Abstract
A number of agents are used clinically to enhance the efficacy of radiotherapy today, many of which are cytotoxic chemotherapies. Agents that enhance radiation induced tumor cell killing or protect normal tissues from the deleterious effects of ionizing radiation are collectively termed radiation modifiers. A significant effort in radiobiological research is geared towards describing and testing radiation modifiers with the intent of enhancing the therapeutic effects of radiation while minimizing normal tissue toxicity. In this review, we discuss the characteristics of these agents, the testing required to translate these agents into clinical trials, and highlight some challenges in these efforts.
Introduction
Radiation is a local and regional therapeutic modality that is used in the palliative care and definitive management of approximately two thirds of cancer patients in the United States. Radiation therapy was one of the first effective therapeutic modalities for lymphomas and solid tumors (1-3). Although it is one of the oldest therapies for malignancies, the use of radiation has changed a great deal in regards to indications, dose, and delivery over the past several decades. Imaging modalities such as magnetic resonance imaging and positron emission tomography have improved the ability to delineate tumor within the body. Linear accelerators have allowed high energy beams with better penetration in tissue and thus improved homogeneity in tumor and reduced normal tissue doses. Novel radiation delivery techniques, such as intensity modulated radiation therapy, have allowed more conformal therapy. The use of daily imaging during treatment has reduced improved the accuracy of treatment and has reduced the margins of normal tissue that must be treated to adequately dose tumor. Collectively, these advances have led to an improved efficacy of radiation treatment and a major reduction in normal tissue exposure and injury.
Radiation is a unique modality in that the dose delivered to tumor is relatively uniform, typically within a few percent of the prescription dose. This is in contrast to chemotherapies and other medications prescribed for cancer, for which the dose delivered to tumor can be heterogeneous as a result of the unique aspects of tumor physiology, such as variations in the degree and functionality of tumor vasculature, elevated interstitial pressure, altered permeability, physiochemical properties of the drug, and target expression (4). One obvious limitation is that radiation is only effective when targeted to the appropriate location. Thus, the benefit of radiotherapy in that it is selectively targeted may also be seen as a negative.
Despite the ability of radiation to treat a tumor homogenously and conformally, the tolerance of normal tissues continues to limit the maximum dose that can safely be delivered. For some tumor types, there is some evidence that radiation dose escalation can improve the efficacy of radiotherapy (5-7), but at a potential cost of increased toxicity of normal tissues. Thus, in many circumstances, the efficacy of radiation continues to be limited by the tolerance of normal tissues adjacent to the tumor.
The importance of local control
In cancer therapy, the primary endpoint for many trials is overall survival. Although local therapies, such as surgery and radiotherapy, are capable in some instances of improving overall survival (8-10), the primary rational for their use in the definitive treatment of cancer is often to enhance the control of disease at the site of the primary. Frequently, a debate arises over the appropriateness of recommending local therapies that do not extend survival, especially when those therapies have real and measureable toxicities. The primary rationale for the development of radiation modifiers is the belief that local and regional control is an important oncologic endpoint. Perhaps the most important question to ask in this regard is “What is the benefit of local and regional control on survival?”
An excellent example to explore this question is breast cancer. In the 1880's Dr. William Halstead developed the radical mastectomy, guided by his belief that breast cancer could be cured by an aggressive regional treatment. Indeed, the radical surgery was effective and rendering a number of women disease free and became the standard treatment. In the 1950's this dogma was questioned, eventually leading to the comparison of radical mastectomy with a more limited surgery and post-mastectomy radiotherapy by the NSABP (11). Indeed, the outcomes were similar for these approaches, but distant failure was the most common site of failure, occurring in nearly 30% of these women. As chemotherapy regimens advanced and were able to more effectively sterilize distant disease, local control took on increasing importance. (12) The importance of local control became evident when the Early Breast Cancer Trialists’ Collaborative Group published updated data regarding breast conserving therapy and radiotherapy in patients with early stage breast cancer (8). This metaanalysis further showed an improvement in breast cancer specific survival at late time points after adjuvant locoregional radiotherapy in patients with positive or negative lymph nodes. These data suggested that avoidance of 4 local failures at 5 years resulted in prevention of one breast cancer death at 15 years. Thus, these data support the concept that local and regional disease may act as a reservoir for distant metastases. Further, these data suggest that in the setting of systemic therapy that is increasingly capable of controlling distant disease, durable local control becomes increasingly important in the eventual survival of the patient. The paradox of local control is simply that, as systemic treatments improve gradually, local control will become increasingly more important to curative strategies.
A benefit in terms of overall survival is not the only reason to deliver a local therapy. Local recurrence may in some instances result in severe pain, obstruction, and decrement in quality of life without a loss in survival. Thus, although survival is often chosen as the most appropriate end point to determine if a therapy is needed, other considerations such as prevention of a morbid local recurrence can be factored into therapeutic decisions.
Finally, there are cancers for which metastasis is rare, such as glioblastoma multiforme, which more typically recurs locally despite aggressive local therapy. For diseases with a low rate of metastatic failure and a high rate of local failure, local control is of utmost importance. In these diseases, local failure often leads to death. Improving the local efficacy of radiotherapy in this setting would provide the opportunity to extend survival to a greater degree than in other diseases dominated by metastatic failure patterns.
The therapeutic ratio
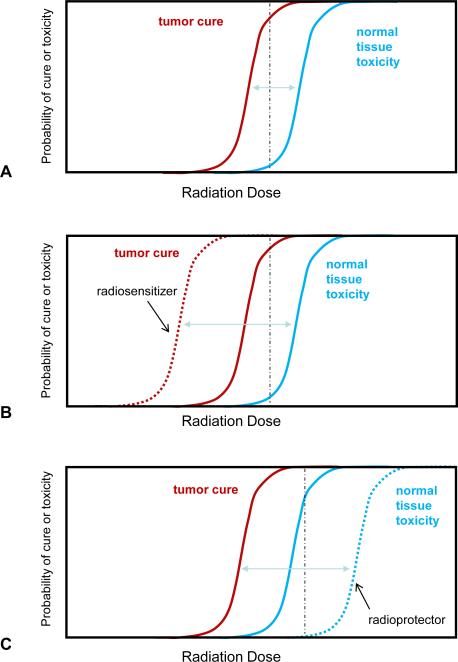
The therapeutic ratio is a concept well known to physicians who prescribe treatment. The therapeutic ratio is a comparison of the amount of a therapeutic agent that causes the desired therapeutic effect, in this case tumor control, to that amount which causes toxicity (Figure 1A). In radiation oncology as in other disciplines, treatment is prescribed at a dose that will limit moderate to severe toxicity to a tolerable level, typically less than a 5% risk of major toxicity, while achieving local control in a substantial percentage. For some more locally aggressive diseases, a higher rate of toxicity may be acceptable to obtain local control.
