Abstract
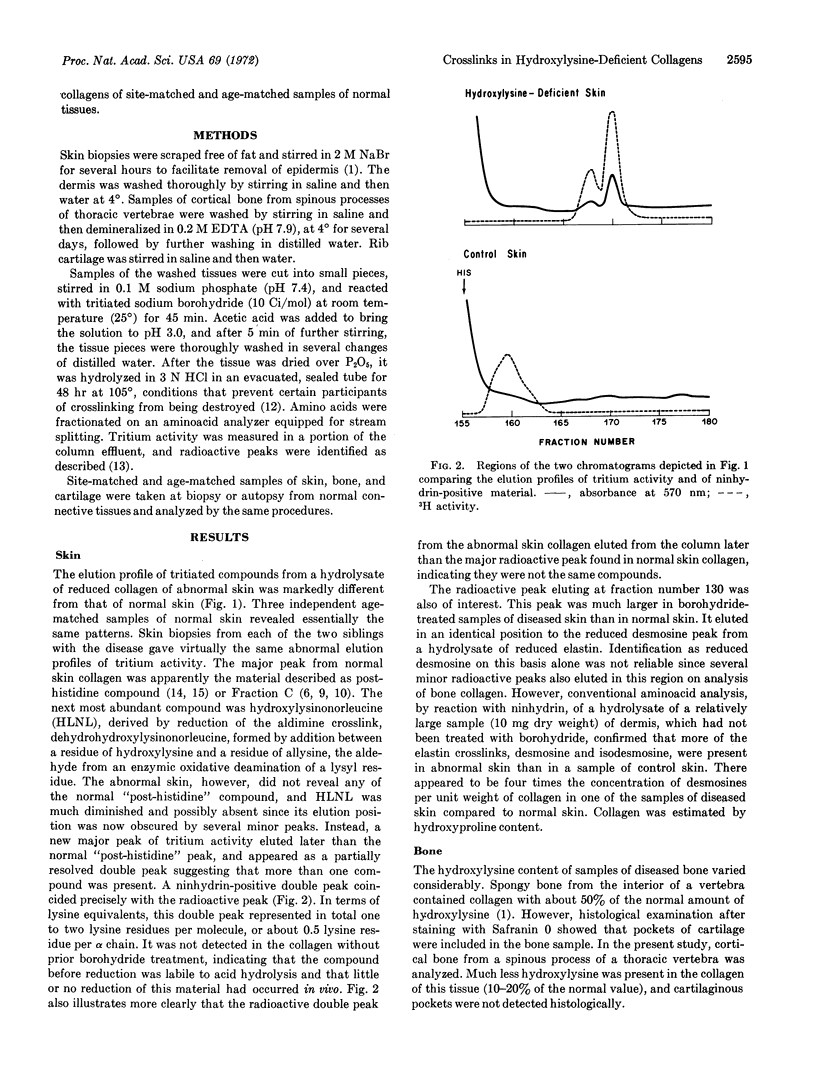
Reducible compounds that participate in crosslinking were analyzed in hydroxylysine-deficient collagens of patients with a heritable disorder of connective tissue. After treatment with [3H]sodium borohydride, new compounds, as well as a totally different pattern of tritiated compounds, were found in hydroxylysine-deficient collagen from skin as compared with age-matched controls. The amount of desmosines detected indicated that more elastin was present in abnormal skin than in control skin.
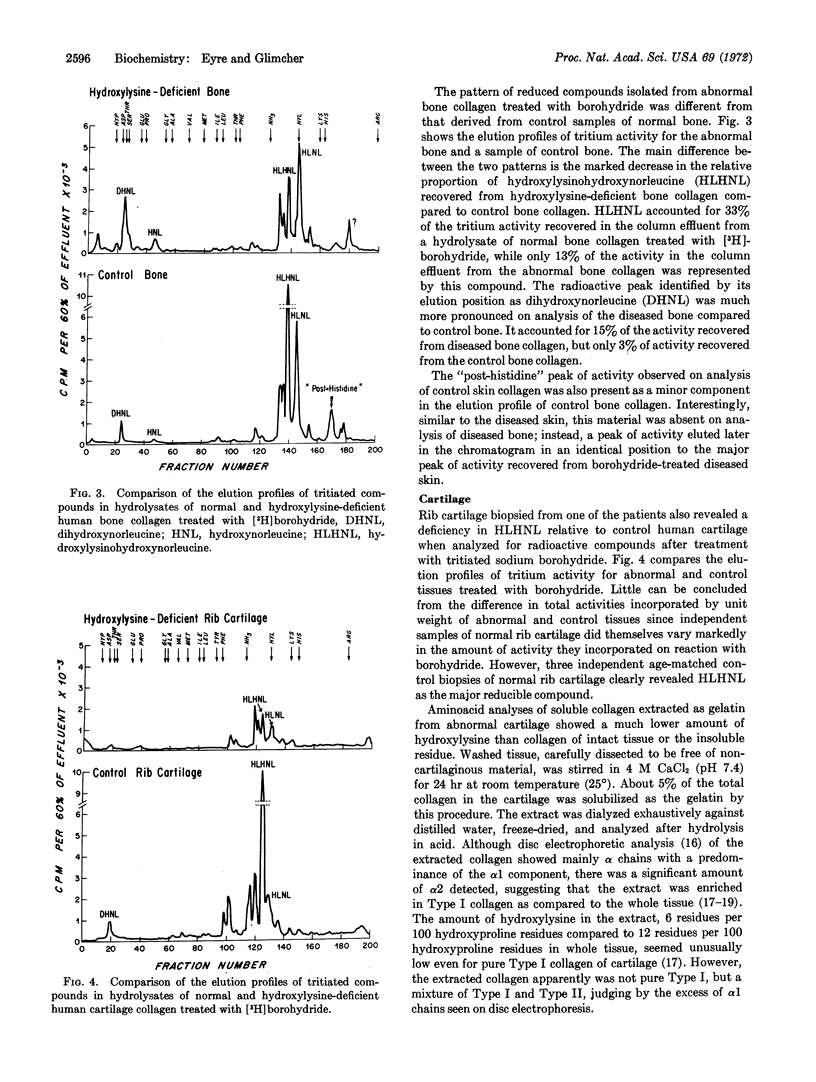
Bone collagen, which was not as deficient in hydroxylysine as skin collagen, had the same compounds as normal bone collagen, but their relative proportions were altered, consistent with a deficiency of hydroxylysine, a precursor of the crosslinks. Although the content of hydroxylysine in collagen of cartilage is essentially normal in these patients, analysis after reduction revealed a different pattern of reduced compounds from that of normal cartilage. It is speculated that Type II collagen, the major collagen component in cartilage, contains a normal amount of hydroxylysine, while Type I collagen, which is the major source of the crosslinks, is hydroxylysine-deficient. This distribution would explain the findings of an abnormal profile of reducible compounds despite an almost normal total hydroxylysine content.
The finding that the deficiency of hydroxylysine in the collagen of these patients is accompanied by changes in number, chemical nature, and, probably, distribution of crosslinkages, and the previously reported alterations in the solubility characteristics, suggest that at least some skeletal and connective tissue abnormalities are directly related to underlying molecular pathology.
Keywords: skin, bone, cartilage, aminoacid analysis
Full text
PDF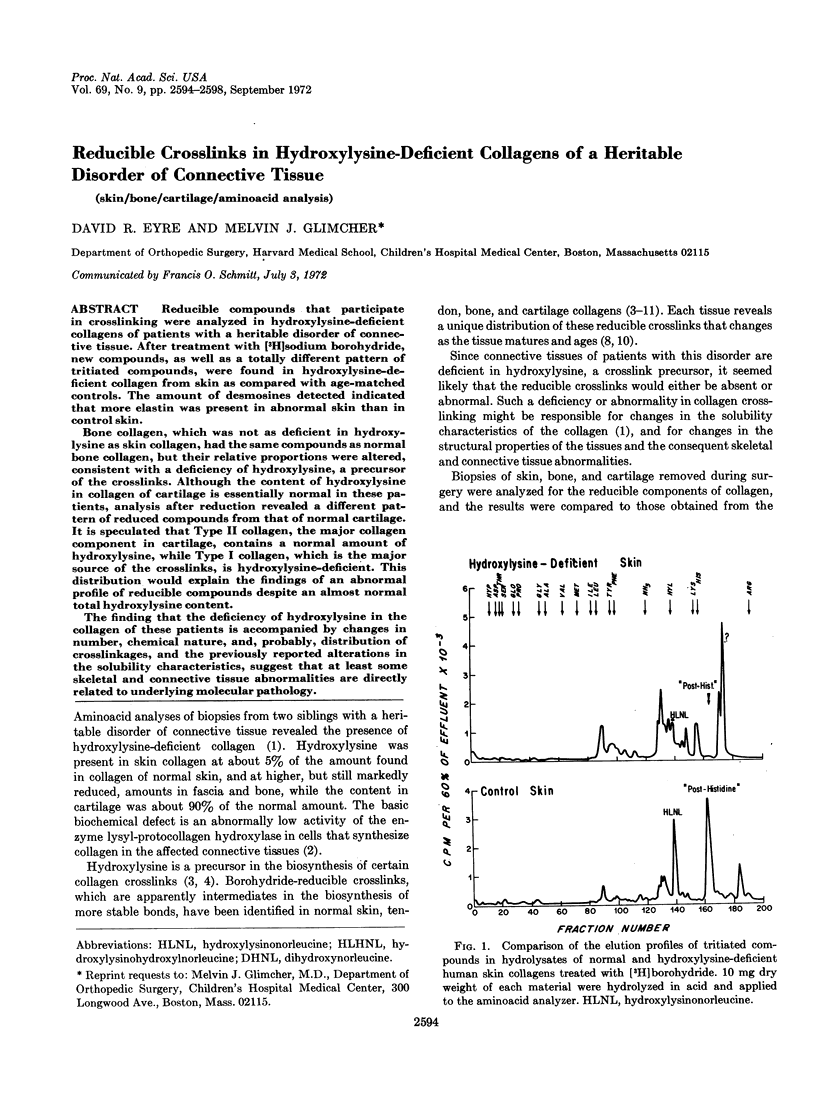


