Abstract
The tentative amino-acid sequence and three-dimensional structure of the lectin concanavalin A have been determined. The amino-acid sequence, which was determined chemically, contains 238 residues. The sequences of three short stretches were assigned on the basis of x-ray crystallographic data. Interpretation of an electron density map at 2-Å resolution indicates that the predominant structural element is extended polypeptide chain arranged in two anti-parallel pleated sheets or β-structures. Residues not included in the β-structures are arranged in regions of random coil. One of the pleated sheets contributes extensively to the interactions among the monomers to form both dimers and tetramers. The positions at which Mn2+, Ca2+, and saccharide are bound to the protein, and the point of cleavage for the formation of the naturally occurring fragments A1 and A2, have been tentatively assigned. Both metal-binding sites are at least 20-Å removed from the position at which saccharides are bound. The saccharide-binding site is a deep pocket of approximately 6Å × 7.5Å × 18Å, the inner portion of which is occupied by hydrophobic residues.
Keywords: x-ray crystallography, sequence, 2-Å resolution, binding sites, lectin
Full text
PDF

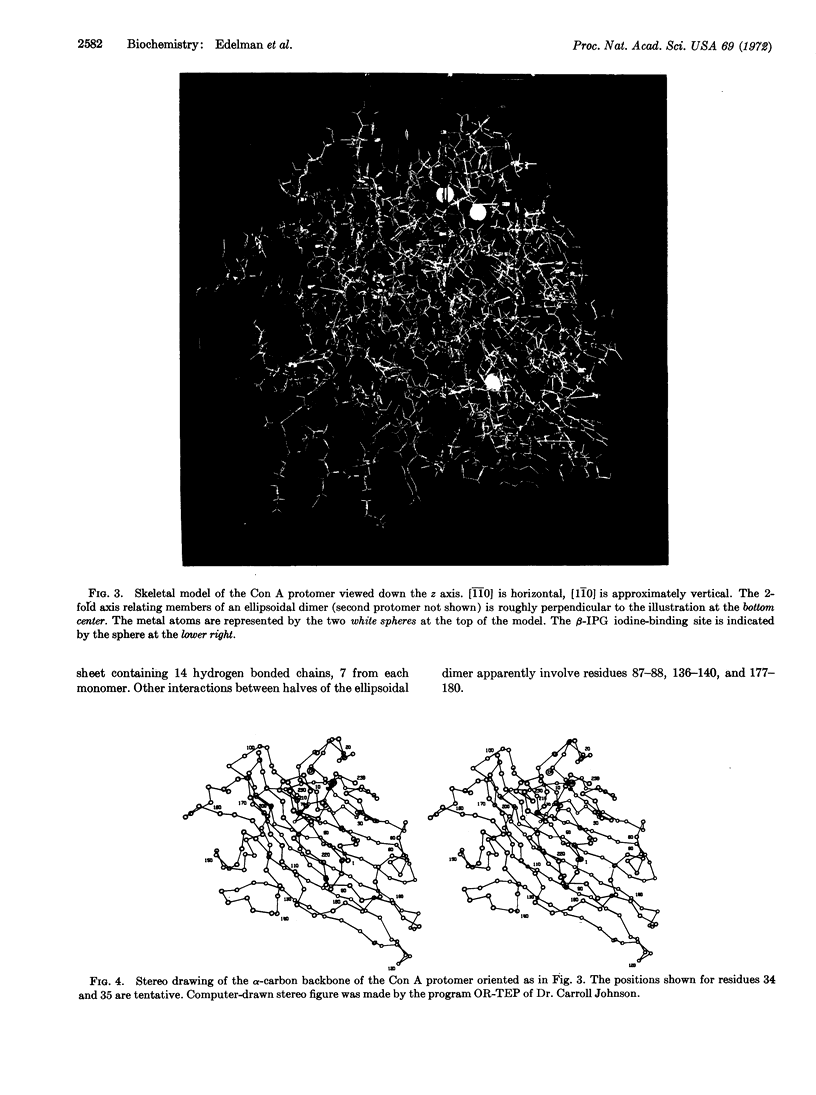


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Edelman G. M., Möller G., Sjöberg O. Activation of B lymphocytes by locally concentrated concanavalin A. Eur J Immunol. 1972 Jun;2(3):233–235. doi: 10.1002/eji.1830020307. [DOI] [PubMed] [Google Scholar]
- BOYD W. C. The lectins: their present status. Vox Sang. 1963 Jan-Feb;8:1–32. doi: 10.1111/j.1423-0410.1963.tb04146.x. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr, Edelman G. M. Location of the saccharide binding site of concanavalin A. J Biol Chem. 1971 Oct 10;246(19):6123–6125. [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Cummingham B. A., Gottlieb P. D., Konigsberg W. H., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. V. Partial amino acid sequence of the light chain. Biochemistry. 1968 May;7(5):1983–1994. doi: 10.1021/bi00845a049. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Millette C. F. Molecular probes of spermatozoan structures. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2436–2440. doi: 10.1073/pnas.68.10.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Gottlieb P. D., Cunningham B. A., Rutishauser U., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. VI. Amino acid sequence of the light chain. Biochemistry. 1970 Aug 4;9(16):3155–3161. doi: 10.1021/bi00818a007. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat New Biol. 1972 Jan 19;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Hardman K. D., Ainsworth C. F. Myo-inositol binding site of concanavalin A. Nat New Biol. 1972 May 10;237(71):54–55. doi: 10.1038/newbio237054a0. [DOI] [PubMed] [Google Scholar]