Figure 1. Therapeutic ratio and radiation modifiers.
A) The probability of tumor cure (red) and normal tissue toxicity (blue) can be plotted as a factor of radiation dose. Note that these are parallel idealized sigmoidal curves which may not be representative of the clinical condition. A therapeutic dose may be selected that delivers a high chance of tumor cure with an acceptable rate of toxicity (black dashed line). B) A tumor selective radiation sensitizer increases the probability of tumor cure for a given dose (red dashed line), hence the tumor control curve is shifted to the left. This results in a higher chance of cure for the same radiation dose as delivered in A) (black dashed line). Alternatively, a lower dose may be delivered to yield the same rate of cure with less normal tissue toxicity. C) A normal tissue radioprotector decreases the probability of normal tissue toxicity for a given dose of radiation, thus pushing the toxicity curve to the right (blue dashed line). This allows a higher dose to be given to obtain a higher rate of cure with less or equivalent normal tissue injury.
For many cancers, the potential toxicity of normal tissue limits the dose of radiotherapy that can safely be delivered to tumor. As a result, the rate of local control is considerably less than 100%. The explosion of new technologies and techniques that allow more conformal therapies and more accurate delivery have allowed dose escalation to tumor while minimizing normal tissue toxicity. However, the need to include regions of possible microscopic disease and the need for additional margin for account for setup error and motion of the target during the treatment limit the ability to completely exclude surrounding normal tissues from the prescription dose.
Radiation Modifiers
An alternative method to widen the therapeutic ratio for radiotherapy is the use of agents that selectively sensitize tumor to radiation or selectively protect normal tissues from the effects of radiation. Collectively, these agents are described as radiation modifiers, as they are capable of modifying tumor or normal tissue response to irradiation. Importantly, these agents must exhibit selectivity to be clinically useful as modifying the response of both tumor and normal tissue to the same degree offers no therapeutic gain.
Agents that enhance the ability of radiation to kill tumor cells are frequently described as radiation sensitizers (Figure 1B). By enhancing the ability of radiation to kill tumor cells, while not altering the radioresponse of normal tissues, these agents are able to broaden the therapeutic window, resulting in a higher chance of cure for an equivalent degree of toxicity. Depending on the degree of sensitization of tumor, dose reduction could be considered to minimize normal tissue toxicity while reducing normal tissue injury.
The term radiation sensitizer is used to describe agents that selectively enhance the cell killing from irradiation in tumor cells while exhibiting no single agent toxicity on tumor or normal tissues.(13) In practice, few agents have been described that meet these criteria, as most agents used in combination with radiotherapy in the clinic or in the laboratory setting have some toxicity as a single agent. Thus, the majority of agents used in this context clinically, such as cisplatin and 5-fluorouracil, are more accurately described as radiation modifiers, despite the common usage of the term “sensitizer.” A true sensitizer is synergistic with radiation: the combination has greater impact on tumor cell killing than the sum of its component parts.
Conversely, agents that protect normal tissues from the deleterious effects of radiation are described as radioprotectors (Figure 1C). By protecting the normal tissue from the deleterious effects of radiation, these agents broaden the therapeutic window (14). In the presence of a radioprotector, the radiation dose may be escalated to provide enhancement in local control, or a similar dose may be used with a reduction in the risk of normal tissue injury. Radiation protection can be achieved by “chemical radiation protectors,” which require high tissue concentrations of the agent immediately before and during the radiation exposure. For post-radiation exposure a variety of radiation mitigators are under evaluation to lessen radiation-induced normal tissue damage. (44)
The selectivity of radiation modifiers can be a result of a number of characteristics of the agent or its pharmacology. One possible method of selectivity is the rate or degree of uptake of the agent into tumor cells or normal tissues. This may be related to selective uptake, selective retention, or more rapid uptake, metabolism, or clearance in tumor versus normal tissue. Another possible mechanism of selectivity is the presence of the target of the modifier on only or at a higher level in tumor cells (for sensitizers) or normal tissues (for protectors).
Radiation Sensitizers
The vast majority of agents used clinically and tested in the laboratory are more appropriately termed radiation modifiers; however in clinical practice, they are often described as radiosensitizing. These agents may work in several ways to enhance the effectiveness of radiation. In some instances the agents directly enhance individual tumor cell radiosensitivity. Most radiation sensitizers in this class target the cell cycle, DNA repair, or pathways known to be involved in survival after irradiation. Alternatively, agents may take advantage of the unique aspects of tumor physiology to enhance the sensitivity of resistant tumor clones, such as by targeting vasculature or selectively sensitizing hypoxic cells. Other agents may have minimal radiation enhancing effects but may provide an enhancement of local control via a direct cytotoxic effect which in turn reduces the number of surviving tumor clones that require sterilization by radiation. These agents, although considered additive and not synergistic, may have therapeutic efficacy.
The distinction between additivity and synergy is an important concept. Agents that kill tumor cells without any alteration of the radiation response provide a therapeutic benefit by reducing the number of surviving tumor cells, but any efficacy is additive. These agents may be useful clinically if the toxicity of therapy is tolerable and if the additivity can lead to enhanced tumor control. In contrast, agents which enhance the efficacy of radiotherapy through synergy directly function to enhance the cytotoxicity of radiotherapy, thus providing a level of tumor cell killing superior to that which would be obtained with either agent given alone or sequentially.
