Abstract
OBJECTIVE
Neonatal diagnoses are often used as surrogate endpoints for longer-term outcomes. We sought to characterize the correlation between neonatal diagnoses and early childhood neurodevelopment.
STUDY DESIGN
We conducted secondary analysis of a multicenter randomized controlled trial of antenatal magnesium sulfate vs placebo administered to women at imminent risk for delivery <32.0 weeks to prevent death and cerebral palsy in their offspring. Singletons and twins delivering 23.0–33.9 weeks who survived to hospital discharge and had 2-year-old outcome data were included. Those surviving to age 2 years were assessed by trained physicians and the Bayley II Scales of Infant Development Mental Development and Psychomotor Development Indices. Neonatal diagnoses at the time of each baby’s initial hospital discharge were examined singly and in combination to determine those most predictive of childhood neurodevelopmental impairment, defined as a childhood diagnosis of moderate/severe cerebral palsy and/or Bayley scores >2 SD below the mean. Data were analyzed by multiple regression models and area under receiver operating characteristic curves.
RESULTS
A total of 1771 children met criteria. Children were delivered at a mean of 29.4 weeks’ gestation. In all, 459 (25.9%) had neuro-developmental impairment. In models controlling for gestational age at delivery, maternal education, maternal race, tobacco/alcohol/drug use during pregnancy, randomization to magnesium, fetal sex, and chorioamnionitis, individual neonatal morbidities were moderately predictive of childhood neurodevelopmental impairment (best model area under receiver operating characteristic curve, 0.68; 95% confidence interval, 0.65–0.71). Combinations of 2, 3, and 4 morbidities did not improve the prediction of neurodevelopmental impairment.
CONCLUSION
Approximately 1 in 4 previously preterm children had neurodevelopmental impairment at age 2 years. Prediction of childhood outcomes from neonatal diagnoses remains imperfect.
Keywords: neonatal outcomes, neurodevelopment, prematurity
Preterm delivery <37 weeks’ gestation remains the leading cause of neonatal and childhood morbidity among nonanomalous infants in the United States and the developed world.1,2 Recent advances in perinatal and neonatal medicine over the last 2 decades have resulted in substantial increases in survival among premature infants.3 However, this survival increase may be accompanied by an increase in survival with subsequent major morbidities, resulting in sicker children who require intensive postnatal medical care and costly developmental services.4 Among the most premature, those children born <1000 g, approximately 10–15% develop moderate to severe cerebral palsy and 30% have deficits in cognitive development.5–7
Frequently, obstetric and pediatric researchers use neonatal morbidity as a surrogate outcome for longer-term, childhood outcomes when studying pregnancy exposures and/or interventions. While risks factors such as extremely low birthweight (<750 g), early gestational age (<28 weeks’ gestation), chorioamnionitis, intracranial hemorrhage, and fetal sex have been identified, the correlation between neonatal and childhood outcomes is imprecisely defined.8,9 Additionally, many preterm infants acquire multiple neonatal morbidities, but it remains uncertain if this confers an additive risk for adverse childhood neurodevelopment.
Previous studies have been limited. Schmidt et al9 recently examined 3 neonatal diagnoses: bronchopulmonary dysplasia (BPD), brain injury (defined as intraventricular hemorrhage, ventriculomegaly, and/or periventricular leukomalacia), and severe retinopathy of prematurity (ROP), diagnosed singly or in combination, with adverse neuro-developmental outcomes at 18 months among infants delivered very prematurely (birthweight 500–999 g). These researchers found a correlation between the number of neonatal diagnoses and 18-month outcomes. Babies with all 3 of the studied diagnoses had an 88% chance of adverse childhood outcomes compared to an 18% chance if the infant had none of these diagnoses. The impact of other factors, including other major neonatal morbidity such as necrotizing enterocolitis, and pregnancy or antenatal characteristics such as chorioamnionitis, could not be assessed. Furthermore, it is unknown if these results are applicable to a wider range of the preterm population or are limited to the extremely low birthweight neonate.
The purpose of this study was to determine the relationship between neonatal diagnoses prior to initial hospital discharge and neurodevelopmental outcomes at age 2 years among a large, prospectively collected cohort of infants delivered preterm between 23–34 weeks’ gestation.
Materials and Methods
This is a secondary analysis of a multi-center randomized controlled trial conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal Fetal Medicine Units Network of antenatal magnesium sulfate vs placebo administered to women at imminent risk for preterm delivery <32.0 weeks’ gestation. The aim of this study was to investigate the role of magnesium in the prevention of death and cerebral palsy in their offspring. The methods and results from the primary study have been previously published. Briefly, the main trial found that fetal exposure to magnesium sulfate did not reduce the combined risk of moderate or severe cerebral palsy or death, but the rate of cerebral palsy was reduced among survivors.10 All participants provided written informed consent at the time of enrollment in the original study. This secondary analysis was performed on a deidentified dataset, and was reviewed by our local institutional review board and determined to be nonhuman subjects research and therefore exempt from institutional review board approval.
