Abstract
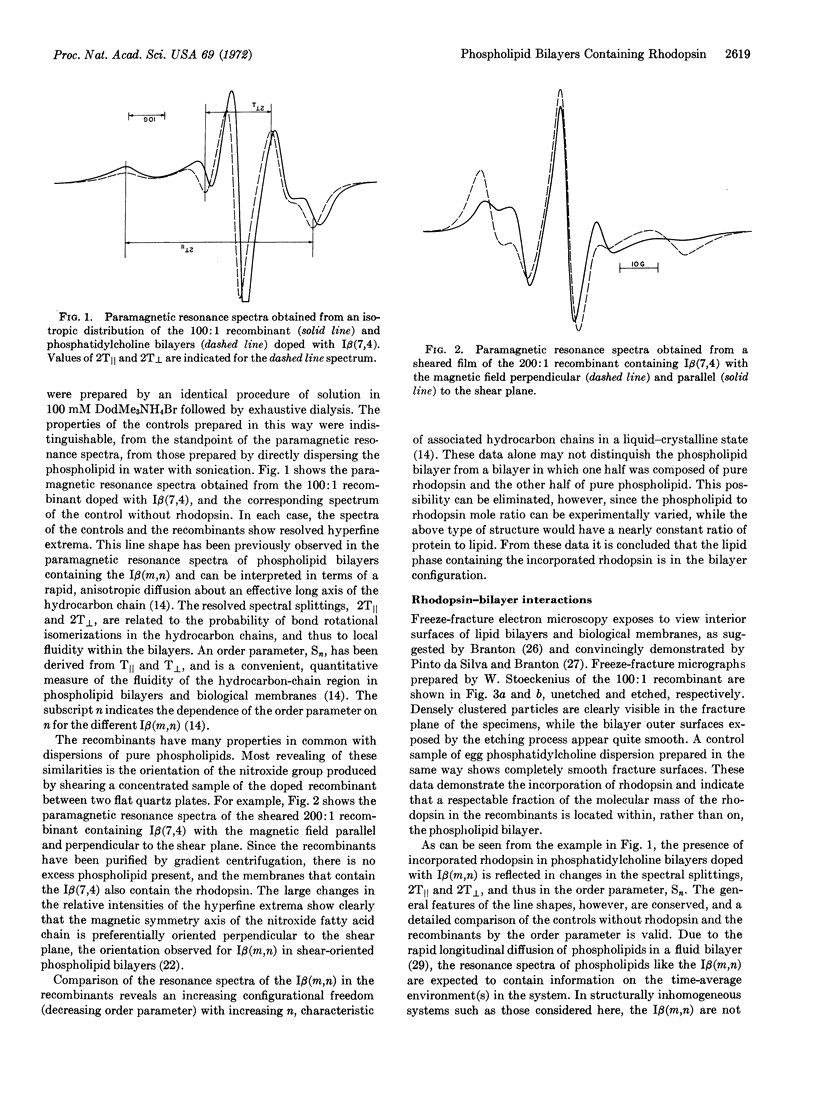
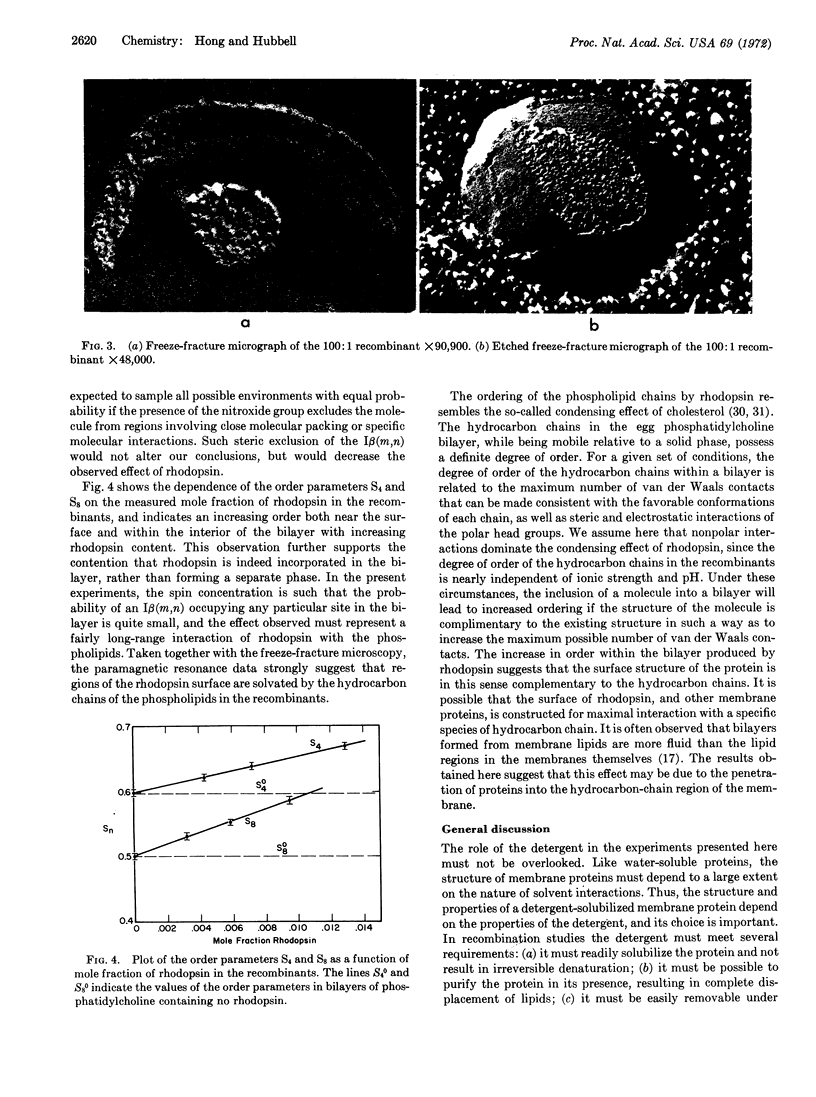
Purified rhodopsin has been prepared containing less than 1.1 mol of phosphate per mol of protein. The purified rhodopsin has been incorporated into phosphatidylcholine bilayers, and the molecular interactions within the bilayers were investigated by the use of spin-labeled phosphatidylcholines. Rhodopsin appears to inhibit segmental motions of the hydrocarbon chains, an effect similar to that of cholesterol on phospholipid bilayers.
Keywords: photoreceptor, membrane, spin-label, retinal rod outer segments
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT G. Acetylrhodopsin. Science. 1957 Jan 11;125(3237):70–72. doi: 10.1126/science.125.3237.70. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blasie J. K. The location of photopigment molecules in the cross-section of frog retinal receptor disk membranes. Biophys J. 1972 Feb;12(2):191–204. doi: 10.1016/S0006-3495(72)86079-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasie J. K., Worthington C. R. Planar liquid-like arrangement of photopigment molecules in frog retinal receptor disk membranes. J Mol Biol. 1969 Feb 14;39(3):417–439. doi: 10.1016/0022-2836(69)90136-3. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Wilkins M. H. Structure of frog photoreceptor membranes. Nature. 1969 Aug 30;223(5209):906–909. doi: 10.1038/223906a0. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. K. Rhodopsin rotates in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):35–38. doi: 10.1038/newbio236035a0. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Corless J. M. Lamellar structure of bleached and unbleached rod photoreceptor membranes. Nature. 1972 May 26;237(5352):229–231. doi: 10.1038/237229a0. