were approved by FDA in May 2011. Boceprevir or telaprevir was used as triple therapy with pegylated α-interferon and ribavirin for hepatitis C patients with genotype 1[9]. These DAAs are inhibitors against HCV NS3/4A serine protease and bind covalently with active site of the enzyme[21-23]. The triple therapy using boceprevir or telaprevir significantly increased the rate of sustained virological response (SVR) for naive or previous treated hepatitis C patients with HCV genotype 1[24-29]. After that, next generation DAAs, ABT-450/r, simeprevir, and faldaprevir, which are also NS3/4A protease inhibitors, have been reported to have advantages of their convenience and improved side effects profile[30-32]. Further, daclatasvir and sofosbuvir, which are an NS5A replication complex inhibitor and a nucleotide analogue NS5B polymerase inhibitor, respectively, also increased SVR rate[33-35]. Notably, the combination of these DAAs only was the highly effective treatment for patients with HCV genotype 1[36,37] and it is feasible to treat HCV without interferon and ribavirin.
While patients with hepatitis C can be treated by above mentioned DAAs without significant side-effects, it requires high medical costs and limits access to the therapy in cost-sensitive countries[38]. Of the 20 countries with the high prevalence of HCV, 12 are categorized as low or lower-middle income countries[39]. Therefore, new anti-HCV agents that are safe, economical, and complementary with present therapies, are still required and we focus attention on functional foods and their ingredients.
FUNCTIONAL FOOD INGREDIENTS EFFECTIVE FOR HCV
The development of HCV-related liver cirrhosis and hepatocellular carcinoma requires a prolonged period (20-30 years). Therefore, the progression of the disease and HCV infectivity may be influenced by a diet including dairy products. Functional foods and their ingredients are known to be capable of modulating various biological processes such as apoptosis and have been attracting interest as natural resources for the prevention and treatment of cancer[10,11,40]. Dietary polyphenols derived from various fruits and vegetables have been suggested to be effective in cancer prevention. Although the importance of functional food ingredients as DAAs against HCV is not fully recognized, these findings suggest that they contribute to the elimination of the virus.
Several functional food ingredients have been reported to interfere with different steps of the HCV life cycle. Epigallocatechin-3-gallate (EGCG) (Figure 2A) and curcumin (Figure 2B), which are ingredients of green tea (Camellia sinensis) and the Indian spice turmeric (Curcuma longa), respectively, inhibit the entry of HCV into host cells[41,42]. Quercetin (Figure 2C), a flavonoid that is abundantly contained in onions, apples, berries, and red wine, has been shown to inhibit NS3 protease activity[43]. Punicalagin (Figure 2D) and its related substance punicalin from the pomegranate (Punica granatum L.) reduced the replication of HCV[44]. Naringenin (Figure 2E) from the grapefruit (Citrus X paradisi Macfady.) has been identified as an ingredient that interferes with viral assembly[45,46]. Diosgenin (Figure 2F) and epicatechin (Figure 2G), which are contained in yams (Dioscorea spp.) and green tea, respectively, also affect the signal transduction pathways of host cells and inhibit HCV replication via the signal transducer and activator of transcription 3 and cycloxygenase-2 pathways, respectively[47,48]. The finding that curcumin and quercetin also inhibited HCV replication by associating with sterol regulatory element binding protein-1 and heat shock proteins, respectively, indicated the existence of multifunctional ingredients[49,50]. Silymarin, which is an extract from milk thistle (Silybum marianum) and consists of at least 7 flavonoid compounds, was also found to interfere with several steps of HCV infectious process, such as NS5B polymerase activity and virus entry and transmission[51]. As shown in Figure 2, most ingredients are polyphenol compounds and, EGCG (A), quercetin (C), naringenin (E), and epicatechin (G) have similar chemical structures. There may be a characteristic structure modulating viral proteins and their associations with host proteins.
Figure 2.