Singleton and twin infants admitted and randomized between 23.0–31.9 weeks’ gestation and delivered <34.0 weeks’ gestation who survived to hospital discharge postbirth and had childhood outcome data at age 2 years were included in this secondary analysis. Infants delivered with chromosomal abnormalities or major congenital malformations and/or with incomplete outcome data at hospital discharge or 2 years of age were excluded. Gestational age was determined by the best obstetric estimate per previously published criteria.11 Trained research nurses obtained data on neonatal outcomes during hospitalization and at discharge, and at scheduled follow-up visits at 6, 12, and 24 months of age (corrected for prematurity). Specifically, each neonate was assessed for the presence or history of intraventricular hemorrhage, periventricular leukomalacia, BPD, ROP, and necrotizing enterocolitis. Additionally, charts were reviewed to determine if the neonate had ≥1 documented (culture-proven) episode(s) of sepsis during their hospitalization.10
Trained pediatricians or pediatric neurologists also evaluated those children who survived to age 2 years. Each child was assessed for the presence of cerebral palsy. When cerebral palsy was diagnosed, the Gross Motor Function Classification System was used to assess severity. Additionally, each child was evaluated with the Bayley II Scales of Infant Development Mental Development Index (MDI) and Psychomotor Development Index (PDI). We defined childhood neuro-developmental impairment as a diagnosis of moderate or severe cerebral palsy and/or Bayley MDI and/or PDI scores >2 SD below the mean. Babies who survived to initial hospital discharge but died prior to 2-year follow-up were considered to have met the primary adverse childhood outcome.
Demographics, pregnancy characteristics, and neonatal courses were compared between children with and without neurodevelopmental impairment at age 2 years. These univariate analyses were conducted using Student t test and χ2 as appropriate. Next, data were analyzed by multiple logistic regression models and area under receiver operating characteristic curves (AUC). Separate regression models were created for each individual neonatal diagnosis and combinations of 2, 3, and 4 neonatal diagnoses and correlated with childhood neurodevelopmental impairment. Other covariables included in each regression model were gestational age at delivery, maternal education, maternal race/ethnicity, maternal use of tobacco, alcohol, and/or drugs during pregnancy, treatment group assignment (magnesium sulfate vs placebo), fetal sex, and chorioamnionitis. For each model, the odds of neurodevelopmental impairment were calculated for infants with neonatal morbidities compared to infants without neonatal morbidities. A limited subgroup analysis was also performed for neonates delivered <28 weeks’ gestation and for twins. Data were analyzed using software (Stata, version 12.1; StataCorp, College Station, TX).
Results
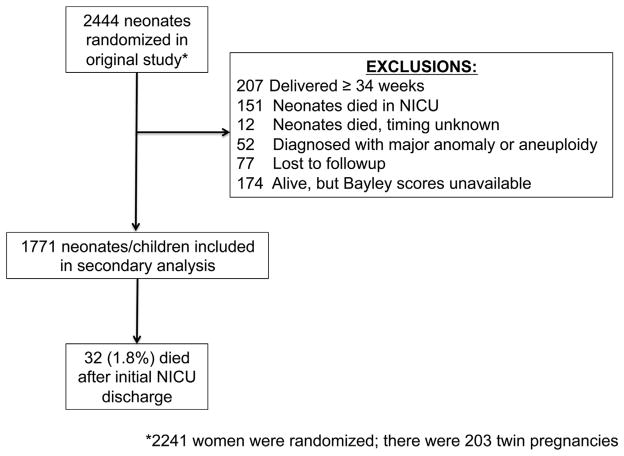
Of 2444 neonates randomized in the original study, 1771 neonates/children met inclusion criteria for this secondary analysis (Figure). Of these, 32 (1.8%) died during the follow-up period between initial hospital discharge and 2-year-old evaluation. The last childhood evaluation occurred at a mean 28.6 months of age, corrected for prematurity (range, 22.5–44.5 months). In total, 459 (25.9%) children (including the 32 deceased) met criteria for neuro-developmental impairment at age 2 years. Demographics and pregnancy characteristics are shown in Table 1. Delivery characteristics and initial neonatal outcomes are shown in Table 2. Children with neuro-developmental impairment were smaller and were enrolled and subsequently delivered, on average, approximately 1 week earlier than those without neuro-developmental impairment.
FIGURE. Eligibility and inclusion in current study.
NICU, neonatal intensive care unit.
TABLE 1.