Evaluating Radiation Sensitizers
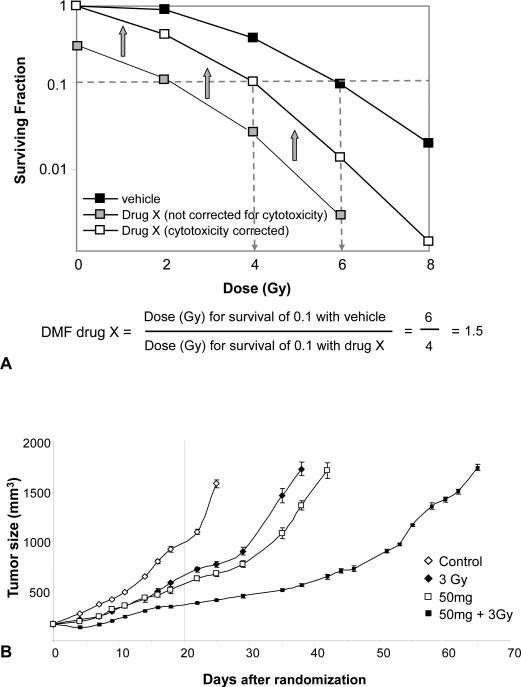
The preclinical and clinical evaluation of radiation sensitizers is tailored to the target of the drug, the chemical and pharmacokinetic and dynamic properties of the drug, and the anticipated clinical outlet. Preclinical assessment of an agent typically involves several steps including enhancement of radiation-induced cell killing, timing of drug administration in relation to radiation exposure, mechanistic studies, and finally in vivo assessment (xenografts). Preclinical evaluation of potential radiation sensitizers should include assessment of cell survival by use of clonogenic assays. The clonogenic assay is the gold standard for in vitro assessment of radiation modification, and directly assesses the ability of single cells to form macroscopic colonies.(15, 16) While automated cell assays such as the dye exclusion or MTT assays may be useful in large scale screening of agents, they should not be used as the primary means of assessing an agent for radiation modification.(17) As shown in Figure 2A, radiation survival curves obtained through at least 2 logs of survival allow for adequate assessment of potential radiation modifiers. Note that it is imperative to correct for the cytotoxicity of the drug alone. Omission of this correction can result in the appearance of synergy when none exists. Figure 2A also illustrates the concept of the radiation dose modification factor (DMF), defined by the ratio of radiation doses without and with the modifier at an iso-survival level (typically at 1 log of cell kill). Hence the DMF provides a measure of the treatment response over a range of doses as opposed to a modification factor for a single radiation dose. For the hypothetical sensitizer Drug X in Figure 2A, the DMF is 1.5, meaning that the addition of Drug X results in the radiation dose being equivalent to 1.5 fold the effect obtained with radiation alone. The DMF for different modifiers can be compared for different cell lines and conditions. Agents with larger DMFs are anticipated to deliver a greater degree of sensitization; however, this depends on the agent being studied as some agents might target vasculature or other physiologic processes that are inadequately modeled in vitro. Determining the DMF with regard to timing of the agent and radiation exposure can greatly facilitate translation to in vivo models as well as identifying if possible the mechanism of action and biomarkers that might inform whether the drug is actually achieving its potential in vivo.
Figure 2. Evaluation of radiation dose modification in vitro and in vivo.
A) Cells are plated at varying densities after exposure to Drug X or vehicle followed by exposure to radiation. Following incubation for 10-14 days, colonies of 50 or more cells are counted. The number of colonies that are counted is compared to the number of cells plated and a surviving fraction is generated. Plotting the surviving fraction over a range of radiation doses allows for the generation of a survival curve. The survival of the cell line when treated with vehicle and radiation is compared to that obtained when cells are treated with Drug X and radiation. The surviving fraction of the drug and radiation combination must include a correction for the cytotoxicity of the drug alone (open squares). If this correction is not made, a dose modifying factor cannot be calculated and the ability to sensitize or protect cannot be accurately determined. In this example, lack of correction for cytotoxicity (grey squares) appears to make the drug much more effective as a sensitizer than it actually is. B) A dose modifying factor may also be calculated for tumor regrowth. Mice are implanted with a tumor xenograft. Once tumors reach a measurable and consistent size, mice are randomized to treatment with vehicle, vehicle with radiation (in this example 3Gy), drug (in this example 50 mg of AZD6244), or radiation with drug. The dose modifying factor is calculated by determining the number of days it takes tumors in each group triple in volume. Comparing volumes at only one time point (dotted line at 20 days) does not allow a calculation of DMF and may over or underestimate the efficacy of the investigational agent. Figure 2B reproduced from (31) with permission.
Agents that have efficacy in clonogenic studies are typically further evaluated in xenograft studies. The two most common xenograft models used evaluate tumor cure (TCD50, tumor control dose in 50% of the mice) or tumor regrowth delay. Both single radiation doses or fractionated radiation delivery are used; however, most researchers favor fractionated radiation delivery as it is more relevant to the radiation clinic. Because of the high cost and long evaluation times, TCD50 studies are used less than regrowth delay studies.