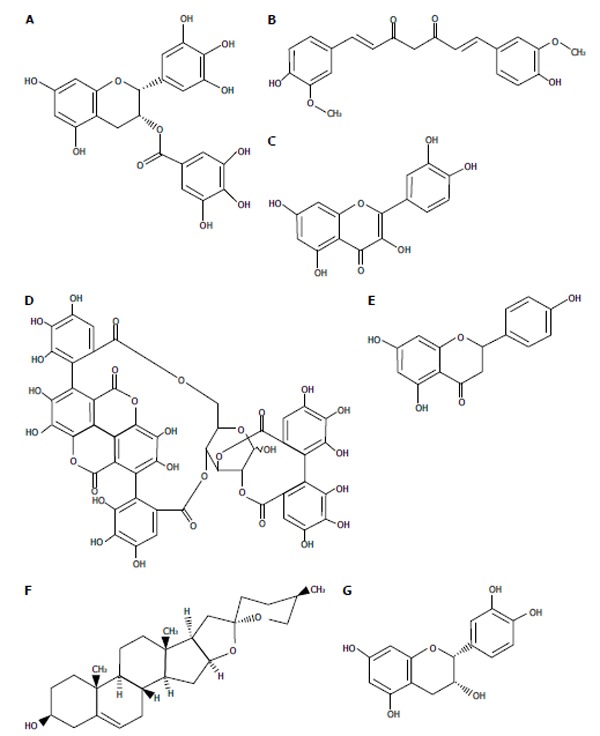
Chemical structure of functional food ingredients with anti-hepatitis C virus activities. A: Epigallocatechin-3-gallate; B: Curcumin; C: Quercetin; D: Punicalagin; E: Naringenin; F: Diosgenin; G: (-)-epicatechin.
Clinically, the supplementation of vitamin group has been reported to increase SVR rates in chronic hepatitis C patients who underwent the standard therapy with pegylated α-interferon and ribavirin[52-54]. Regarding significant side-effects of the standard therapy, a tomato-based functional food abundant in natural antioxidants alleviated the severity of anemia caused by ribavirin and improved the tolerance to the drug[55].
OLIGOMERIC PROANTHOCYANIDIN FROM BLUEBERRY LEAVES HAS SUPPRESSIVE ACTIVITY AGAINST HCV SUBGENOME REPLICATION IN VITRO
To identify functional food ingredients effective for hepatitis C, we comprehensively screened the extracts of commonly ingested agricultural products (1700 samples from 283 species) grown in Miyazaki prefecture, Japan using an HCV replicon cell system[13]. Samples having high antioxidative activities were first selected irrespective of edible part or non-edible part, and then the inhibitory activities against HCV subgenomic RNA replication were examined using the system. We found that extracts of blueberry leaves significantly suppressed the replication. Furthermore, by comparing the inhibitory activities using leaves from various kinds of blueberry species, it was found that the leaves of rabbit-eye blueberry (Vaccinium virgatum Aiton) had the highest activity[13]. Rabbit-eye blueberry is cultivated in a region with a warm climate, such as the southern areas of Japan, including Miyazaki prefecture. Its leaves have been also reported to be good sources of polyphenols and natural antioxidants[56].
We identified oligomeric proanthocyanidin as the blueberry leaf-derived inhibitor of HCV subgenomic RNA replication[13]. Proanthocyanidin is a polyphenol and has polymerized structures in which more than two flavan-3-ol units such as catechin (Figure 3A) and epicatechin (Figure 2G) are covalently linked. Figure 3B shows an example of the chemical structure of proanthocyanidin. Proanthocyanidin possesses two interflavan bonds, in which the A-type and B-type have two bond linkages (C4→C8 and O7→C2) and one linkage (C4→C8 or C4→C6), respectively[57], and both types co-exist in proanthocyanidin from the rabbit-eye blueberry plant[13]. While catechin, epicatechin, EGCG, and dimers such as procyanidin B2 did not exhibit inhibitory activity against HCV subgenomic expression in our experimental system, proanthocyanidin oligomer having polymerization degree of 8 to 9 markedly inhibited this expression[13]. This finding suggested that the HCV inhibitory activity of oligomeric proanthocyanidin in the replicon assay may require an oligomerized structure.
Figure 3.

Chemical structures of a flavan-3-ol and proanthocyanidin. A: (+)-catechin; B: An example of a procyanidin B-type polymer with an (-)-epicatechin based structure.
Proanthocyanidins are abundantly contained in various plants and foods[58] and contribute to organoleptic properties such as bitterness and astringency[59]. Proanthocyanidin-containing foods and nutritional supplements are known to have benefits in health promotion. United States Department of Agriculture Database reported proanthocyanidin contents of various foods, showing that apple peel, red kidney beans, pinto beans, cacao beans, cocoa, grape seeds, several nuts (almonds, hazelnuts, pecans, and pistachios), sorghum, and cinnamon are proanthocyanidin-rich[60]. Blueberry fruits are also relatively proanthocyanidin-rich; however, the fruits did not show significant HCV inhibitory activity compared to the leaves (unpublished data). In the fruits, proanthocyanidin contents of monomer, dimer, trimer, 4-6mer, 7-10mer, and polymer with degrees of polymerization greater than 10mer are 3.46, 5.71, 4.15, 19.57, 14.55, and 129.05 mg per 100 g edible portion, respectively[60]. As the inhibitory activity required the oligomeric structure of proanthocyanidin having a polymerization degree of 8 to 9 but not polymer and fresh blueberry leaf contained 3000-4000 mg proanthocyanidins per 100 g total extracts[13], leaves but not fruits from blueberry are likely suitable for the prevention of HCV-related diseases. With regard to the oral uptake, oligomeric proanthocyanidin seems to elute off by boiling for cooking as shown with pint beans[60]. Therefore, oligomeric proanthocyanidin from blueberry leaves might be ingested as a hot water extract such as herbal tea. However, absorption efficiency of oligomeric proanthocyanidin in the intestine may be very low.
