Abstract
Using a feline model, erections caused by a new class of vasodilator agents that specifically activate potassium (K+-ATP) channels (lemakalim, nicorandil, and pinacidil) were compared to baseline and maximal erections induced by a standard drug combination (1.65 mg papaverine, 25 μg phentolamine and 0.5 μg PGE1) injected intracavernosally. The responses were characterized by the maximal intracavernosal pressure, the duration of maximal pressure, the total duration of drug effect, the change in penile length, and alterations in systemic arterial blood pressure.
All three K+-ATP channel openers caused erections in a dose-dependent manner. All agents caused similar increases in penile length with full erection, but the duration of maximal pressure and duration of action were significantly shorter (P<0.01) than with the standard drug combination. Lemakalim did not decrease systemic blood pressure as did nicorandil, pinacidil, and the standard drug combination.
This study supports the use of an in-vivo feline model for the evaluation of vasoactive agents and suggests that a new class of agents can pharmacologically activate the erectile response selectively by an alternate pathway.
Keywords: pharmacologic erection, feline model, potassium channel agonists
Introduction
While investigating the role of the autonomic nervous system in the control of the urinary bladder in the cat, Domer et al. serendipitously noted the erection-producing properties of the intravenously administered nonselective alpha receptor antagonist, phentolamine and beta2 receptor agonists1. After the initial clinical reports by Virag2 and Brindley3 regarding the use of vasoactive agents in the therapy of erectile dys–function, self-administration of drugs intracavernosally has become an alternative for men suffering from impotence.
Understanding of the physiology and pharmacology of relaxation of corporal smooth muscle remains incomplete. In addition to the presence of cholinergic and adrenergic pathways, it is postulated that there are other nonadrenegic–noncholinergic pathways that mediate relaxation of smooth muscle and penile erection4–6.
Vasoactive intestinal peptide (VIP) has been suggested as a putative neuro–transmitter that not only mediates relaxation of the smooth muscle of the corpus cavernosum but also may stimulate sexual behavior7,8. Still, there are many classes of pharmacological agents that induce erection but do not have smooth muscle relaxing effects9. With continued basic research, therapeutic approaches using intracavernous injection of other drugs may further develop the treatment of erectile dysfunction.
Each patient with the diagnosis of impotence has a condition that may result from a variety of causes. Objective evaluation of potentially effective classes of drugs must rely, therefore, on in-vivo comparisons which use animal models. For our studies, we developed a feline model to analyze the potential responses of erection-producing drugs.
There is active investigation of the applications of drugs that act upon ATP-sensitive potassium channels to cause relaxation of smooth muscle10. The purpose of the present study is to evaluate this class of agents, with regard to the potential of their pharmacologic effects on erectile function.
Materials and methods
Thirty-six adult male cats weighing 4.0–5.3 kg were sedated with ketamine hydrochloride (10–15 mg/kg im) and anesthetized with pentobarbital sodium (30 mg/kg iv). Supplemental doses of pentobarbital were given as needed to maintain a uniform level of anesthesia.
In each animal the trachea was cannulated and the cat was allowed to breathe room air spontaneously. One polyethylene catheter was inserted into an external jugular vein for intravenous administration of drugs and another into a carotid artery for the measurement of systemic arterial pressure.
The penis was shaved and prepped. A vertical, circumcision-like incision was made to expose the two ventral corpora cavernosa and the dorsal corpus spongiosum. A 25-gauge 0.5-inch needle attached to polyethylene tubing (PE 10) was positioned midway into the left corpus for measurement of intracavernosal pressure. Both systemic arterial and intracavernosal pressures were measured with Statham P23 transducers connected to a Grass model 7 polygraph zeroed at the right atrial level. A 30-gauge needle with similar polyethylene tubing was placed midway into the right corpus at a 45° angle to the shaft to permit administration of drugs directly into the penis.