To test a candidate radiation modifier, xenograft studies typically include at least 4 conditions: control, radiation with vehicle, radiation with the candidate drug, and the candidate drug alone. These studies compare the various groups by determining the length of time (days) it takes a tumor to grow to a pre-specified volume (typically at least a tripling in size of the tumor volume from the initiation of treatment) (Figure 2B). From these data, a DMF of the agent in vivo can be calculated. Comparing tumor volume at a single point is not sufficient to determine the degree of tumor growth delay and can inadvertently over or underestimate the degree of effect. For these studies, it is also critical that tumors are a similar size at the time of treatment as sensitivity and radiation can vary with tumor size due to hypoxia or other physiologic processes.(18)
Mechanisms of Radiation Sensitization
The vast majority of agents used clinically as radiation sensitizers are cytotoxic chemotherapies that independently induce DNA damage or inhibit DNA repair. DNA damage, particularly unrepaired DNA double strand breaks, are a potentially lethal event following exposure to ionizing radiation.(19) Agents that increase the extent of DNA damage or that inhibit DNA double strand break repair often sensitize tumor cells to irradiation.(20) An excellent example of a clinically useful modifier in this category is 5-Fluorouracil (5-FU). Although 5-FU may sensitize tumor cells to irradiation by several different mechanisms,(21) impaired DNA double strand break repair is a major contributing factor.(22) A number of agents that target DNA repair, such as PARP inhibitors and ATM/ATR inhibitors have been tested pre-clinically and are in varying stages of clinical development.(20, 23, 24)
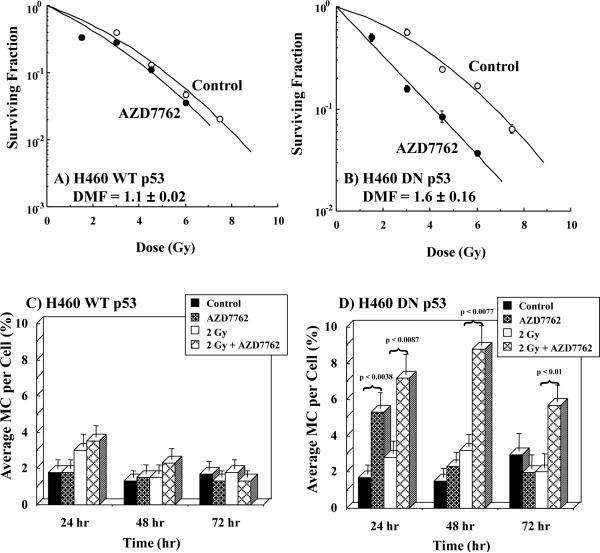
Aside from DNA damage, other aspects of tumor cell biology can alter radiation sensitivity. For example, the location of a tumor cell in the cell cycle can alter the sensitivity to radiation.(25) Cells in G1 and late S are relatively resistant to radiation compared to cells in G2 and M. This is likely partially due to the ability of cells in G1 and S to activate cell cycle checkpoints that allow for DNA repair. Thus, agents that redistribute tumor cells into a more sensitive phase of the cell cycle or that prevent checkpoint activation are capable of sensitizing cells to irradiation. An example of such an agent is AZD7762, and inhibitor of Chk1. This agent selectively sensitizes p53 mutant cells to radiation through abrogation of the G2 checkpoint after irradiation (Figure 3).(26) It is important to reiterate that agents targeting this and other pathways must have selective uptake, retention, or activity in regards to tumor versus normal tissues or no improvement in therapeutic ratio will be realized with their use.
Figure 3.
The effects of radiation with and without the Chk1/2 inhibitor AZD7762 (100 nM, added 1 hr prior to and 24 hr post-radiation) on radiation-induced cell killing and mitotic catastrophe. In this study the influence of p53 on Chk1/2 inhibition/radiation response was evaluated using a H460 lung cancer cell line (wild type p53) as opposed to H460 cells containing a dominant negative p53 construct (H460 DN p53). Wild type p53 cells (A & C) were not sensitized by the combination of AZD7762 and radiation; whereas, the radiosensitivity H460 DN p53 cells were significantly enhanced by AZD7762 (B,) as well as DNA damage as measured by mitotic catastrophe (D). (Adapted from reference (26) with permission)
A number of other signaling pathways are known to be activated after exposure to ionizing radiation. In some cases, basal activation of these pathways though mutation or amplification can result in a reduction in radioresposiveness. An example of such a pathway is the Ras-MAPK pathway, which is rapidly activated following ionizing radiation.(27, 28) Tumor cells expressing mutant conditionally active Ras are more resistant to radiation induced cell death.(29, 30) Targeting of these pathways, such as with MEK inhibition, is capable of sensitizing tumor cells in vitro and in vivo to radiation.(31, 32) An ever increasing number of modifiers that target such pro-survival pathways, such as EGFR, Akt, and mTOR, have been described.(24, 33) Indeed, EGFR inhibition has been shown to sensitize to irradiation both in vitro and in vivo (34, 35), and is the only molecularly targeted radiation sensitizing strategy to be successfully translated to the clinic with FDA approval.(36)
The majority of pre-clinical in vitro research on radiation modifiers and for that matter molecularly targeted agents use tumor cells in log phase growth maintained under ambient oxygen conditions (21%). While cycling tumor cells are an important component of solid tumors, many other sub-populations can be present in solid tumors whose response to radiation and radiation modifiers may differ from aerobic log phase cells. Abnormal vasculature in solid tumors can result in a complex local microenvironment consisting of reduced levels of oxygenation (hypoxia), areas of low nutrient status, and low pH.(37) Cells under hypoxic conditions (<0.5 % oxygen) have long been known to be resistant to radiation by a factor of 3.(38, 39) Hypoxia has also been shown to decrease the efficacy of cytotoxic chemotherapy agents.(40) The combination of reduced oxygen levels and low nutrient status can result in non-cycling cell populations (plateau phase, G0 arrested), whose response to cell-cycle specific agents combined with radiation can be compromised.(41) Further, low nutrient status and defects in metabolism can result in compromised production of ATP, needed to fuel DNA damage repair systems.(42) Lastly, genetic mutation status of specific driver genes has been shown to differ markedly when multiple biopsies are taken from the same tumor (43), potentially complicating the application of effective molecularly targeted therapy. Thus, the inherent heterogeneity of solid tumors across microenvironmental and genetic landscapes presents a formidable challenge to the identification of radiation modifiers. This reality speaks to the importance of including xenograft models in pre-clinical regrowth delay studies when evaluating radiation modifiers/sensitizers.
Radiation Protectors
Sensitizing tumor cells to irradiation is one possible method to enhance the therapeutic ratio. Another alternative is to prevent radiation injury in normal tissues. Improvements in radiation delivery and imaging technology have reduced the amount of normal tissues exposed to high dose radiation, however a margin of clinically uninvolved normal tissue is often treated to account for set up error and microscopic tumor extension that cannot be visualized with modern imaging technology. Thus, technological improvements can improve the therapeutic ratio only to a point, with residual normal tissue toxicity dominated by the effects of irradiation on the tissues included in this obligatory margin.
Radiation injury in normal tissues is highly specific to the location of exposure and can occur as either an acute injury and/or a late injury. Acute radiation injury occurs during or shortly after treatment, and typically involves rapidly dividing cells such as skin and mucosa. Examples of acute injury are dermatitis and mucositis, but these are temporary and not permanent. Late radiation injury occurs months to years after treatment. Examples of late radiation injury are myelopathy, fibrosis, fracture, and ulceration. These injuries can be permanent, progressive even fatal. These toxicities are typically manifestations of cell loss, tissue/organ atrophy, and sublethal injury to surviving cells.