Proanthocyanidin has also been reported to possess anti-viral activity against other viruses, herpes simplex virus and human immunodeficiency virus type 1[61-65]. To the best of our knowledge, we first reported that the proanthocyanidin oligomer inhibited the expression of HCV subgenomic RNA[13]. However, the effects of oligomeric proanthocyanidin on HCV replication in hepatocytes in vivo currently remain unknown.
ACTION MECHANISM OF OLIGOMERIC PROANTHOCYANIDIN IN HCV REPLICON CELLS
The suppression of HCV subgenomic RNA replication by oligomeric proanthocyanidin has been attracting increasing attention. Polyphenolic compounds generally have high antioxidant activities[10,11,58]. Therefore, the nonspecific antioxidant activity of polyphenols may contribute to the suppression of HCV subgenomic RNA replication by oligomeric proanthocyanidin. However, we examined other polyphenolic compounds in our HCV replicon assay, and found that constitutional units such as catechin and epicatechin did not display suppressive activity, which requires the oligomerized structure of proanthocyanidin[13]. While it currently remains unknown whether proanthocyanidin oligomer can be translocated within the cells in spite of the structure, the ingredient has been reported to be absorbed from the digestive tract[66,67], implying the internalization into cells. Oligomeric proanthocyanidin appears to suppress HCV subgenomic RNA replication via a specific association with certain intracellular molecules.
Proteomic approach using two-dimensional differential gel electrophoresis combined with mass spectrometry provides a powerful tool to determine the cellular response to functional foods[40]. To clarify the action mechanism of oligomeric proanthocyanidin in HCV replicon cells, we performed proteomic analysis of proanthocyanidin-binding proteins purified by affinity chromatography[13]. Then, cellular proteins from replicon cells having higher affinity to proanthocyanidin than catechin were identified by a mass spectrometric analysis, and whether the proteins identified were associated with HCV RNA expression was further examined using a siRNA-based replicon assay (Figure 4). Four heterogeneous nuclear ribonucleoproteins (hnRNPs), hnRNP A/B, A2/B1, K, and L, were suggested to be possible cellular binding proteins of oligomeric proanthocyanidin. While siRNA targeting hnRNP A/B, K, and L showed weak inhibitory activities, the knockdown of hnRNP A2/B1 significantly suppressed HCV subgenomic replication[13].
Figure 4.
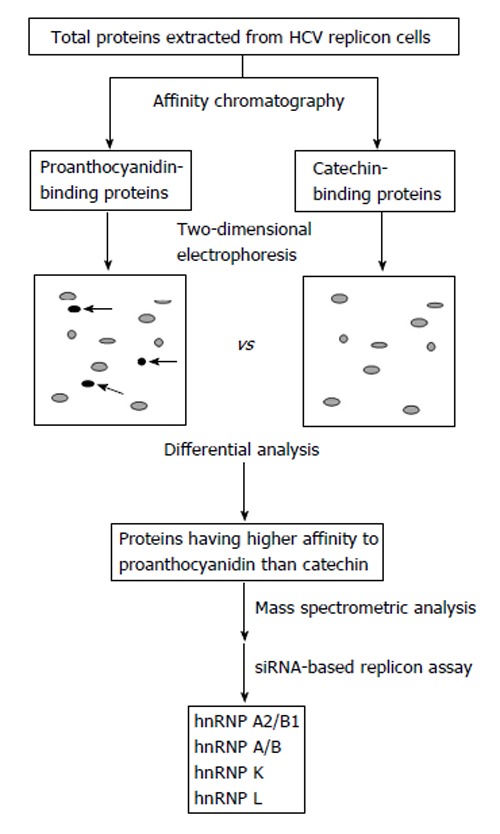
Identification strategy of candidate proteins involved in the proanthocyanidin-mediated inhibition of hepatitis C virus subgenomic expression[13]. Total proteins were extracted from hepatitis C virus (HCV) replicon cells and then proanthocyanidin-binding and catechin-binding proteins were purified by affinity chromatography using sepharose beads coupled with proanthocyanidin and catechin, respectively. Purified proteins were separated by two-dimensional electrophoresis followed by detecting spots of proteins having higher affinity to proanthocyanidin than catechin (arrows). Mass spectrometric analysis and further screening by a siRNA-based replicon assay showed that hnRNP A2/B1, A/B, K, and L are candidate proteins involved in the oligomeric proanthocyanidin-mediated inhibition of HCV subgenomic expression. hnRNP: Heterogeneous nuclear ribonucleoprotein.