Demographic and pregnancy characteristics
| Characteristic | Neurodevelopmental impairment at age 2 y, n = 459 | No neurodevelopmental impairment at age 2 y, n = 1312 | P value |
|---|---|---|---|
| Maternal age, y | 26.3 ± 6.5a | 26.4 ± 6.1a | .848 |
| Maternal prepregnancy body mass index, kg/m2 | 27.2 ± 7.2a | 26.1 ± 6.4a | .003 |
| Married | 212 (46.3) | 682 (52.0) | .035 |
| Maternal race or ethnic group | < .001 | ||
| Black | 217 (47.3) | 555 (42.3) | |
| White | 137 (29.9) | 527 (40.2) | |
| Hispanic | 96 (20.9) | 202 (15.4) | |
| Other | 9 (2.0) | 28 (2.1) | |
| Maternal education–highest level completed | 11.5 ± 2.6a | 12.1 ± 2.6a | < .001 |
| Prior preterm delivery | 124 (27.0) | 328 (25.0) | .394 |
| No prenatal care | 29 (6.3) | 84 (6.4) | .949 |
| Smoking during pregnancy | 135 (29.4) | 326 (24.9) | .055 |
| Alcohol use during pregnancy | 47 (10.2) | 103 (7.9) | .114 |
| Illicit drug use during pregnancy | 56 (12.2) | 114 (8.7) | .028 |
| Gestational age at randomization, wk | 27.6 ± 2.5a | 28.6 ± 2.3a | <.001 |
| Randomized to magnesium sulfate | 213 (46.4) | 641 (48.9) | .366 |
| Preterm premature rupture of membranes | 394 (85.8) | 1152 (87.8) | .276 |
| Product of a twin gestation | 82 (17.9) | 220 (16.8) | .591 |
Data are compared between those diagnosed with and without neurodevelopmental impairment at age 2 y. Unless otherwise specified, data are listed as n (%).
SD (the preceding value is the mean).
TABLE 2.
Delivery characteristics and initial neonatal outcomes
| Characteristic | Neurodevelopmental impairment at age 2 y, n = 459 | No neurodevelopmental impairment at age 2 y, n = 1312 | P value | Unadjusted OR for neurodevelopmental impairment (95% CI) |
|---|---|---|---|---|
| Gestational age at delivery, wk | 28.6 ± 2.6a | 29.7 ± 2.3a | < .001 | – |
| Received antenatal corticosteroids | 445 (97.0) | 1280 (97.6) | .479 | 0.79 (0.42–1.50) |
| Chorioamnionitis | 62 (13.5) | 161 (12.3) | .492 | 1.12 (0.82–1.53) |
| Cesarean delivery | 200 (43.6) | 523 (39.9) | .164 | 1.16 (0.94–1.44) |
| Male fetus | 270 (58.8) | 659 (50.2) | .002 | 1.42 (1.13–1.76) |
| Birthweight, g | 1199 ± 407a | 1407 ± 438a | < .001 | – |
| Culture-proven sepsis | 106 (23.1) | 176 (13.4) | < .001 | 1.94 (1.48–2.54) |
| Any necrotizing enterocolitis | 53 (11.6) | 80 (6.10) | < .001 | 2.01 (1.40–2.90) |
| Severe (stage II/III) necrotizing enterocolitis | 24 (5.2) | 33 (2.5) | .005 | 2.14 (1.25–3.66) |
| Retinopathy of prematurity | 178 (38.8) | 275 (21.0) | < .001 | 2.39 (1.90–3.01) |
| Bronchopulmonary dysplasia | 136 (29.6) | 189 (14.4) | < .001 | 2.50 (1.94–3.22) |
| Neonatal seizures | 15 (3.3) | 9 (0.70) | < .001 | 4.89 (2.12–11.25) |
| Any grade intraventricular hemorrhage and/or periventricular leukomalacia | 118 (25.7) | 252 (19.2) | .003 | 1.46 (1.13–1.87) |
| Grade III or IV intraventricular hemorrhage and/or periventricular leukomalacia | 38 (8.3) | 25 (1.9) | < .001 | 4.64 (2.77–7.79) |
Data are compared between those with and without neurodevelopmental impairment at age 2 y. Unless otherwise specified, data are listed as n (%).
CI, confidence interval; OR, odds ratio.
SD (the preceding value is the mean).