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Fowler L. J., Peach C. M. Identification of two interchain crosslinks of bone and dentine collagen. Biochem Biophys Res Commun. 1969 Jun 6;35(5):663–671. doi: 10.1016/0006-291x(69)90456-2. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M., Fowler L. J. Chemistry of the collagen cross-links. Isolation and characterization of two intermediate intermolecular cross-links in collagen. Biochem J. 1970 May;117(5):819–831. doi: 10.1042/bj1170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M. Isolation and structural identification of a labile intermolecular crosslink in collagen. Biochem Biophys Res Commun. 1968 Dec 9;33(5):812–819. doi: 10.1016/0006-291x(68)90233-7. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Shimokomaki M. S. Age related changes in the reducible cross-links of collagen. FEBS Lett. 1971 Aug 1;16(2):86–88. doi: 10.1016/0014-5793(71)80338-1. [DOI] [PubMed] [Google Scholar]
- Bailey Allen J. Comparative studies on the nature of the cross-links stabilizing the collagen fibres of invertebrates, cyclostomes and elasmobranchs. FEBS Lett. 1971 Oct 15;18(1):154–158. doi: 10.1016/0014-5793(71)80433-7. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Comparative biochemistry of collagen crosslinks: reducible bonds in invertebrate collagens. Biochim Biophys Acta. 1971 Sep 28;243(3):525–529. doi: 10.1016/0005-2795(71)90027-4. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Julkunen H., Rokkanen P., Inoue H. Scanning electron microscopic study of the collagen bundles of the skin in the Ehlers-Danlos syndrome. Ann Med Exp Biol Fenn. 1970;48(4):201–204. [PubMed] [Google Scholar]
- Kang A. H., Faris B., Franzblau C. The in vitro formation of intermolecular cross-links in chick skin collagen. Biochem Biophys Res Commun. 1970 Apr 8;39(1):175–182. doi: 10.1016/0006-291x(70)90774-6. [DOI] [PubMed] [Google Scholar]
- Mechanic G. Crosslinking of collagen in a heritable disorder of connective tissue: Ehlers-Danlos syndrome. Biochem Biophys Res Commun. 1972 Apr 14;47(1):267–272. doi: 10.1016/s0006-291x(72)80038-x. [DOI] [PubMed] [Google Scholar]