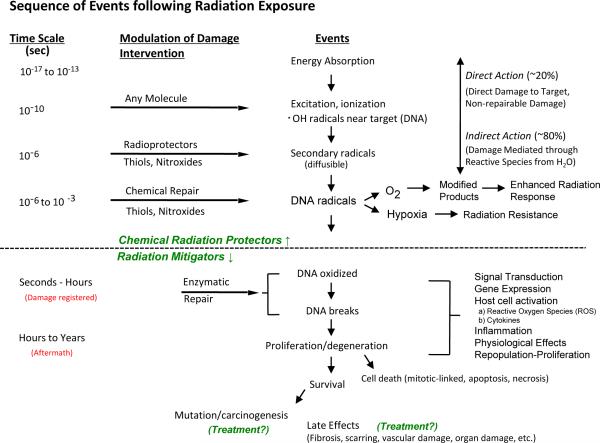
The DNA damage and free radical generation that result from irradiation occur rapidly after exposure, however the deleterious effects of this injury may not be manifest until months to years after treatment is completed (Figure 4). Agents that prevent the initial injury from occurring or reduce the extent of the injury are termed radioprotectors. Radioprotectors are expected to reduce both acute and late toxicities as they reduce the initial extent of normal tissue damage. Radiation mitigators are agents that function after the initial insult but prior to the manifestation of toxicity. These agents target the late toxicities of radiation exposure, such as fibrosis.(44) An example is delivery of bone morrow stromal cells six weeks after exposure to a fibrosing dose of radiation. These stromal cells are capable of reducing subsequent fibrosis.(45) Finally, agents that are aimed at reducing or reversing the injury after it is manifest are called treatment. An example of such an agent is pentoxifylline and Vitamin E which have been used in combination to effectively treat cutaneous radiation fibrosis.(46)
Figure 4. Sequence of events following radiation exposure.
The chart is divided into three parts by dashed lines suggesting events and reactions that might be modified by radiation protectors (top), radiation mitigators, and treatment (bottom). Reproduced from (14).
As described above, radiation protectors are agents that prevent the initial injury caused by radiation exposure. The vast majority of these agents prevent DNA damage by scavenging free radicals, the molecules that are responsible the majority of DNA damage after ionizing radiation. These chemical radioprotectors are often anti-oxidants; however, not all anti-oxidants are radioprotectors.(47) To be an effective radioprotector of this class, and agent must be able to enter the cell nucleus and reside in close proximity to DNA as free radicals have an extremely short half-life and range. By scavenging the free radicals that are generated in this process, the agents prevent DNA double strand breaks and the resulting negative consequences. Two examples of chemical radioprotectors are the aminothiol Amifostine (48) and the nitroxide Tempol.(49)
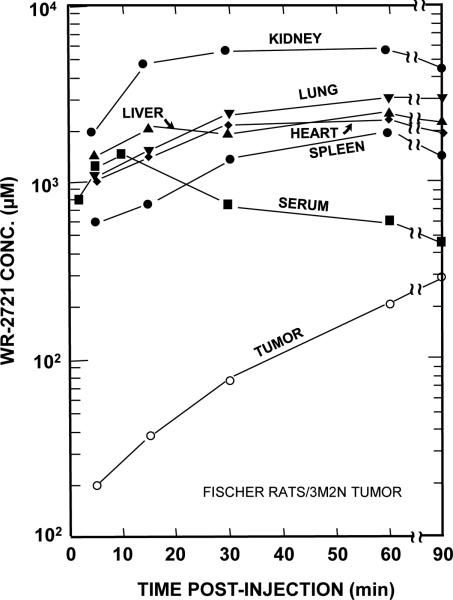
Amifostine is a drug that is metabolized into a free radical scavenging thiol compound and is an example of a chemical radioprotector. Amifostine is thought to selectively protect normal tissues from irradiation due to transiently increased uptake in normal tissues coupled with the higher pH of normal tissues and differential activity of alkaline phosphates in tumor versus normal tissues.(50) Levels of Amifostine in experimental tumors eventually reached comparable levels to normal tissue, making the timing of the radiation treatment critical to achieve maximal radioprotection (Figure 5)(48). Amifostine is the only FDA approved radiation protector, however controversy remains about the potential for tumor protection.(51)
Figure 5. Serum, tissue, and tumor levels of WR-2721 (Amifostine) in Fischer 344 rats as a function of time after an i.p. injection.
Notice that normal tissue levels rapidly achieve mM concentrations of WR-2721; whereas, tumor levels are quite low initially. With time tumor levels gradually increase, while normal tissue levels begin to decrease. This study demonstrates that chemical radioprotector tissue concentrations in the mM range can be achieved quickly. Further the study underscores the importance of the timing of radiation exposure with within 15-30 min after injection to take advantage of the differential concentration of WR-2721 in normal as opposed to tumor tissue. (Adapted from reference (64), with permission)
Evaluating Radiation Protectors
Many of the techniques used to evaluate candidate radiation sensitizers can also be used in the evaluation of radiation protectors. Clonogenic assays can provide evidence of cellular radioprotection. Typically, these include the evaluation of non-malignant cell lines, such as fibroblasts, and tumor cells lines to evaluate if radioprotectors have selectivity for normal cells versus tumor. Data from these assays can be used to determine a DMF in a similar fashion to that used for radiation sensitizers.
For in vivo testing of radiation protectors, a number of models exist. Total body irradiation is usually used to test the ability of agents to protect bone marrow (52, 53) and intestines from irradiation.(54) Lung fibrosis and pneumonitis can be assessed after pulmonary irradiation using objective endpoints such as survival, inflammation, and collagen content.(55-57) Models for mucositis, dermatitis, and skin fibrosis have also been developed to test radiation protectors. (58-60) In addition to assessment of survival, measures of fibrosis, and scores for acute toxicity, functional assays such as breathing rate for lung injury models, can be used as end points.(61)
Lastly, but perhaps most importantly, pre-clinical studies identifying radioprotectors should also determine if the agent will protect against tumor regrowth delay. Agents that protect both tumor and normal tissue to the same degree offer no benefit in regards to the therapeutic ratio.