In all, 898 infants (50.7%) were discharged from the neonatal intensive care unit (NICU) without any major neonatal morbidities (no diagnoses of necrotizing enterocolitis, intraventricular hemorrhage, periventricular leukomalacia, seizures, ROP, or BPD). Of these, 174/898 (19.4%) were ultimately diagnosed with neurodevelopmental impairment at age 2 years, compared to 285/873 (32.7%) of those who had ≥1 adverse neonatal outcomes (P <.001). In univariate analyses, those with neurodevelopmental impairment were more likely to be diagnosed with culture-proven neonatal sepsis, BPD, any ROP, necrotizing enterocolitis and severe (stage II or III) necrotizing enterocolitis, any grade intraventricular hemorrhage and/or periventricular leukomalacia, neonatal seizures, and severe intraventricular hemorrhage and/or periventricular leukomalacia in unadjusted models (Table 2). When adjusting for confounders including gestational age at delivery; maternal education; maternal race; maternal use of tobacco, alcohol, and/or drugs during pregnancy; treatment group assignment (magnesium sulfate vs placebo); fetal sex; and chorioamnionitis, each individual neonatal morbidity with the exception of culture-proven neonatal sepsis, severe necrotizing enterocolitis, and any grade intraventricular hemorrhage remained associated with adverse neurodevelopmental outcomes at age 2 years (Table 3).
TABLE 3.
Relationship between individual neonatal morbidities and early childhood neurodevelopmental impairment
| Individual neonatal morbidity | n (%) | Adjusted OR for neurodevelopmental impairment (95% CI) | AUC (95% CI) |
|---|---|---|---|
| Culture-proven sepsis | 282 (15.9) | 1.27 (0.94–1.72) | 0.66 (0.63–0.69) |
| Any necrotizing enterocolitis | 133 (7.5) | 1.61 (1.10–2.35) | 0.66 (0.63–0.69) |
| Severe (stage II/III) necrotizing enterocolitis | 57 (3.2) | 1.55 (0.89–2.69) | 0.66 (0.63–0.69) |
| Retinopathy of prematurity | 453 (25.6) | 1.48 (1.10–2.00) | 0.66 (0.63–0.69) |
| Bronchopulmonary dysplasia | 325 (18.4) | 1.58 (1.14–2.17) | 0.66 (0.63–0.70) |
| Any grade intraventricular hemorrhage and/or periventricular leukomalacia | 370 (20.9) | 1.24 (0.96–1.62) | 0.66 (0.63–0.69) |
| Neurologic injury: diagnosis of grade III or IV intraventricular hemorrhage and/or periventricular leukomalacia and/or neonatal seizures | 81 (4.6) | 3.91 (2.41–6.34) | 0.68 (0.65–0.71) |
The number of babies diagnosed with each neonatal morbidity listed as n (%). All models controlled for gestational age; chorioamnionitis; maternal education; maternal race; maternal use of tobacco, alcohol, and/or drugs during pregnancy; fetal sex; and treatment group assignment (magnesium sulfate vs placebo).
AUC, area under receiver operating characteristic curve; CI, confidence interval; OR, odds ratio.
Next, we examined the correlation between specific combinations of 2, 3, and 4 neonatal diagnoses and adverse early childhood neurodevelopment (Table 4). The majority of combinations significantly increased the odds of neurodevelopmental impairment, but the predictive value remained fair with AUC values in the range of 0.61–0.68. Overall, the combination of BPD and ROP was the most predictive, with an adjusted odds ratio of 2.89 (95% confidence interval, 1.75–4.79) and AUC of 0.68 (95% confidence interval, 0.64–0.72). However, this was only marginally better than a large number of other combinations.
TABLE 4.
Relationship between combinations of neonatal morbidities and early childhood neurodevelopmental impairment
| Neonatal morbidities | n (%) | Adjusted OR (95% CI) | AUC (95% CI) |
|---|---|---|---|
| 2 diagnoses | |||
| BPD + sepsis | 129 (7.3) | 3.02 (1.71–5.32) | 0.65 (0.61–0.69) |
| BPD + any NEC | 47 (2.7) | 3.34 (1.58–7.06) | 0.63 (0.58–0.67) |