- Mechanic G., Gallop P. M., Tanzer M. L. The nature of crosslinking in collagens from mineralized tissues. Biochem Biophys Res Commun. 1971 Nov 5;45(3):644–653. doi: 10.1016/0006-291x(71)90465-7. [DOI] [PubMed] [Google Scholar]
- Mechanic G., Tanzer M. L. Biochemistry of collagen crosslinking. Isolation of a new crosslink, hydroxylysinohydroxynorleucine, and its reduced precursor, dihydroxynorleucine, from bovine tendon. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1597–1604. doi: 10.1016/0006-291x(70)90571-1. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Fox R., Krane S. M. Human collagens: differences in glycosylated hydroxylysines in skin and bone. Biochim Biophys Acta. 1971 Jan 19;229(1):119–122. doi: 10.1016/0005-2795(71)90325-4. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Krane S. M., Kenzora J. E., Glimcher M. J. A heritable disorder of connective tissue. Hydroxylysine-deficient collagen disease. N Engl J Med. 1972 May 11;286(19):1013–1020. doi: 10.1056/NEJM197205112861901. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. A subtle disease and a dilemma: can cells secrete collagen that does not contain a sugar-tag? N Engl J Med. 1972 May 11;286(19):1055–1056. doi: 10.1056/NEJM197205112861910. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L. Collagen reduction by sodium borohydride: effects of reconstitution, maturation and lathyrism. Biochem Biophys Res Commun. 1968 Sep 6;32(5):885–892. doi: 10.1016/0006-291x(68)90324-0. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Mechanic G., Gallop P. M. Isolation of hydroxylsinonorleucine and its lactone from reconstituted collagen fibrils. Biochim Biophys Acta. 1970 Jun 23;207(3):548–552. doi: 10.1016/s0005-2795(70)80017-4. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Mechanic G. Isolation of lysinonorleucine from collagen. Biochem Biophys Res Commun. 1970 Apr 8;39(1):183–189. doi: 10.1016/0006-291x(70)90775-8. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Igarashi S., Gross J. Isolation of two distinct collagens from chick cartilage. Biochemistry. 1970 Dec 8;9(25):4993–4998. doi: 10.1021/bi00827a025. [DOI] [PubMed] [Google Scholar]