HnRNPs comprise a family of RNA-binding proteins that are involved in diverse RNA-related biological processes[68]. They are multifunctional proteins composed of major and minor hnRNP proteins, and hnRNP A/B, A2/B1, K, and L that we identified belonged to the major hnRNPs[69]. Previous studies demonstrated that these hnRNPs regulated the metabolism of RNA such as pre-mRNA splicing and transcription[70-76]. For example, hnRNP A2/B1 was shown to affect the alternative splicing of several tumor suppressors and oncogenes in glioblastoma cells[72]. Furthermore, several studies reported interactions and cooperation between these hnRNPs[77-79]. hnRNP A2 and hnRNP L have also been shown to exist as a complex and regulate the expression of glucose transporter-1 by binding to mRNA 3’NTR[80,81].
In the HCV life cycle, hnRNPs are associated with HCV genome RNA and regulate its replication. hnRNP A1, which exhibits high homology with hnRNP A2/B1, was shown to facilitate HCV replication via binding to the HCV 5’ and 3’NTRs (Figure 1), and the replication was significantly suppressed by the double knockdown of hnRNP A1 and hnRNP A2[82]. hnRNP K and hnRNP L are also NTR-binding proteins[83-85]. Furthermore, all the hnRNPs we identified as the target protein candidates of oligomeric proanthocyanidin were included in HCV 3’NTR-binding proteins[86]. Collectively, these findings suggested that a complex composed of hnRNP A2/B1, A/B, K, and L may serve in HCV genome replication by binding to NTRs and oligomeric proanthocyanidin is an inhibitor of the replication complex. This possibility should be addressed in a further study.
CONCLUSION
Currently, a combination of pegylated recombinant interferons and ribavirin is used as the standard therapy for hepatitis C patients. Recently emerged DAAs are expected to provide new promising treatment options in hepatitis C patients. However, their high medical costs may make difficult to disseminate worldwide. We demonstrated that extracts of blueberry leaves suppressed HCV subgenome replication in vitro, and their active ingredient was oligomeric proanthocyanidin[13]. Investigations into the underlying action mechanism suggested that proanthocyanidin may be an inhibitor of several hnRNPs such as hnRNP A2/B1[13]. On the other hand, it currently remains unknown whether the oligomeric form of proanthocyanidin, which is required for the inhibition of HCV replication, can be efficiently absorbed from the digestive tract to maintain effective plasma concentrations in vivo. However, further basic research on the action mechanism of oligomeric proanthocyanidin against HCV replication may open ways to develop novel anti-HCV drugs and supplements for hepatitis C patients worldwide.
ACKNOWLEDGMENTS
We dedicate this work to Mr. Fumiaki Mieno (deceased, March 19, 2013), who inspired our work in the protection and exploitation of intellectual property. We thank Sachiko Tomiyama, Tokoyo Imai, Toshiro Morishita, and Makoto Kodama (Miyazaki Prefectural Industrial Support Foundation) for coordinating our study.