| BPD + severe (stage II/III) NEC | 23 (1.3) | 2.21 (0.83–5.88) | 0.61 (0.56–0.66) |
| BPD + any grade IVH and/or PVL | 111 (6.3) | 3.45 (1.96–6.06) | 0.65 (0.61–0.70) |
| BPD + grade III/IV IVH and/or PVL and/or neonatal seizures | 81 (4.6) | 7.52 (1.26–44.9) | 0.61 (0.57–0.66) |
| BPD + ROP | 235 (13.3) | 2.89 (1.75–4.79) | 0.68 (0.64–0.72) |
| Sepsis + any NEC | 54 (3.1) | 2.79 (1.46–5.35) | 0.63 (0.58–0.67) |
| Sepsis + severe (stage II/III) NEC | 29 (1.6) | 2.41 (1.05–5.53) | 0.62 (0.57–0.66) |
| Sepsis + any grade IVH and/or PVL | 80 (4.5) | 2.76 (1.50–5.06) | 0.63 (0.59–0.68) |
| Sepsis + grade III/IV IVH and/or PVL and/or neonatal seizures | 27 (1.5) | 5.78 (2.33–14.34) | 0.63 (0.58–0.67) |
| Sepsis + ROP | 164 (9.3) | 2.53 (1.48–4.33) | 0.66 (0.61–0.70) |
| Any NEC + any grade IVH and/or PVL | 20 (1.4) | 3.97 (1.76–8.97) | 0.62 (0.58–0.67) |
| Any NEC + grade III/IV IVH and/or PVL and/or neonatal seizures | 13 (0.7) | 14.2 (3.54–56.6) | 0.62 (0.58–0.67) |
| Any NEC + ROP | 53 (3.0) | 3.90 (1.91–7.98) | 0.63 (0.59–0.68) |
| Severe (stage II/III) NEC + any grade IVH and/or PVL | 13 (0.7) | 2.81 (0.88–9.01) | 0.61 (0.56–0.66) |
| Severe (stage II/III) NEC + grade III/IV IVH and/or PVL and/or neonatal seizures | 7 (0.4) | 10.01 (1.76–56.8) | 0.61 (0.56–0.66) |
| ROP + severe (stage II/III) NEC | 26 (1.5) | 2.93 (1.18–7.27) | 0.62 (0.57–0.66) |
| ROP + any grade IVH and/or PVL | 131 (7.4) | 3.68 (2.16–6.27) | 0.66 (0.62–0.70) |
| ROP + grade III/IV IVH and/or PVL and/or neonatal seizures | 47 (2.7) | 8.28 (3.83–17.9) | 0.65 (0.61–0.70) |
| 3 diagnosesa | |||
| BPD + sepsis + any NEC | 24 (1.4) | 2.20 (0.81–5.98) | 0.61 (0.56–0.65) |
| BPD + sepsis + any grade IVH and/or PVL | 49 (2.8) | 3.90 (1.86–8.19) | 0.63 (0.59–0.68) |
| BPD + sepsis + ROP | 100 (5.7) | 3.93 (2.08–7.42) | 0.66 (0.61–0.70) |
| BPD + any NEC + any grade IVH and/or PVL | 11 (0.8) | 7.08 (1.84–27.3) | 0.61 (0.56–0.67) |
| BPD + any NEC + ROP | 37 (2.1) | 5.44 (2.34–12.62) | 0.63 (0.59–0.68) |
| BPD + any grade IVH and/or PVL + ROP | 84 (4.7) | 4.56 (2.38–8.73) | 0.66 (0.61–0.70) |
| Sepsis + any grade IVH and/or PVL + ROP | 61 (3.4) | 3.57 (1.78–7.17) | 0.64 (0.59–0.68) |
| Sepsis + any NEC + any grade IVH and/or PVL | 12 (0.7) | 3.17 (0.95–10.6) | 0.61 (0.57–0.66) |
| Sepsis + any NEC + ROP | 28 (1.6) | 3.18 (1.27–7.94) | 0.61 (0.57–0.66) |
| Any NEC + any grade IVH and/or PVL + ROP | 14 (0.8) | 11.0 (3.05–39.6) | 0.62 (0.58–0.67) |
| 4 diagnosesa | |||
| BPD + sepsis + any NEC + any grade IVH and/or PVL | 7 (0.4) | 2.81 (0.58–13.8) | 0.61 (0.56–0.65) |
| BPD + sepsis + any NEC + ROP | 19 (1.1) | 3.50 (1.18–10.4) | 0.61 (0.56–0.66) |
| BPD + sepsis + any grade IVH and/or PVL + ROP | 42 (2.4) | 5.19 (2.31–11.7) | 0.64 (0.59–0.68) |
| Sepsis + any NEC + any grade IVH and/or PVL + ROP | 7 (0.4) | 4.92 (0.99–24.3) | 0.61 (0.56–0.66) |
| BPD + any NEC + any grade IVH and/or PVL + ROP | 13 (0.7) | 10.2 (2.79–37.7) | 0.62 (0.57–0.67) |
All models controlled for gestational age; chorioamnionitis; maternal education; maternal race; maternal use of tobacco, alcohol, and/or drugs during pregnancy; fetal sex; and treatment group assignment (magnesium sulfate vs placebo).
AUC, area under receiver operating characteristic curve; BPD, bronchopulmonary dysplasia; CI, confidence interval; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; OR, odds ratio; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity.
Due to small numbers, severities of NEC and IVH are not listed for 3 and 4 diagnoses.
We also examined the correlation between the absolute number of neonatal diagnoses and adverse childhood neurodevelopment; these results are shown in Table 5. We observed an increased risk of neurodevelopmental impairment with each additional neonatal diagnosis, for up to 4 neonatal diagnoses.
TABLE 5.