Considerations in the development of novel radiation modifiers
It is clear that current in vitro and in vivo models used to identify radiation modifiers have limitations. There is always concern as to whether in vitro models are relevant to the in vivo condition. There is a huge disparity between the growth rates of tumor xenografts and tumors in humans. The xenograft is further complicated in that the tumor is supported by a rodent vascular system and infiltrated by rodent host cells. The question also arises as to the location of the tumor on the mouse (leg, flank, or orthotopic). Gene expression profiles of some glioblastoma cell lines have been shown to vary considerably when grown in vitro, in the flank of mice, or orthotopic placement in the brain of mice.(62, 63) Whether this variation in gene expression governs the response to radiation with or without modifiers is not known. The relevance of radiation-induced toxicities in rodent normal tissues to human injury can also be questioned. Despite these limitations, pre-clinical studies are able to identify agents that can sensitize or protect at the cellular and tissue level that may hold clinical promise.
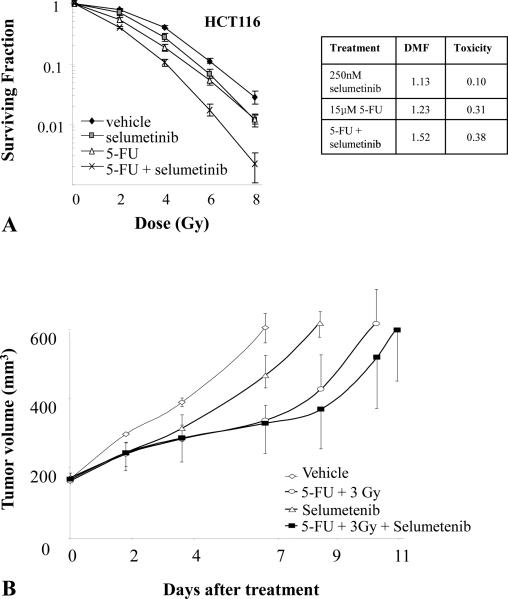
An additional complication of developing novel radiation modifiers is the frequent use of sensitizing chemotherapy in modern treatment regimens. As a result, the use of a radiosensitizer or protector must include an evaluation of any interaction of the new agent with the established regimen. For example, the addition of a MEK inhibitor to a standard regimen of radiation and 5-FU for eventual clinical translation for rectal cancer required the evaluation of the effects of MEK inhibition on 5-FU efficacy in tumor, an evaluation of the effects of MEK inhibition on radiation sensitivity of tumor cells when given with radiation or the combination of 5-FU and radiation (Figure 6).(32) The evaluation of the chemotherapy agent and the modifier is critical to rule out any decrease in sensitivity to chemotherapy with the combination. Many candidate radiation sensitizers that target signal transduction also result in cell cycle arrest in G1. As cycling is often important for sensitivity to cytotoxic chemotherapy, this interaction must be probed. The sequencing of the agents for both preclinical testing and clinical translation become of critical importance to understanding possible positive and negative interactions.
Figure 6. The effects of selumetinib and 5-FU on tumor cell and xenograft radiosensitivity.
A) The HCT116 cell line was exposed to 15 μmol/L of 5-FU (or vehicle) for 18 hours and selumetinib (or vehicle) for 2 hours and irradiated with graded doses of X-rays. Colony forming efficiency was determined 10 to 12 days later and survival curves generated after normalizing for cell killing by selumetinib, 5-FU or selumetinib + 5FU in the absence of IR. B) Mice harboring HCT116 xenografts were randomized into four groups: vehicle, selumetinib, radiation + 5-FU, or selumetinib + radiation + 5-FU. Each drug was given as a single dose prior to a single dose of radiation. The study demonstrates that radiation modifiers can be evaluated in the context of chemoradiotherapy and highlights the need to account for the toxicity of both the investigational agent and chemotherapy in calculations of modifying capability. Adapted from reference (32) with permission.
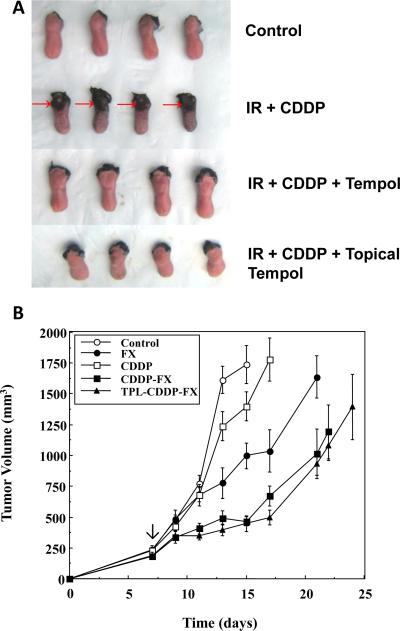
Similarly, the addition of a protector to these standard chemoradiation regimens presents similar concerns, namely that the radiation protector may interfere with the efficacy of the chemotherapy as a single agent or with the radiation modification offered by the chemotherapy. An example of this scenario is the addition of Tempol, a radioprotector, to the regimen of radiation and cisplatin in anticipation of use in the treatment of head and neck cancer.(58) As shown in Figure 7A, Tempol protected against radiation/cisplatin mucositis, but a crucial question in such studies is whether Tempol would lessen the radiation/cisplatin tumor response. Figure 7B shows that Tempol did not interfere with the chemoradiation enhancement in the tumor. Preclinical studies that include an evaluation of radiation, chemotherapy, and an investigational radiation modifier are by nature complex, costly, and time consuming. Nonetheless as the standard of care in many cancers has moved toward the use of concurrent chemotherapy with radiation, radioprotector studies must be evaluated in pre-clinical models that include these agents.
Figure 7. The effect of the radioprotector Tempol on oral mucositis resulting from fractionated irradiation and cisplatin (CDDP).
To measure ulceration tongues were removed from the various groups and stained with Toluidine blue. Ulcerations were clearly present in tongues from mice treated with the combination of CDDP and radiation (Panel A, second row, marked by arrows). Tempol either delivered systemically or topically afforded near complete protection (A, third and fourth rows). Using the same Tempol/CDDP/radiation protocol as used in A, SCCVII tumor regrowth for the various groups is shown in B. Tempol did not interfere with CDDP mediated enhancement of radiation. (Adapted from reference (58), with permission).