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Leonard R., Tardieu A., Branton D. Lamellar and hexagonal lipid phases visualized by freeze-etching. Biochim Biophys Acta. 1970;219(1):47–60. doi: 10.1016/0005-2736(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Tinoco J. Monolayer interactions of individual lecithins with natural sterols. Biochim Biophys Acta. 1972 Apr 14;266(1):41–49. doi: 10.1016/0005-2736(72)90117-4. [DOI] [PubMed] [Google Scholar]
- Gras W. J., Worthington C. R. X-ray analysis of retinal photoreceptors. Proc Natl Acad Sci U S A. 1969 Jun;63(2):233–238. doi: 10.1073/pnas.63.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. H., Libertini L. J., Birrell G. B. The role of lipid spin labels in membrane biophysics. J Phys Chem. 1971 Oct 28;75(22):3417–3425. doi: 10.1021/j100691a004. [DOI] [PubMed] [Google Scholar]
- Heitzmann H. Rhodopsin is the predominant protein of rod outer segment membranes. Nat New Biol. 1972 Jan 26;235(56):114–114. doi: 10.1038/newbio235114a0. [DOI] [PubMed] [Google Scholar]
- Heller J. Structure of visual pigments. I. Purification, molecular weight, and composition of bovine visual pigment500. Biochemistry. 1968 Aug;7(8):2906–2913. doi: 10.1021/bi00848a030. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., Metcalfe J. C., Metcalfe S. M., McConnell H. M. The interaction of small molecules with spin-labelled erythrocyte membranes. Biochim Biophys Acta. 1970 Dec 1;219(2):415–427. doi: 10.1016/0005-2736(70)90219-1. [DOI] [PubMed] [Google Scholar]
- Irreverre F., Stone A. L., Shichi H., Lewis M. S. Biochemistry of visual pigments. I. Purification and properties of bovine rhodopsin. J Biol Chem. 1969 Feb 25;244(4):529–536. [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Lateral diffusion of phospholipids in a vesicle membrane. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2564–2568. doi: 10.1073/pnas.68.10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Wright K. L., McFarland B. G. The fraction of the lipid in a biological membrane that is in a fluid state: a spin label assay. Biochem Biophys Res Commun. 1972 Apr 14;47(1):273–281. doi: 10.1016/s0006-291x(72)80039-1. [DOI] [PubMed] [Google Scholar]