Relationship between number of neonatal morbidities and early childhood neurodevelopmental impairment
| No. of neonatal morbidities | n (%) | Severe neurodevelopmental impairment at age 2 y, n (%) | Adjusted OR for neurodevelopmental impairment (95% CI) | AUC (95% CI) |
|---|---|---|---|---|
| None | 898 (50.7) | 174 (19.4) | 0.75 (0.58–0.98) | 0.66 (0.63–0.69) |
| Any 1 neonatal morbidity | 449 (25.3) | 107 (23.8) | 1.15 (0.85–1.56) | 0.62 (0.58–0.66) |
| Any 2 neonatal morbidities | 228 (12.9) | 89 (39.0) | 2.20 (1.44–3.34) | 0.65 (0.61–0.69) |
| Any 3 neonatal morbidities | 132 (7.5) | 53 (40.2) | 2.41 (1.41–4.13) | 0.64 (0.60–0.69) |
| Any 4 neonatal morbidities | 58 (3.3) | 33 (56.9) | 5.44 (2.62–11.3) | 0.65 (0.60–0.69) |
| All 5 neonatal morbidities | 6 (0.34) | 3 (50) | 4.03 (0.74–22.1) | 0.61 (0.56–0.65) |
All models controlled for gestational age; chorioamnionitis; maternal education; maternal race; maternal use of tobacco, alcohol, and/or drugs during pregnancy; fetal sex; and treatment group assignment (magnesium sulfate vs placebo).
AUC, area under receiver operating characteristic curve; CI, confidence interval; OR, odds ratio.
In all, 506 babies (28.6%) were delivered <28.0 weeks’ gestation. As expected, these very premature neonates were more likely to be diagnosed with ≥1 neonatal morbidities (444/506, 87.8% vs 429/1265, 33.9%, of babies delivered 28.0–33.9 weeks, P ≤ .001), and more likely to have adverse child neurodevelopmental outcomes (199/506, 39.3% vs 260/1265, 20.6%, P < .001). There was a moderate increase in the correlation between neonatal and neuro-developmental impairment among this subset compared to the whole group, with AUC values ranging from 0.64–0.77 (Table 6).
TABLE 6.
Neonatal morbidities and neurodevelopment impairment for neonates delivered <28 weeks’ gestation
| Neonatal morbidity/morbidities | n (%) | Adjusted OR (95% CI) | AUC (95% CI) |
|---|---|---|---|
| None of the listed diagnoses | 62 (12.3) | 0.52 (0.27–0.98) | 0.64 (0.59–0.69) |
| Any 1 diagnosis | 131 (25.9) | 1.41 (0.68–2.95) | 0.66 (0.58–0.74) |
| Best 1 diagnosis: grade III/IV IVH and/or PVL and/or neonatal seizures | 55 (10.9) | 3.27 (1.78–6.03) | 0.67 (0.62–0.72) |
| Any 2 diagnoses | 150 (29.6) | 2.21 (1.07–4.53) | 0.66 (0.58–0.73) |
| Best 2 diagnoses: ROP + grade III/IV IVH and/or PVL and/or neonatal seizures | 39 (7.7) | 9.40 (2.51–35.2) | 0.77 (0.68–0.86) |
| Any 3 diagnoses | 103 (20.4) | 2.65 (1.14–6.17) | 0.69 (0.60–0.77) |
| Best 3 diagnoses: any grade IVH and/or PVL + any NEC + ROP | 13 (2.6) | 15.5 (2.68–89.5) | 0.75 (0.63–0.87) |
| Any 4 diagnoses | 54 (10.7) | 4.92 (1.66–14.6) | 0.74 (0.64–0.83) |
| Best 4 diagnoses: BPD + sepsis + any grade IVH and/or PVL + ROP | 40 (7.9) | 3.28 (0.95–11.3) | 0.71 (0.61–0.82) |
| All 5 diagnoses | 6 (1.2) | 5.52 (0.68–45.0) | 0.67 (0.53–0.82) |
The probability of neurodevelopmental impairment at age 2 y is given based on the number of neonatal diagnoses and from the best model (highest AUC) of specific individual and combined neonatal diagnosis. All models controlled for delivery gestational age; chorioamnionitis; maternal education; maternal race; maternal use of tobacco, alcohol, and/or drugs during pregnancy; fetal sex; and treatment group assignment (magnesium sulfate vs placebo).
AUC, area under receiver operating characteristic curve; BPD, bronchopulmonary dysplasia; CI, confidence interval; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; OR, odds ratio; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity.
In all, 302 babies (17.1%) were products of a twin gestation. Overall, rates of neurodevelopmental impairment at age 2 years were similar between twins and singletons (Table 1). In a limited subgroup analysis, the predictive value of the number of neonatal diagnoses in determining neurodevelopmental outcomes in twins was similar to the overall group analysis (Table 7). Unfortunately, we were unable to examine specific combinations of morbidities for the subset of twins due to the relatively small sample size.