An additional complication to the clinical translation of radiation modifiers is determining and delivering the optimal schedule for sensitization. Many drugs that are developed that may have radiosensitizing properties are tested with dosing schedules geared towards chemotherapy. As radiotherapy is given more continuously than many chemotherapy regimens, these schedules must be tested pre-clinically to provide safety data for clinical translation.
Finally, incorporation of biomarkers and surrogate markers into clinical trials is of increasing importance. These markers may be used to screen for presence of the target, effective modulation of the target with the investigational drug, and up regulation of the target with radiotherapy. Ideally, a non-invasive or minimally invasive marker, such as a circulating marker or imaging test, could be used for this purpose.
Future Directions and Conclusions
A number of promising targets for radiation sensitization and protection have been described, and agents targeting these pathways are in development. Successful clinical translation requires rigorous preclinical testing, careful study of sequencing and interactions with standard therapy. Thoughtful clinical translation requires the consideration of available preclinical and clinical data to rationally design the appropriate method and sequence of delivery. Incomplete assessment of agents, such as lack of assessment of effects on both normal and tumor tissue, incomplete studies on sequencing, and incomplete understanding of mechanism of effect may result in failure at the time of clinical translation.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures and Conflicts of Interests: The authors have no conflicts of interests or relationships to disclose.
Contributor Information
Deborah E. Citrin, National Cancer Institute Bethesda, MD..
James B. Mitchell, National Cancer Institute Bethesda, MD. 20892 (301) 496-7511 (phone) Jm6w@nih.gov.
References
- 1.Gilbert M. Radiotherapy in Hodgkin's Disease (malignant granulomatosis); anatomic and clinical foundations; governing principles, results. Am J Roentgenol. 1939;41:198–241. [Google Scholar]
- 2.Peters M. A study in survivals in Hodgkin's disease treated radiologically. Am J Roentgenol. 1950;63:299–311. [Google Scholar]
- 3.Coutard H. Roentgen therapy of epitheliomas of the tonsillar region, hypopharynx, anf larynx from 1920 to 1926. AJR Am J Roentgenol 1932. 28:313. [Google Scholar]
- 4.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 5.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 6.Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 7.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 8.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 10.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 12.Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 13.Russo A, Mitchell J, Kinsella T, Morstyn G, Glatstein E. Determinants of radiosensitivity. Semin Oncol. 1985;12:332–349. [PubMed] [Google Scholar]
- 14.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puck TT, Marcus PI, Cieciura SJ. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956;103:273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 17.Harrington KJ, Billingham LJ, Brunner TB, Burnet NG, Chan CS, Hoskin P, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br J Cancer. 2011;105:628–639. doi: 10.1038/bjc.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suit H, Schlachter L, Andrews JR. “Oxygen effect” and tumor size as related to response of C3H/Ba adenocarcinoma to local X irradiation. J Natl Cancer Inst. 1960;24:1271–1281. [PubMed] [Google Scholar]
- 19.Cornforth MN, Bedford JS. A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat Res. 1987;111:385–405. [PubMed] [Google Scholar]
- 20.Thoms J, Bristow RG. DNA repair targeting and radiotherapy: a focus on the therapeutic ratio. Semin Radiat Oncol. 2010;20:217–222. doi: 10.1016/j.semradonc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence TS, Tepper JE, Blackstock AW. Fluoropyrimidine-Radiation Interactions in Cells and Tumors. Semin Radiat Oncol. 1997;7:260–266. doi: 10.1053/SRAO00700260. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence TS, Davis MA, Tang HY, Maybaum J. Fluorodeoxyuridine-mediated cytotoxicity and radiosensitization require S phase progression. Int J Radiat Biol. 1996;70:273–280. doi: 10.1080/095530096145003. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers AJ, Lakshman M, Chan N, Bristow RG. Poly(ADP-ribose) polymerase inhibition as a model for synthetic lethality in developing radiation oncology targets. Semin Radiat Oncol. 2010;20:274–281. doi: 10.1016/j.semradonc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Choudhury A, Cuddihy A, Bristow RG. Radiation and new molecular agents part I: targeting ATM-ATR checkpoints, DNA repair, and the proteasome. Semin Radiat Oncol. 2006;16:51–58. doi: 10.1016/j.semradonc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Terasima T, Tolmach LJ. Changes in x-ray sensitivity of HeLa cells during the division cycle. Nature. 1961;190:1210–1211. doi: 10.1038/1901210a0. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JB, Choudhuri R, Fabre K, Sowers AL, Citrin D, Zabludoff SD, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res. 2010;16:2076–2084. doi: 10.1158/1078-0432.CCR-09-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasid U, Suy S, Dent P, Ray S, Whiteside TL, Sturgill TW. Activation of Raf by ionizing radiation. Nature. 1996;382:813–816. doi: 10.1038/382813a0. [DOI] [PubMed] [Google Scholar]
- 28.Kharbanda S, Saleem A, Shafman T, Emoto Y, Weichselbaum R, Kufe D. Activation of the pp90rsk and mitogen-activated serine/threonine protein kinases by ionizing radiation. Proc Natl Acad Sci U S A. 1994;91:5416–5420. doi: 10.1073/pnas.91.12.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sklar MD. The ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation. Science. 1988;239:645–647. doi: 10.1126/science.3277276. [DOI] [PubMed] [Google Scholar]
- 30.Bernhard EJ, Stanbridge EJ, Gupta S, Gupta AK, Soto D, Bakanauskas VJ, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60:6597–6600. [PubMed] [Google Scholar]