Footnotes
Supported by The Collaboration of Regional Entities for the Advancement of Technological Excellence from Japan Science and Technology Agency
P- Reviewer: Ampuero J, Chuang WL, Conti B, Hernanda PY, Tijera MFH, Qin JM S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
References
- 1.Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15–22. doi: 10.1002/hep.21283. [DOI] [PubMed] [Google Scholar]
- 2.Webster DP, Klenerman P, Collier J, Jeffery KJ. Development of novel treatments for hepatitis C. Lancet Infect Dis. 2009;9:108–117. doi: 10.1016/S1473-3099(09)70020-9. [DOI] [PubMed] [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Ni ZJ, Wagman AS. Progress and development of small molecule HCV antivirals. Curr Opin Drug Discov Devel. 2004;7:446–459. [PubMed] [Google Scholar]
- 6.Gane E. Future hepatitis C virus treatment: interferon-sparing combinations. Liver Int. 2011;31 Suppl 1:62–67. doi: 10.1111/j.1478-3231.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 7.Pol S, Corouge M, Sogni P. Oral antiviral therapies for chronic hepatitis C infection. Ther Adv Infect Dis. 2013;1:107–116. doi: 10.1177/2049936113488359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ampuero J, Romero-Gómez M, Reddy KR. Review article: HCV genotype 3 – the new treatment challenge. Aliment Pharmacol Ther. 2014;39:686–698. doi: 10.1111/apt.12646. [DOI] [PubMed] [Google Scholar]
- 9.Tungol A, Rademacher K, Schafer JA. Formulary management of the protease inhibitors boceprevir and telaprevir for chronic hepatitis C virus. J Manag Care Pharm. 2011;17:685–694. doi: 10.18553/jmcp.2011.17.9.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 11.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 12.Manson MM. Cancer prevention -- the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;9:11–18. doi: 10.1016/s1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita M, Ishida Y, Akamatsu E, Ohmori Y, Sudoh M, Uto H, Tsubouchi H, Kataoka H. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J Biol Chem. 2009;284:21165–21176. doi: 10.1074/jbc.M109.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray SC, Bailey JR, Thomas DL. Fields virology. 6th ed. Knipe DM, Howley PM, editors. Lippincott Williams & Wilkins (PA): Hepatitis C virus; 2013. pp. 795–824. [Google Scholar]
- 15.Griffin SD, Beales LP, Clarke DS, Worsfold O, Evans SD, Jaeger J, Harris MP, Rowlands DJ. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett. 2003;535:34–38. doi: 10.1016/s0014-5793(02)03851-6. [DOI] [PubMed] [Google Scholar]
- 16.Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto H, Okamoto K, Aoki M, Kato H, Katsume A, Ohta A, Tsukuda T, Shimma N, Aoki Y, Arisawa M, et al. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat Chem Biol. 2005;1:333–337. doi: 10.1038/nchembio742. [DOI] [PubMed] [Google Scholar]
- 18.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 19.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 20.El-Shamy A, Hotta H. Impact of hepatitis C virus heterogeneity on interferon sensitivity: an overview. World J Gastroenterol. 2014;20:7555–7569. doi: 10.3748/wjg.v20.i24.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perni RB, Farmer LJ, Cottrell KM, Court JJ, Courtney LF, Deininger DD, Gates CA, Harbeson SL, Kim JL, Lin C, et al. Inhibitors of hepatitis C virus NS3.4A protease. Part 3: P2 proline variants. Bioorg Med Chem Lett. 2004;14:1939–1942. doi: 10.1016/j.bmcl.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 22.Malcolm BA, Liu R, Lahser F, Agrawal S, Belanger B, Butkiewicz N, Chase R, Gheyas F, Hart A, Hesk D, et al. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob Agents Chemother. 2006;50:1013–1020. doi: 10.1128/AAC.50.3.1013-1020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu P, Sanfiorenzo V, Curry S, Guo Z, Liu S, Skelton A, Xia E, Cullen C, Ralston R, Greene J, et al. Identification of HCV protease inhibitor resistance mutations by selection pressure-based method. Nucleic Acids Res. 2009;37:e74. doi: 10.1093/nar/gkp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 27.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 28.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vierling JM, Davis M, Flamm S, Gordon SC, Lawitz E, Yoshida EM, Galati J, Luketic V, McCone J, Jacobson I, et al. Boceprevir for chronic HCV genotype 1 infection in patients with prior treatment failure to peginterferon/ribavirin, including prior null response. J Hepatol. 2014;60:748–756. doi: 10.1016/j.jhep.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, Everson GT, Kwo P, Foster GR, Sulkowski MS, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 31.Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 32.Sulkowski MS, Asselah T, Lalezari J, Ferenci P, Fainboim H, Leggett B, Bessone F, Mauss S, Heo J, Datsenko Y, et al. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology. 2013;57:2143–2154. doi: 10.1002/hep.26276. [DOI] [PubMed] [Google Scholar]