- Hardman K. D., Wood M. K., Schiffer M., Edmundson A. B., Ainsworth C. F. X-ray crystallographic studies of concanavalin A. Cold Spring Harb Symp Quant Biol. 1972;36:271–276. doi: 10.1101/sqb.1972.036.01.036. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Levitzki A. Metal-binding sites of concanavalin A and their role in the binding of alpha-methyl d-glucopyranoside. Biochem J. 1968 Oct;109(4):669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Lustig A. The molecular weight of concanavalin A. Biochim Biophys Acta. 1968 Oct 21;168(2):366–367. doi: 10.1016/0005-2795(68)90161-x. [DOI] [PubMed] [Google Scholar]
- Pflumm M. N., Wang J. L., Edelman G. M. Conformational changes in concanavalin A. J Biol Chem. 1971 Jul 10;246(13):4369–4370. [PubMed] [Google Scholar]
- Poretz R. D., Goldstein I. J. Protein-carbohydrate interaction. On the mode of bonding of aromatic moieties to concanavalin A, the phytohemagglutinin of the jack bean. Biochem Pharmacol. 1971 Oct;20(10):2727–2739. doi: 10.1016/0006-2952(71)90182-1. [DOI] [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Becker J. W., Quiocho F. A. The structure of concanavalin A at 4 A resolution. Cold Spring Harb Symp Quant Biol. 1972;36:277–284. doi: 10.1101/sqb.1972.036.01.037. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The matching of physical models to three-dimensional electron-density maps: a simple optical device. J Mol Biol. 1968 Oct 14;37(1):225–230. doi: 10.1016/0022-2836(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Edelman G. M. Unusual fragments in the subunit structure of concanavalin A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1130–1134. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxdal M. J., Wang J. L., Pflumm M. N., Edelman G. M. Isolation and order of the cyanogen bromide fragments of concanavalin A. Biochemistry. 1971 Aug 31;10(18):3343–3347. doi: 10.1021/bi00794a004. [DOI] [PubMed] [Google Scholar]
- Weinzierl J., Kalb A. J. The transition metal-binding site of concanavalin A at 2.8 A resolution. FEBS Lett. 1971 Nov 1;18(2):268–270. doi: 10.1016/0014-5793(71)80461-1. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]