In initial experiments (n=10), a control maximal erectile response was established with a mixture of 0.5 μg PGE1 (Upjohn), 25 μg phentolamine HCl (Ciba-Geigy) and 1.65 mg papaverine HCl (Lilly) in 200 μl of normal saline. This drug mixture was flushed in with 200 μl saline.
Next, we evaluated the actions of three chemically distinct potassium channel openers: lemakalim (Beecham, UK), nicorandil (Bekloff Associates), and pinacidil (Lilly). These agents were dissolved in 100% ethanol at a concentration of 10 mg/ml and diluted with 0.9% NaCl on the day of the experiments.
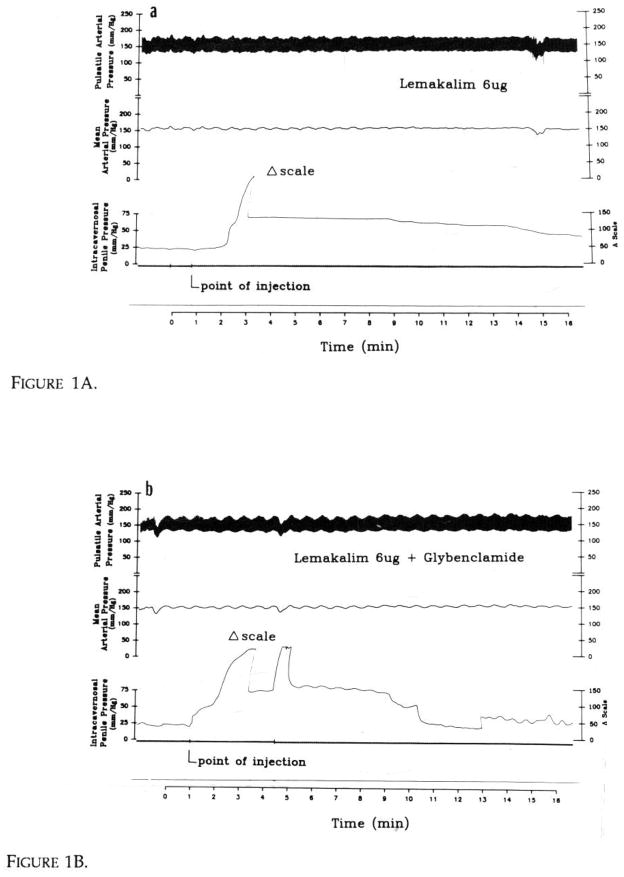
Lemakalim (n=10) was injected into the corpus in incremental doses (0.3, 0.6, 1, 1.5, 3, 6 μg) in 200 μl of 0.9% NaCl and the catheter was subsequently flushed with 200 μl saline. Intracavernosal pressures were recorded continuously before injection of the drug, during the response and until return to the baseline (Fig. 1A). Later, when the peak erectile response was obtained with lemakalim, glybenclamide (0.8–1.2 mg) (Sigma) was injected and the duration of pressure rise was compared to that recorded in the absence of the blocking agent (Fig. 1B).
Figure 1.
Figure 1A. Typical recording response after intracavernosal injection of lemakalim with increase in intracavernosal penile pressure (mmHg) and lack of change in systemic arterial pressures (mmHg). Scale changed by one half where indicated. Abscissa recorded in min.
Figure 1B. Typical intracavernosal penile pressure response when glybenclamide administered 4 min after lemakalim. Pressure rise at time of second injection represents fluid flush.
The systemic arterial pressure, intracavernosal pressure, changes in maximal penile length, the delay before reaching the maximal pressure, the duration of peak pressure, and the duration of action were recorded (Fig. 1A and B).
In a similar manner, nicorandil (n=9) was administered in incremental doses (0.01, 0.03, 0.05, 0.1, 0.3, 0.5, 1 μg) and pinacidil (n=7) was administered in increasing doses (1.0, 5.0, 10, 30, 50, 100, 300, 500 μg).
All data were expressed as mean ± SE and were analyzed by t-tests. P≤0.05 was used as the criterion for statistical significance.