- 33.Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, Hézode C, Lim JK, Bronowicki JP, Abrams GA, et al. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12:671–677. doi: 10.1016/S1473-3099(12)70138-X. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki F, Toyota J, Ikeda K, Chayama K, Mochida S, Hayashi N, Ishikawa H, Miyagoshi H, Hu W, McPhee F, et al. A randomized trial of daclatasvir with peginterferon alfa-2b and ribavirin for HCV genotype 1 infection. Antivir Ther. 2014;19:491–499. doi: 10.3851/IMP2730. [DOI] [PubMed] [Google Scholar]
- 35.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 36.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 37.Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, Chang TT, Everson GT, Heo J, Gerken G, Yoffe B, Towner WJ, Bourliere M, Metivier S, Chu CJ, Sievert W, Bronowicki JP, Thabut D, Lee YJ, Kao JH, McPhee F, Kopit J, Mendez P, Linaberry M, Hughes E, Noviello S; HALLMARK-DUAL Study Team. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597–1605. doi: 10.1016/S0140-6736(14)61059-X. [DOI] [PubMed] [Google Scholar]
- 38.Ford N, Singh K, Cooke GS, Mills EJ, von Schoen-Angerer T, Kamarulzaman A, du Cros P. Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis. 2012;54:1465–1472. doi: 10.1093/cid/cis227. [DOI] [PubMed] [Google Scholar]
- 39.Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58:928–936. doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishida Y, Yamasaki M, Yukizaki C, Nishiyama K, Tsubouchi H, Okayama A, Kataoka H. Carnosol, rosemary ingredient, induces apoptosis in adult T-cell leukemia/lymphoma cells via glutathione depletion: proteomic approach using fluorescent two-dimensional differential gel electrophoresis. Hum Cell. 2014;27:68–77. doi: 10.1007/s13577-013-0083-6. [DOI] [PubMed] [Google Scholar]
- 41.Calland N, Albecka A, Belouzard S, Wychowski C, Duverlie G, Descamps V, Hober D, Dubuisson J, Rouillé Y, Séron K. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology. 2012;55:720–729. doi: 10.1002/hep.24803. [DOI] [PubMed] [Google Scholar]
- 42.Anggakusuma CC, Schang LM, Rachmawati H, Frentzen A, Pfaender S, Behrendt P, Brown RJ, Bankwitz D, Steinmann J, Ott M, et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137–1149. doi: 10.1136/gutjnl-2012-304299. [DOI] [PubMed] [Google Scholar]
- 43.Bachmetov L, Gal-Tanamy M, Shapira A, Vorobeychik M, Giterman-Galam T, Sathiyamoorthy P, Golan-Goldhirsh A, Benhar I, Tur-Kaspa R, Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat. 2012;19:e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 44.Reddy BU, Mullick R, Kumar A, Sudha G, Srinivasan N, Das S. Small molecule inhibitors of HCV replication from pomegranate. Sci Rep. 2014;4:5411. doi: 10.1038/srep05411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahmias Y, Goldwasser J, Casali M, van Poll D, Wakita T, Chung RT, Yarmush ML. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaguer P, Polyak SJ, Chung RT, Yarmush ML, Nahmias Y. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol. 2011;55:963–971. doi: 10.1016/j.jhep.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YJ, Pan KL, Hsieh TC, Chang TY, Lin WH, Hsu JT. Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. J Nat Prod. 2011;74:580–584. doi: 10.1021/np100578u. [DOI] [PubMed] [Google Scholar]