TABLE 7.
Neonatal morbidities and neurodevelopment impairment for neonates from twin gestation
| Neonatal morbidity/morbidities | n (%) | Adjusted OR (95% CI) | AUC (95% CI) |
|---|---|---|---|
| None of the listed diagnoses | 156 (51.7) | 0.54 (0.28–1.01) | 0.66 (0.59–0.73) |
| Any 1 diagnosis | 76 (32.8) | 1.63 (0.76–3.54) | 0.69 (0.62–0.76) |
| Any 2 diagnoses | 35 (18.3) | 3.50 (1.13–10.8) | 0.67 (0.58–0.75) |
| Any 3 diagnoses | 25 (13.8) | 5.55 (1.44–21.3) | 0.72 (0.63–0.81) |
| Any 4 diagnoses | 8 (4.9) | 8.19 (1.10–61.1) | 0.68 (0.59–0.77) |
| All 5 diagnoses | 2 (1.3) | 13.3 (0.46–389) | 0.68 (0.58–0.77) |
The probability of neurodevelopmental impairment at age 2 y is given based on the number of neonatal diagnoses. All models controlled for delivery gestational age; chorioamnionitis; maternal education; maternal race; maternal use of tobacco, alcohol, and/or drugs during pregnancy; fetal sex; and treatment group assignment (magnesium sulfate vs placebo).
AUC, area under receiver operating characteristic curve; CI, confidence interval; OR, odds ratio.
Comment
Approximately 1 in 4 children delivered preterm <34 weeks’ gestation were diagnosed with severe childhood morbidity at age 2 years. We found that individual neonatal morbidities (including BPD, necrotizing enterocolitis, sepsis, severe intraventricular hemorrhage, neonatal seizures, and/or periventricular leukomalacia) had only modest predictive value for subsequent adverse early childhood neurodevelopmental impairment. However, the presence of ≥1 neonatal diagnoses conferred a nearly 1 in 3 chance of neurodevelopmental impairment at age 2 years. In contrast, although babies discharged from the NICU without any major diagnoses did not necessarily remain “healthy” at age 2 years, they had a significantly reduced risk of neurodevelopmental impairment (19.4% vs 32.7%). In general, the diagnosis of neurologic abnormalities during the neonatal period, in particular grade III/IV intraventricular hemorrhage, periventricular leukomalacia, and/or neonatal seizures, alone or in combination with other diagnoses conferred the highest odds of adverse childhood outcomes.
Of the diagnoses studied, combinations of 2, 3, and 4 neonatal morbidities were similar in prognostic value with similar AUC values to individual morbidities. These results remained consistent when the earliest subset of babies was examined separately. The combined neonatal morbidity data, particularly when >2 specific diagnoses are considered, are limited by the small sample size and small numbers of babies with these combinations of multiple diagnoses. We attempted to address this issue by also separately examining the relationship between the absolute number of diagnoses (regardless of which specific ones) and outcomes (Table 4). Although there was a higher chance of adverse childhood neurodevelopmental outcomes with each additional neonatal diagnosis through 4 total diagnoses, the majority of babies with multiple neonatal diagnoses did not meet criteria for severe neurodevelopmental impairment at age 2 years.
These data are useful when counseling patients regarding anticipated long-term outcomes following premature delivery. Although there was a definite relationship between the number of neonatal diagnoses and neurodevelopmental impairment in early childhood, this correlation was imperfect, and remained imperfect even among the highest-risk subset, those delivered <28 weeks’ gestation. Importantly, discharge from the NICU without major morbidity did not ensure normal neurodevelopment at age 2 years. Nearly 1 in 5 infants without evidence of BPD, intracranial hemorrhage, periventricular leukomalacia, sepsis, necrotizing enterocolitis, or ROP during their initial hospital stay (discharged “healthy”) ultimately had moderate or severe cerebral palsy and/or Bayley MDI and/or PDI scores >2 SD below the mean when evaluated at age 2 years. In contrast, >40% of infants carrying up to 4 neonatal diagnoses did not have adverse outcomes at age 2 years.
Our study had several strengths. This was a large, prospectively collected cohort. All children were evaluated in a standardized fashion by trained research nurses and physicians. Outcomes were determined, in part, by using an objective, previously validated assessment tool (Bayley II Scales of Infant Development). Other outcomes were obtained by trained pediatricians. We had comprehensive pregnancy information, combined with neonatal outcomes and early childhood outcomes, and were able to incorporate these data into the analyses. We also had data on twin gestations, and did not observe a difference in rates of neurodevelopmental impairment between singleton and twins. Babies delivered <28 weeks’ gestation are most likely to experience adverse neonatal and childhood outcomes and therefore have been the focus of many recent outcome-based studies in neonatal medicine. However, while the focus of obstetric research and intervention has been on this extremely premature subset, these data again confirm that adverse outcomes remain widespread across a broad spectrum of preterm gestational ages.
