Abstract
This study aimed to examine the association between obstructive sleep apnea (OSA) and obesity among young adults. A total of 2911 college students in Thailand participated in the study. Anthropometric measurements and blood pressure were taken by trained research staff. Overall, 6.3% of college students had OSA determined by the Berlin Questionnaire, 9.6% were overweight (BMI: 25–29 kg/m2), 4.5% were obese (BMI≥30 kg/m2); 12.4% had abdominal obesity (men: waist circumference≥90 cm; women: waist circumference≥80 cm). There were significant associations between OSA and overweight (odds ratio (OR)=1.72; 95% confidence interval (CI)=1.04–1.85) and obesity (OR=24.23; 95% CI=15.20–38.61), independent of demographic and lifestyle factors, blood pressure, and psychological distress. Students with OSA were more likely to have abdominal obesity than those without OSA (OR=2.09; 95% CI=1.19–3.67). OSA was significantly related to joint effects of general and abdominal obesity. The OSA-obesity associations were robust and evident for both genders, individuals with normal and elevated blood pressure, and those with and without psychological distress. This study shows independent associations of OSA with general and abdominal obesity among young adults. OSA could be a risk factor for obesity and consequent cardiovascular morbidities. OSA screening and treatment might be important for young adults.
Keywords: obstructive sleep apnea, obesity, abdominal obesity, college student, Asia, Thailand
1. Introduction
The obesity epidemic is a worldwide public health problem, with the prevalence of obesity up to 36% for US adults [1]. Obesity predicts various health outcomes such as diabetes, cardiovascular disease, and mortality, and is also related to increased health care costs, decreased productivity, and lower quality of life [2, 3]. Body mass index (BMI) has been employed widely for classifying general obesity [1]. Abdominal fat deposition measured by waist circumference (WC) or waist-to-hip ratio (WHR) has been suggested as a better indicator of obesity in relation to health outcomes than BMI [4, 5]. The rapid economic growth in Asia has led to a rapid increase in the prevalence of obesity, especially abdominal obesity in Asia [6, 7].
One of possible identifiable risks of obesity is obstructive sleep apnea (OSA), a common disorder characterized by repetitive episodes of upper airway obstruction that occur during sleep [8]. OSA may contribute to obesity or excess weight through increased sympathetic activation, sleep deprivation, and disrupted metabolism [8, 9]. Furthermore, OSA may be related to changes in leptin, ghrelin, and orexin levels, and thus may increase individuals’ appetite and caloric intake, which exacerbate obesity [8]. OSA is highly prevalent among middle-aged and older adults [10–12], but remains underdiagnosed in the general population. A recent systematic review has highlighted the lack of data regarding the prevalence of OSA in Asia [13].
A growing body of evidence suggests that OSA is independently associated with health outcomes (e.g., hypertension, cardiovascular disease) [14, 15]. It has been reported that untreated OSA is associated with increased mortality [16], and successful treatment has been shown to reduce mortality [17]. It is unclear whether OSA is associated with obesity. Two earlier small longitudinal studies found no statistically significant correlations between change in OSA and change in BMI [18, 19], whereas several recent epidemiologic studies have demonstrated significant associations between OSA and obesity [20, 21].
There is a paucity of research evaluating OSA and its related general and abdominal obesity among young adults, especially among healthy college students in Asian countries. To our knowledge, only one study examined OSA and its association with general and abdominal obesity concurrently but focused on middle-aged adults in Korea [21]. To fill the research gap, we examined the associations of OSA with general and abdominal obesity in a large cross-sectional study of college students in Thailand.
2. Methods
2.1 Study Population
This cross-sectional study was conducted between December 2010 and February 2011 at seven colleges in Thailand. The study procedures have been described elsewhere [22]. A total of 3000 full-time undergraduate students participated in the study. Students with incomplete questionnaires and missing data for OSA were excluded (n=89). These students were similar to the total population with regards to their demographic and lifestyle characteristics. The final analyzed sample included 2911 (97.0%) college students (964 males and 1947 females) with complete information on OSA, demographic and lifestyle factors, anthropometric measurements, and psychological distress. All the completed questionnaires were anonymous, and no personal identifiers were collected.
All study procedures were approved by the institutional review boards of the Faculty of Medicine Chulalongkorn University and Walailak University in Thailand, and the University of Washington, USA. The Harvard School of Public Health Office of Human Research Administration, USA, granted approval to use the anonymous data set for analysis.
2.2 Measures
Recruitment flyers were posted on each campus to invite college students to participate in the study. Students who expressed an interest in participating were asked to meet in a large classroom or an auditorium where they were informed the purpose of the study. Students who consented to participate were asked to complete a self-administered questionnaire survey regarding demographic information, psychological distress status, lifestyle factors, as well as sleep related questions including the Berlin Questionnaire. Measurements of height, weight, waist circumference (WC), hip circumference (HC), and blood pressure (BP) were taken by trained researchers.
2.2.1 General Obesity
Measured weight and height were used to calculate body mass index (BMI) and to define normal weight (BMI < 25 kg/m2), overweight (BMI: 25–29.9 kg/m2), and obesity (BMI ≥ 30 kg/m2). Students were divided into the non-obese group and obese group if their BMI was <30 kg/m2 or ≥30 kg/m2, respectively.