- 48.Lin YT, Wu YH, Tseng CK, Lin CK, Chen WC, Hsu YC, Lee JC. Green tea phenolic epicatechins inhibit hepatitis C virus replication via cycloxygenase-2 and attenuate virus-induced inflammation. PLoS One. 2013;8:e54466. doi: 10.1371/journal.pone.0054466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez O, Fontanes V, Raychaudhuri S, Loo R, Loo J, Arumugaswami V, Sun R, Dasgupta A, French SW. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50:1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim K, Kim KH, Kim HY, Cho HK, Sakamoto N, Cheong J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010;584:707–712. doi: 10.1016/j.febslet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Wagoner J, Negash A, Kane OJ, Martinez LE, Nahmias Y, Bourne N, Owen DM, Grove J, Brimacombe C, McKeating JA, et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010;51:1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nimer A, Mouch A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J Gastroenterol. 2012;18:800–805. doi: 10.3748/wjg.v18.i8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rocco A, Compare D, Coccoli P, Esposito C, Di Spirito A, Barbato A, Strazzullo P, Nardone G. Vitamin B12 supplementation improves rates of sustained viral response in patients chronically infected with hepatitis C virus. Gut. 2013;62:766–773. doi: 10.1136/gutjnl-2012-302344. [DOI] [PubMed] [Google Scholar]
- 55.Morisco F, Vitaglione P, Carbone A, Stingo S, Scarpati S, Ascione A, Marmo R, Fogliano V, Caporaso N. Tomato-based functional food as interferon adjuvant in HCV eradication therapy. J Clin Gastroenterol. 2004;38:S118–S120. doi: 10.1097/01.mcg.0000128935.48082.f9. [DOI] [PubMed] [Google Scholar]
- 56.Li C, Feng J, Huang WY, An XT. Composition of polyphenols and antioxidant activity of rabbiteye blueberry (Vaccinium ashei) in Nanjing. J Agric Food Chem. 2013;61:523–531. doi: 10.1021/jf3046158. [DOI] [PubMed] [Google Scholar]
- 57.Porter LJ. The flavonoids. Advances in research since 1980. Harborne JB, editor. Springer US (NY): Flavans and proanthocyanidins; 1988. pp. 21–62. [Google Scholar]
- 58.Dixon RA, Xie DY, Sharma SB. Proanthocyanidins--a final frontier in flavonoid research? New Phytol. 2005;165:9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 59.Brossaud F, Cheynier V, Noble AC. Bitterness and astringency of grape and wine polyphenols. Aust J Grape Wine Res. 2001;7:33–39. [Google Scholar]
- 60.United States Department of Agriculture. USDA database for the proanthocyanidin content of selected foods. 2004. [Google Scholar]
- 61.Iwasawa A, Niwano Y, Mokudai T, Kohno M. Antiviral activity of proanthocyanidin against feline calicivirus used as a surrogate for noroviruses, and coxsackievirus used as a representative enteric virus. Biocontrol Sci. 2009;14:107–111. doi: 10.4265/bio.14.107. [DOI] [PubMed] [Google Scholar]
- 62.Xu X, Xie H, Wang Y, Wei X. A-type proanthocyanidins from lychee seeds and their antioxidant and antiviral activities. J Agric Food Chem. 2010;58:11667–11672. doi: 10.1021/jf1033202. [DOI] [PubMed] [Google Scholar]
- 63.Gescher K, Hensel A, Hafezi W, Derksen A, Kühn J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antiviral Res. 2011;89:9–18. doi: 10.1016/j.antiviral.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Gescher K, Kühn J, Lorentzen E, Hafezi W, Derksen A, Deters A, Hensel A. Proanthocyanidin-enriched extract from Myrothamnus flabellifolia Welw. exerts antiviral activity against herpes simplex virus type 1 by inhibition of viral adsorption and penetration. J Ethnopharmacol. 2011;134:468–474. doi: 10.1016/j.jep.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 65.Suedee A, Tewtrakul S, Panichayupakaranant P. Anti-HIV-1 integrase compound from Pometia pinnata leaves. Pharm Biol. 2013;51:1256–1261. doi: 10.3109/13880209.2013.786098. [DOI] [PubMed] [Google Scholar]
- 66.Shoji T, Masumoto S, Moriichi N, Akiyama H, Kanda T, Ohtake Y, Goda Y. Apple procyanidin oligomers absorption in rats after oral administration: analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J Agric Food Chem. 2006;54:884–892. doi: 10.1021/jf052260b. [DOI] [PubMed] [Google Scholar]
- 67.Xu L, Li B, Cheng M, Zhang W, Pan J, Zhang C, Gao H. Oral administration of grape seed proanthocyanidin extracts downregulate RAGE dependant nuclear factor- kappa BP65 expression in the hippocampus of streptozotocin induced diabetic rats. Exp Clin Endocrinol Diabetes. 2008;116:215–224. doi: 10.1055/s-2007-993188. [DOI] [PubMed] [Google Scholar]
- 68.Dreyfuss G, Matunis MJ, Piñol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 69.Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1’s multifunctional regulatory roles. RNA. 2010;16:1449–1462. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hou VC, Lersch R, Gee SL, Ponthier JL, Lo AJ, Wu M, Turck CW, Koury M, Krainer AR, Mayeda A, et al. Decrease in hnRNP A/B expression during erythropoiesis mediates a pre-mRNA splicing switch. EMBO J. 2002;21:6195–6204. doi: 10.1093/emboj/cdf625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Szaro BG. hnRNP K post-transcriptionally co-regulates multiple cytoskeletal genes needed for axonogenesis. Development. 2011;138:3079–3090. doi: 10.1242/dev.066993. [DOI] [PubMed] [Google Scholar]