This study is not without limitations. Due to the enrollment criteria for the primary study, the vast majority of children were delivered following pre-term premature rupture of membranes. It is unclear if the relationships between neonatal and childhood morbidity vary by the indication for the preterm birth; unfortunately we are unable to address this issue in the current study. However, previous studies of outcomes stratified for preterm birth indication suggest similar outcomes regardless of reason for early delivery.12,13 Children were evaluated using the Bayley II Scale, known to have consistently higher rates of neurodevelopmental impairment compared to the current Bayley III Scale.14 However, we would not anticipate a difference in the correlation between neonatal outcomes and early childhood neurodevelopmental impairment as testing results would be consistently and proportionally lower with the Bayley III Scale. Additionally, we were limited in our ability to assess the correlation between several of the most severe outcomes and combinations of these due to small numbers. Lastly, there may be additional, more predictive or highly correlative neonatal variables. Because this was a secondary analysis, we were limited to the data and variables previously collected; for example, we did not have information on the severity of ROP, and did not have information regarding visual acuity at the time of early childhood follow-up.
In conclusion, in this large, prospectively enrolled cohort of preterm infants initially presenting <32 weeks’ gestation and delivering <34 weeks’ gestation, the relationship between neonatal diagnoses at the time of initial hospital discharge and severe neurodevelopmental outcomes in early childhood was limited. Neither the combination of multiple neonatal diagnoses nor the absolute number of neonatal diagnoses improved the prediction of early childhood neurodevelopmental impairment. Of preterm infants, >1 in 4 were diagnosed with adverse neurodevelopmental outcomes in early childhood. These data are useful in counseling patients, both those with infants receiving ≥1 neonatal diagnoses, and those whose babies are considered “healthy” at the time of discharge from the NICU. Early childhood evaluation and/or intervention should not be withheld from seemingly “healthy” previous preterm children. Additionally, obstetric and pediatric researchers and clinicians should keep these data in mind when designing outcome and/or intervention studies or determining eligibility criteria for early childhood intervention.
Acknowledgments
The authors acknowledge the assistance of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Maternal-Fetal Medicine Units Network, and the BEAM Protocol Subcommittee in making the database available for these analyses.
Footnotes
The authors report no conflict of interest.
The contents of this report represent the views of the authors and do not represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network or the National Institutes of Health.
Presented, in part, in oral format at the 34th annual meeting of the Society for Maternal-Fetal Medicine, New Orleans, LA, Feb. 3–8, 2014.
Reprints not available from the authors.
References
- 1.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 2.EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS) Acta Paediatr. 2010;99:978–92. doi: 10.1111/j.1651-2227.2010.01846.x. [DOI] [PubMed] [Google Scholar]
- 3.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996; NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 4.de Kleine MJ, den Ouden AL, Kollee LA, et al. Lower mortality but higher neonatal morbidity over a decade in very preterm infants. Paediatr Perinat Epidemiol. 2007;21:15–25. doi: 10.1111/j.1365-3016.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaucher YE, Peralta-Carcelen M, Finer NN, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367:2495–504. doi: 10.1056/NEJMoa1208506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hintz SR, Kendrick DE, Wilson-Costello DE, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics. 2011;127:62–70. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne AH, Hintz SR, Hibbs AM, et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 2013;167:451–9. doi: 10.1001/jamapediatrics.2013.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teune MJ, van Wassenaer AG, van Dommelen P, Mol BW, Opmeer BC. Perinatal risk indicators for long-term neurological morbidity among preterm neonates. Am J Obstet Gynecol. 2011;204:396, e1–14. doi: 10.1016/j.ajog.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–9. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 10.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 12.Kimberlin DF, Hauth JC, Owen J, et al. Indicated versus spontaneous preterm delivery: an evaluation of neonatal morbidity among infants weighing </=1000 grams at birth. Am J Obstet Gynecol. 1999;180:683–9. doi: 10.1016/s0002-9378(99)70273-5. [DOI] [PubMed] [Google Scholar]
- 13.Bottoms SF, Paul RH, Mercer BM, et al. Obstetric determinants of neonatal survival: antenatal predictors of neonatal survival and morbidity in extremely low birth weight infants. Am J Obstet Gynecol. 1999;180:665–9. doi: 10.1016/s0002-9378(99)70270-x. [DOI] [PubMed] [Google Scholar]
- 14.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161:222–228. e3. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]