Results
The initial series of experiments was carried out in 10 cats to establish the characteristics of the penile response to a standard combination of drugs composed of PGE1 0.5 μg, phentolamine 25 μg and papaverine 1.65 mg in 200 μl of 0.9% saline. Injection of the standard combination into the corpora cavernosa caused a rise in intracavernous pressure from a control value of 17 ± 1 mmHg to a maximal increase of 101 ± 11 mmHg. The time to the maximal increase in pressure was 1.6 ± 0.2 min with a duration of peak action of 25.9 ± 8.8 min. The increased pressure in response to the standard combination of drugs lasted 41.3 ± 9.6 min, and penile length increased 6.6 ± 1.6 mm from a baseline value of 22.7 ± 1.8 mm to a maximal erect length of 29.4 ± 1.0 mm. Mean systemic arterial pressure decreased 30 ± 6 mmHg from a baseline value of 140 ± 6 to 110 ± 7 mmHg in response to injection of the standard combination of drugs.
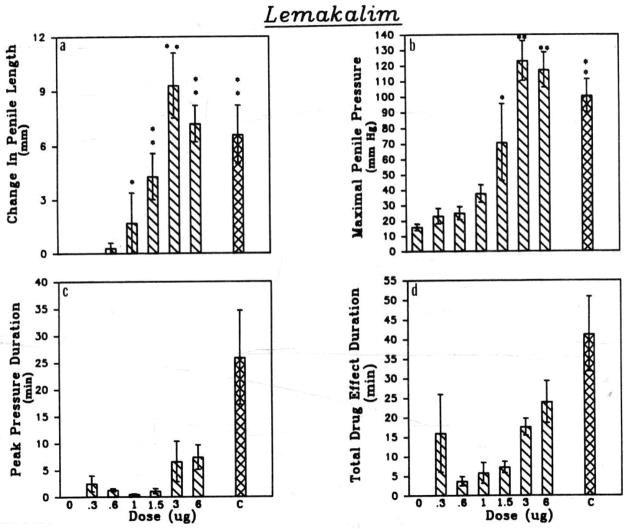
After establishment of the standard response, the effects of the ATP-sensitive potassium channel opener lemakalim on the erectile response were compared to the response to the standard combination of drugs. These data are summarized in Figure 2. Injections of lemakalim 0.3 to 3 μg caused dose-related increases in pressure in the corpora cavernosa. The maximal response occurred at the dose of 3 μg and was significantly greater than the response to the standard combination of drugs (Fig. 2). However, the duration of the response to lemakalim was significantly shorter than that observed in response to the standard combination of drugs.
Figure 2.
Comparisons of cats receiving lemakalim intracavernosally on (a) changes in penile length, (b) maximal penile pressure, (c) duration of peak pressure, and (d) total duration of drug effect. The dose in μg is indicated on the abscissa and ‘C’ represents the standard triple combination. Statistical significance is *P=0.05, **P=0.01, (n=10).
Penile length increased significantly in response to injection of lemakalim in doses of 1 and 3 μg (Fig. 2). Systemic arterial pressure was unchanged when lemakalim was injected into the corpora cavernosa (Fig. 1A), whereas the standard combination of drugs decreased pressure by 30 ± 6 mmHg.
The effects of glybenclamide, an antagonist at ATP-sensitive potassium channels, on the penile response to lemakalim were investigated. After administration of glybenclamide (0.8–1.2 mg), the duration of the response to lemakalim was reduced significantly (Fig. 1B).