- 72.Golan-Gerstl R, Cohen M, Shilo A, Suh SS, Bakàcs A, Coppola L, Karni R. Splicing factor hnRNP A2/B1 regulates tumor suppressor gene splicing and is an oncogenic driver in glioblastoma. Cancer Res. 2011;71:4464–4472. doi: 10.1158/0008-5472.CAN-10-4410. [DOI] [PubMed] [Google Scholar]
- 73.Preussner M, Schreiner S, Hung LH, Porstner M, Jäck HM, Benes V, Rätsch G, Bindereif A. HnRNP L and L-like cooperate in multiple-exon regulation of CD45 alternative splicing. Nucleic Acids Res. 2012;40:5666–5678. doi: 10.1093/nar/gks221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu G, Razanau A, Hai Y, Yu J, Sohail M, Lobo VG, Chu J, Kung SK, Xie J. A conserved serine of heterogeneous nuclear ribonucleoprotein L (hnRNP L) mediates depolarization-regulated alternative splicing of potassium channels. J Biol Chem. 2012;287:22709–22716. doi: 10.1074/jbc.M112.357343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao W, Razanau A, Feng D, Lobo VG, Xie J. Control of alternative splicing by forskolin through hnRNP K during neuronal differentiation. Nucleic Acids Res. 2012;40:8059–8071. doi: 10.1093/nar/gks504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu J, Chen Z, Xia D, Wu J, Xu H, Ye ZQ. Promoter-associated small double-stranded RNA interacts with heterogeneous nuclear ribonucleoprotein A2/B1 to induce transcriptional activation. Biochem J. 2012;447:407–416. doi: 10.1042/BJ20120256. [DOI] [PubMed] [Google Scholar]
- 77.Kim JH, Hahm B, Kim YK, Choi M, Jang SK. Protein-protein interaction among hnRNPs shuttling between nucleus and cytoplasm. J Mol Biol. 2000;298:395–405. doi: 10.1006/jmbi.2000.3687. [DOI] [PubMed] [Google Scholar]
- 78.Shnyreva M, Schullery DS, Suzuki H, Higaki Y, Bomsztyk K. Interaction of two multifunctional proteins. Heterogeneous nuclear ribonucleoprotein K and Y-box-binding protein. J Biol Chem. 2000;275:15498–15503. doi: 10.1074/jbc.275.20.15498. [DOI] [PubMed] [Google Scholar]
- 79.Griffith BN, Walsh CM, Szeszel-Fedorowicz W, Timperman AT, Salati LM. Identification of hnRNPs K, L and A2/B1 as candidate proteins involved in the nutritional regulation of mRNA splicing. Biochim Biophys Acta. 2006;1759:552–561. doi: 10.1016/j.bbaexp.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamilton BJ, Nichols RC, Tsukamoto H, Boado RJ, Pardridge WM, Rigby WF. hnRNP A2 and hnRNP L bind the 3’UTR of glucose transporter 1 mRNA and exist as a complex in vivo. Biochem Biophys Res Commun. 1999;261:646–651. doi: 10.1006/bbrc.1999.1040. [DOI] [PubMed] [Google Scholar]
- 81.Griffin ME, Hamilton BJ, Roy KM, Du M, Willson AM, Keenan BJ, Wang XW, Nichols RC. Post-transcriptional regulation of glucose transporter-1 by an AU-rich element in the 3’UTR and by hnRNP A2. Biochem Biophys Res Commun. 2004;318:977–982. doi: 10.1016/j.bbrc.2004.04.128. [DOI] [PubMed] [Google Scholar]
- 82.Kim CS, Seol SK, Song OK, Park JH, Jang SK. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J Virol. 2007;81:3852–3865. doi: 10.1128/JVI.01311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hahm B, Kim YK, Kim JH, Kim TY, Jang SK. Heterogeneous nuclear ribonucleoprotein L interacts with the 3’ border of the internal ribosomal entry site of hepatitis C virus. J Virol. 1998;72:8782–8788. doi: 10.1128/jvi.72.11.8782-8788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan B, Lu KY, Reymond Sutandy FX, Chen YW, Konan K, Zhu H, Kao CC, Chen CS. A human proteome microarray identifies that the heterogeneous nuclear ribonucleoprotein K (hnRNP K) recognizes the 5’ terminal sequence of the hepatitis C virus RNA. Mol Cell Proteomics. 2014;13:84–92. doi: 10.1074/mcp.M113.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y, Masaki T, Shimakami T, Lemon SM. hnRNP L and NF90 interact with hepatitis C virus 5’-terminal untranslated RNA and promote efficient replication. J Virol. 2014;88:7199–7209. doi: 10.1128/JVI.00225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harris D, Zhang Z, Chaubey B, Pandey VN. Identification of cellular factors associated with the 3’-nontranslated region of the hepatitis C virus genome. Mol Cell Proteomics. 2006;5:1006–1018. doi: 10.1074/mcp.M500429-MCP200. [DOI] [PubMed] [Google Scholar]