- McFarland B. G., McConnell H. M. Bent fatty acid chains in lecithin bilayers. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1274–1278. doi: 10.1073/pnas.68.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. E., Gordon-Walker A., Bownds D. Molecular weight of frog rhodopsin. Nat New Biol. 1972 Jan 26;235(56):112–114. doi: 10.1038/newbio235112a0. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Seelig J., Hasselbach W. A spin label study of sarcoplasmic vesicles. Eur J Biochem. 1971 Jul 15;21(1):17–21. doi: 10.1111/j.1432-1033.1971.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Shichi H. Biochemistry of visual pigments. II. Phospholipid requirement and opsin conformation for regeneration of bovine rhodopsin. J Biol Chem. 1971 Oct 25;246(20):6178–6182. [PubMed] [Google Scholar]
- Shichi H. Circular dichroism of bovine rhodopsin. Photochem Photobiol. 1971 Jun;13(6):499–502. doi: 10.1111/j.1751-1097.1971.tb06144.x. [DOI] [PubMed] [Google Scholar]
- Shields J. E., Dinovo E. C., Henriksen R. A., Kimbel R. L., Jr, Millar P. G. The purification and amino acid composition of bovine rhodopsin. Biochim Biophys Acta. 1967 Oct 23;147(2):238–251. doi: 10.1016/0005-2795(67)90402-3. [DOI] [PubMed] [Google Scholar]
- Tourtellotte M. E., Branton D., Keith A. Membrane structure: spin labeling and freeze etching of Mycoplasma laidawii. Proc Natl Acad Sci U S A. 1970 Jul;66(3):909–916. doi: 10.1073/pnas.66.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn M., Futterman S. Properties of rhodopsin dependent on associated phospholipid. J Biol Chem. 1971 Feb 25;246(4):881–886. [PubMed] [Google Scholar]