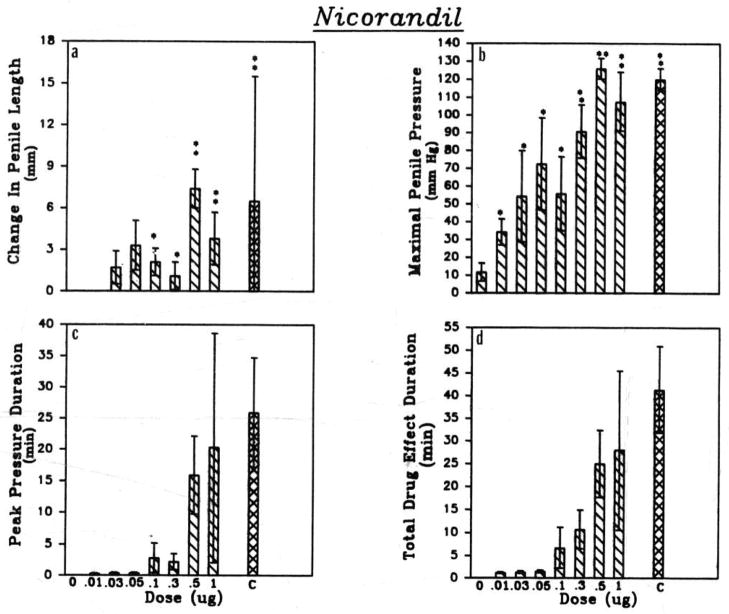
Nicorandil, an agonist at ATP-sensitive potassium channels, also increases levels of cyclic guanosine monophosphate (cGMP) in smooth muscle. Nicorandil induced penile erection in a dose as low as 0.01 μg. This agent also significantly increased intracavernous pressure. The maximal response was observed with a dose of 0.5 μg (Fig. 3). The maximal increase in cavernosal pressure in response to 0.5 μg of nicorandil was significantly greater than that caused by the standard drug combination (Fig. 3). The duration of the maximal increase in pressure and of the duration of the response to the 0.5 μg of nicorandil were significantly shorter than observed with the standard combination of drugs (Fig. 3). The duration of the maximal increase in pressure and the duration of action with nicorandil were decreased significantly after administration of glybenclamide (0.8–1.2 mg).
Figure 3.
Comparisons of cats receiving nicorandil intracavernosally on (a) changes in penile length, (b) maximal penile pressure, (c) duration of peak pressure, and (d) total duration of drug effect. The dose in μg is indicated on the abscissa and ‘C’ represents the standard triple combination. Statistical significance is *P=0.05, **P=0.01, (n=9).
Pinacidil caused an erection at 3.0 μg with a concomitant increase in intracavernous pressure (Fig. 4). The total duration and peak pressure duration were both significantly less than with the standard combination. Similarly, the maximal increase in pressure and the duration of action of pinacidil were decreased significantly after administration of glybenclamide (0.8–1.2 mg).
Figure 4.
Comparisons of cats receiving pinacidil intracavernosally on (a) changes in penile length, (b) maximal penile pressure, (c) duration of peak pressure, and (d) total duration of drug effect. The dose in μg is indicated on the abscissa and ‘C’ represents the standard triple combination. Statistical significance is *P=0.05, **P=0.01, (n=7).
Discussion
Between 10 and 20 million American men suffer from impotence, but programs using drugs offer many of these patients a satisfactory solution to their condition11. The development of new drugs is limited by the difficulty in determining the efficacy of drugs in humans. The development of an animal model with normal cavernosal function could help solve this problem.
The choice of the animal model is of paramount importance. The monkey is prohibitively expensive. The corpora cavernosa in the dog are not in communication with each other12. The rat penis is small, making the measurements of intracavernosal pressure difficult. In addition, certain drugs — such as PGE1 — have failed to induce full erection in rabbits, dogs, and monkeys11. From our earlier experience and current pilot experiments, we have found large adult cats to be a satisfactory model. Both corpora are located ventrally with the corpus spongiosum running in a dorsal position and cradled by the os penis in the distal part of the organ13.