- 31.Chung EJ, Brown AP, Asano H, Mandler M, Burgan WE, Carter D, et al. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinase. Clin Cancer Res. 2009;15:3050–3057. doi: 10.1158/1078-0432.CCR-08-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urick ME, Chung EJ, Shield WP, 3rd, Gerber N, White A, Sowers A, et al. Enhancement of 5-fluorouracil-induced in vitro and in vivo radiosensitization with MEK inhibition. Clin Cancer Res. 2011;17:5038–5047. doi: 10.1158/1078-0432.CCR-11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinnaiyan P, Allen GW, Harari PM. Radiation and new molecular agents, part II: targeting HDAC, HSP90, IGF-1R, PI3K, and Ras. Semin Radiat Oncol. 2006;16:59–64. doi: 10.1016/j.semradonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–2174. [PubMed] [Google Scholar]
- 35.Harari PM, Huang S. Radiation combined with EGFR signal inhibitors: head and neck cancer focus. Semin Radiat Oncol. 2006;16:38–44. doi: 10.1016/j.semradonc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Bonner JA, Harari PM, Giralt J, Azarnia N, Cohen RB, Raben D, et al. Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head and neck: A phase III study of high dose radiation therapy with or without cetuximab. J Clin Oncol (Meeting Abstracts) 2004;22:5507. [Google Scholar]
- 37.Brown JM. Tumor microenvironment and the response to anticancer therapy. Cancer Biol Ther. 2002;1:453–458. doi: 10.4161/cbt.1.5.157. [DOI] [PubMed] [Google Scholar]
- 38.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 40.Teicher BA, Holden SA, al-Achi A, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990;50:3339–3344. [PubMed] [Google Scholar]
- 41.Liebmann J, Cook JA, Fisher J, Teague D, Mitchell JB. In vitro studies of Taxol as a radiation sensitizer in human tumor cells. J Natl Cancer Inst. 1994;86:441–446. doi: 10.1093/jnci/86.6.441. [DOI] [PubMed] [Google Scholar]
- 42.Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ. Targeting the metabolic microenvironment of tumors. Adv Pharmacol. 2012;65:63–107. doi: 10.1016/B978-0-12-397927-8.00004-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams JP, Jackson IL, Shah JR, Czarniecki CW, Maidment BW, DiCarlo AL. Animal models and medical countermeasures development for radiation-induced lung damage: report from an NIAID Workshop. Radiat Res. 177:e0025–0039. doi: 10.1667/rrol04.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horton JA, Hudak KE, Chung EJ, White AO, Scroggins BT, Burkeen JF, et al. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells. 31:2231–2241. doi: 10.1002/stem.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–8579. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 47.Camphausen K, Citrin D, Krishna MC, Mitchell JB. Implications for tumor control during protection of normal tissues with antioxidants. J Clin Oncol. 2005;23:5455–5457. doi: 10.1200/JCO.2005.05.903. [DOI] [PubMed] [Google Scholar]
- 48.Yuhas JM, Storer JB. Differential chemoprotection of normal and malignant tissues. J Natl Cancer Inst. 1969;42:331–335. [PubMed] [Google Scholar]
- 49.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, et al. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 51.Brizel DM, Overgaard J. Does amifostine have a role in chemoradiation treatment? Lancet Oncol. 2003;4:378–381. doi: 10.1016/s1470-2045(03)01132-x. [DOI] [PubMed] [Google Scholar]
- 52.Hahn SM, Tochner Z, Krishna CM, Glass J, Wilson L, Samuni A, et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992;52:1750–1753. [PubMed] [Google Scholar]
- 53.Davis RM, Sowers AL, DeGraff W, Bernardo M, Thetford A, Krishna MC, et al. A novel nitroxide is an effective brain redox imaging contrast agent and in vivo radioprotector. Free Radic Biol Med. 2011;51:780–790. doi: 10.1016/j.freeradbiomed.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Travis EL, Thames HD, Jr., Tucker SL, Watkins TL, Kiss I. Protection of mouse jejunal crypt cells by WR-2721 after small doses of radiation. Int J Radiat Oncol Biol Phys. 1986;12:807–814. doi: 10.1016/0360-3016(86)90040-4. [DOI] [PubMed] [Google Scholar]
- 55.Travis EL, Harley RA, Fenn JO, Klobukowski CJ, Hargrove HB. Pathologic changes in the lung following single and multi-fraction irradiation. Int J Radiat Oncol Biol Phys. 1977;2:475–490. doi: 10.1016/0360-3016(77)90159-6. [DOI] [PubMed] [Google Scholar]
- 56.Citrin DE, Shankavaram U, Horton JA, Shield W, 3rd, Zhao S, Asano H, et al. Role of Type II Pneumocyte Senescence in Radiation-Induced Lung Fibrosis. J Natl Cancer Inst. 2013;105:1474–1484. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, et al. Compartmental responses after thoracic irradiation of mice: strain differences. Int J Radiat Oncol Biol Phys. 2005;62:862–871. doi: 10.1016/j.ijrobp.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 58.Cotrim AP, Yoshikawa M, Sunshine AN, Zheng C, Sowers AL, Thetford AD, et al. Pharmacological protection from radiation +/− cisplatin-induced oral mucositis. Int J Radiat Oncol Biol Phys. 2012;83:1284–1290. doi: 10.1016/j.ijrobp.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleischer G, Dorr W. Amelioration of early radiation effects in oral mucosa (mouse) by intravenous or subcutaneous administration of amifostine. Strahlenther Onkol. 2006;182:567–575. doi: 10.1007/s00066-006-1587-8. [DOI] [PubMed] [Google Scholar]
- 60.Stone HB. Leg contracture in mice: an assay of normal tissue response. Int J Radiat Oncol Biol Phys. 1984;10:1053–1061. doi: 10.1016/0360-3016(84)90177-9. [DOI] [PubMed] [Google Scholar]
- 61.Travis EL, Down JD, Holmes SJ, Hobson B. Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiat Res. 1980;84:133–143. [PubMed] [Google Scholar]
- 62.Camphausen K, Purow B, Sproull M, Scott T, Ozawa T, Deen DF, et al. Influence of in vivo growth on human glioma cell line gene expression: convergent profiles under orthotopic conditions. Proc Natl Acad Sci U S A. 2005;102:8287–8292. doi: 10.1073/pnas.0502887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camphausen K, Purow B, Sproull M, Scott T, Ozawa T, Deen DF, et al. Orthotopic growth of human glioma cells quantitatively and qualitatively influences radiation-induced changes in gene expression. Cancer Res. 2005;65:10389–10393. doi: 10.1158/0008-5472.CAN-05-1904. [DOI] [PubMed] [Google Scholar]
- 64.Yuhas JM. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980;40:1519–1524. [PubMed] [Google Scholar]