Clinical experience from human studies has shown that combinations of vasoactive drugs can be effective in a number of previously non-responding patients14. Our initial studies in cats, using a standard combination (1.65 mg papaverine, 25 μg phentolamine, 0.5 μg PGE1), resulted in a reproducible full erection. Papaverine acts in part by inhibiting phosphodiesterase and thereby causing relaxation of the smooth muscle of the corpus cavernosum. Phentolamine is a competitive, nonselective, alpha-adrenergic blocking agent that may act by causing an unopposed relative increase in beta2 adrenergic tone1. Prostaglandin E1 acts directly via its own receptor to increase cAMP levels in order to cause relaxation of the smooth muscle of the corpus cavernosum. This combination served as our standard positive control.
Intensive ongoing basic research has focused on the benzopyrene group of drugs for the treatment of essential hypertension15. The mechanism of action of this group involves opening of the potassium channels that are sensitive to the intracellular concentration of adenosine triphosphate (ATP). These channels are similar functionally and pharmacologically to those that are opened by acetylcholine, VIP, and calcitonin gene-related peptide (CGRP)16, 17. These actions result in hyperpolarization of the membrane, inhibition of the Ca++ influx, and relaxation of vascular smooth muscle. Urologic interest has been generated by the observed effect of these agents in the inhibition of the detrusor muscle and thereby its potential use in the treatment of the irritable bladder 18–20. There is one preliminary report on the effect of pinacidil on in-vitro human and porcine corpus cavernosum strips and its qualitative effects on in-vivo simian erections21.
In the present study, three chemically dissimilar potassium channel opening agents — lemakalim, nicorandil, and pinacidil — produced dose-dependent erections in cats. This erection was significantly greater than that caused by the standard drug combination in the same animals. However, the duration of action and the peak pressure increase were less than those observed with the standard drug combination. This may have potential therapeutic implications in that the incidence of priapism may be reduced relative to that caused by the compounds in the standard mixture.
Glybenclamide is a sulfonylurea hypoglycemic agent which blocks ATP-sensitive potassium channels competitively22. In the present study, glybenclamide reduced the duration of the penile response to lemakalim, nicorandil, and pinacidil without influencing the erectile response to the standard combination of agonists. Thus, the potassium channel agonists represent a new, separate pathway to induce erections. The exploitation of such an alternative pathway may be of use in men refractory to treatment with traditional agents or in lieu of some of the usual combinations of vasoactive drugs11.
Evaluation of the relative potencies of the three compounds indicated that pinacidil required a much higher concentration to achieve the maximal penile pressure when compared to nicorandil and lemakalim. Of the three drugs evaluated, only lemakalin did not decrease systemic blood pressure. This could be advantageous if it should be associated with a lower incidence of systemic side effects.
This work provides a foundation for further studies on the physiology and pharmacology of erection. Employing the in-vivo feline model, investigators can evaluate and compare the effects of different vasoactive agents on cavernous smooth muscle.
Acknowledgments
T. Higuera for technical help, J. Bellin and R. Minkes for their valuable suggestions, E. Kukuy for production of illustrations and technical assistance, J. Evans and M.B. Shinn for manuscript evaluation and preparation. This work was supported by BRSG Grant number 1090-1-02-110 and NIH Grant number HL15580.
References
- 1.Domer FR, Wessler G, Brown RL, Charles HC. Involvement of the sympathetic nervous system in the urinary bladder internal sphincter and in penile erection in the anaesthetized cat. Invest Urol. 1978;15:404–7. [PubMed] [Google Scholar]
- 2.Virag R. Intracavernous injection of papaverine for erectile failure. Lancet. 1982;2:938. doi: 10.1016/s0140-6736(82)90910-2. [DOI] [PubMed] [Google Scholar]
- 3.Brindley GS. Cavernosal alpha-blockade: a new technique for investigating and treating erectile impotence. Br J Psychiat. 1983;143:332–37. doi: 10.1192/bjp.143.4.332. [DOI] [PubMed] [Google Scholar]
- 4.Saenz de Tejada I, Kim N, Lagan I, Krane RJ, Goldstein I. Regulation of adrenergic activity in penile corpus cavernosum. J Urol. 1989;142:1117–21. doi: 10.1016/s0022-5347(17)39009-2. [DOI] [PubMed] [Google Scholar]
- 5.Saenz de Tejada I, Blanco R, Goldstein I, Azadzoi K, de las Morenas A, Krane RJ, Cohen RA. Cholinergic neurotransmission in human corpus cavernosum. I. Responses of isolated tissue. Am J Physiol. 1988;254:H459–67. doi: 10.1152/ajpheart.1988.254.3.H459. [DOI] [PubMed] [Google Scholar]
- 6.Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Comm. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 7.Saenz de Tejada I, Goldstein I, Krane RJ. Local control of penile erection. The Urol Clin of NA Impotence. 1988;15 (1):915. [PubMed] [Google Scholar]
- 8.Gozes I, Meltzer E, Rubinrout S, Brenneman DE, Fridkin M. Vasoactive intestinal peptide potentiates sexual behavior: inhibition by novel antagonist. Endocrinology. 1989;125:2945–9. doi: 10.1210/endo-125-6-2945. [DOI] [PubMed] [Google Scholar]
- 9.Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989;320:1025–30. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- 10.Weston AH. Smooth muscle K+ channel openers; their pharmacology and clinical potential. Pflugers Arch. 1989;414(Suppl 1):599–105. doi: 10.1007/BF00582256. [DOI] [PubMed] [Google Scholar]
- 11.Junemann KP, Alken P. Pharmacotherapy of erectile dysfunction: a review. Int J Impotence Res. 1989;1:71–93. [Google Scholar]
- 12.Miller ME, Christensen GC, Evans HE. Anatomy of the dog. Philadelphia: W.B. Saunders; 1964. p. 750. [Google Scholar]
- 13.Crouch JE, Lackey MG. Text-Atlas of Cat Anatomy. Philadelphia: Lea and Febiger; 1969. p. 160. [Google Scholar]
- 14.Padma-Nathan H. The efficacy and synergy of polypharmacotherapy in primary and salvage therapy of vasculogenic erectile dysfunction. Int J Impotence Res. 1990;2(Suppl 2):257–8. [Google Scholar]
- 15.Ashwood VA, Buckingham RE, Cassidy F, Evans JM, Faruk EA, Hamilton TC, Nash DJ, Stemp G, Willcocks K. Synthesis and antihypertensive activity of 4-(cyclic amido)-2H-lbenzopyrans. J Med Chem. 1986;29:2194–01. doi: 10.1021/jm00161a011. [DOI] [PubMed] [Google Scholar]
- 16.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature (London) 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 17.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP sensitive K+ channels in arterial smooth muscle. Science (Washington DC) 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 18.Edwards G, Henshaw M, Miller M, Weston AH. Comparison of the effects of several potassium-channel openers on rat bladder and rat portal vein in vitro. Br J Pharmacol. 1991;102:679–86. doi: 10.1111/j.1476-5381.1991.tb12233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malmgren A, Andersson K-E, Andersson PO, Fovaeus M, Sjogren C. Effects of cromakalim (BRL 34915) and pinacidil on normal and hypertrophied rat detrusor in vitro. J Urol. 1990;143:828–34. doi: 10.1016/s0022-5347(17)40111-x. [DOI] [PubMed] [Google Scholar]
- 20.Foster CD, Fujii K, Kingdom J, Brading AF. The effect of cromakalim on the smooth muscle of the guinea-pig urinary bladder. Br J Pharmacol. 1989;97:281. doi: 10.1111/j.1476-5381.1989.tb11952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giraldi A, Wagner G. Effects of pinacidil upon penile erectile tissue, in vitro and in vivo. Pharm Tox. 1990;67:235–8. doi: 10.1111/j.1600-0773.1990.tb00819.x. [DOI] [PubMed] [Google Scholar]
- 22.Eltze M. Competitive antagonism by glybenclamide of cromakalim inhibition of twitch contractions in rabbit vas deferens. Europ J Pharmacol. 1989;161:103–6. doi: 10.1016/0014-2999(89)90187-8. [DOI] [PubMed] [Google Scholar]