We also used the Asian criteria of overweight and obesity [23], and further grouped students into normal weight (BMI < 23 kg/m2), overweight (BMI: 23–26 kg/m2), obesity (BMI ≥ 27 kg/m2). Study participants were divided into the non-obese group and obese group if their BMI was < 27 kg/m2 or ≥ 27 kg/m2, respectively [23].
2.2.2 Abdominal Obesity
Abdominal obesity was defined based on the new International Diabetes Federation (IDF) criteria for the use in South Asia: WC ≥ 90cm for men and WC ≥ 80cm for women [24]. WC, HC, and waist-to-hip ratio (WHR) were categorized by quartile distribution. Abdominal obesity was also determined as WHR equal or higher than the top quartile (WHR ≥ 0.83).
2.2.3 Obstructive Sleep Apnea (OSA)
We used the Berlin Questionnaire for the assessment of OSA. The Berlin Questionnaire has been validated and widely used in previous studies [25, 26]. The questionnaire is divided into three sections. Section 1 is about snoring behavior. Section 2 is about individuals’ feelings of fatigue, tiredness, and daytime sleepiness, and section 3 ascertains the presence of obesity or hypertension. In sections 1 and 2, high risk for OSA is considered when there is a persistent symptom (more than 3–4 times/week). In section 3, high risk for OSA is defined when there is a history of hypertension or BMI ≥30 kg/m2. Individuals are considered at a high risk for OSA if they are qualified as high risk in two or three sections.
2.2.4 Covariates
Students’ demographic information were age, sex, and education level. Lifestyle factors were cigarette smoking history, and participation in moderate or vigorous physical activity, alcohol consumption, and energy drinks. Energy drinks or stimulant beverages are a group of beverages used to provide an extra boost in energy, promote wakefulness, and provide cognitive and mood enhancement [22]. Participants were first asked whether they consumed more than one stimulant or energy drink per week every month during the current academic semester/quarter. Energy drinks were summarized and categorized as dichotomous variables (no vs. yes) [22]. Psychological distress was evaluated by the General Health Questionnaire 12-item scale (GHQ-12). The GHQ-12 is a self-report instrument for the detection of psychological distress in the community and in primary care settings [27]. The GHQ scoring method (0-0-1-1) was used, with the sum scores ranging from 0 to 12. Participants with the total GHQ-12 score ≥ 2 were considered to have psychological distress [27].
2.3 Statistical Analysis
Unpaired t-tests and χ2 tests were conducted to evaluate the differences in covariates on either a continuous or discrete scale across the OSA status. Linear regression analyses were conducted to evaluate the associations of OSA with BMI, WC, HC, and WHR. Multinomial logistic regression models were used to assess associations of OSA with overweight and obesity; those with normal weight served as the reference group. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated using the logistic regression models. We also evaluated whether individuals with OSA had a high risk of joint effects of having general and abdominal obesity compared to those without OSA in logistic regression analyses. Potential confounders included age, sex, education, cigarette smoking, physical activity participation, alcohol consumption, use of energy drinks, BP, and psychological distress. Stratified analyses were conducted to examine whether these associations varied by sex, BP level, and psychological distress. All tests were performed by using Statistical Analysis Software (SAS, version 9.3; SAS Institute, Cary, NC). The significance levels were set at P <0.05 for two-sided analyses.
3. Results
Of 2911 college students, 33.1 % were males and the average age was 20.3 years (standard deviation: 1.3). Overall, 6.3% of college students reported having OSA.
Table 1 shows demographic and lifestyle characteristics of participants by OSA status. A total of 8.5 % of college students reported smoking cigarettes. More than half (54.9 %) of students regularly participated in recreational physical activity. Overall, 9.6% of students were overweight (BMI: 25–29 kg/m2) and 4.5% were obese (BMI≥30 kg/m2) according to the WHO definition. When the Asian criteria were applied, 18.4% were overweight and 8.0% were obese. Based on the IDF criteria of abdominal obesity, 12.4% of students were centrally obese. The factors associated with OSA were male gender, older age, alcohol consumption, cigarette smoking, use of energy drinks, and higher BP. Students with OSA had a higher BMI, WC, HC, and WHR, and a higher percentage of general and abdominal obesity than those without OSA.
Table 1.
Characteristics of 2911 college students in Thailand, according to obstructive sleep apnea status
| Characteristic | Total (n=2911) |
OSA | P valuea |
|
|---|---|---|---|---|
| No (n=2728) | Yes (n=183) | |||
| Demographic characteristics | ||||
| Age, year, mean (SD) | 20.3 (1.3) | 20.3 (1.3) | 20.6 (1.5) | 0.005 |
| Male, % | 33.1 | 31.7 | 54.6 | <0.001 |
| Education (college) | ||||
| First year | 24.5 | 25 | 16.9 | 0.034 |
| Second year | 31.9 | 31.8 | 35.5 | |
| Third year | 26.8 | 26.9 | 26.8 | |
| Senior | 16.7 | 16.4 | 20.8 | |
| Lifestyle factors | ||||
| Recreational PA participation, % | 54.9 | 54.8 | 55.2 | 0.926 |
| Alcohol consumption, % | 16.3 | 15.6 | 25.1 | <0.001 |
| Cigarette smoking, % | 8.5 | 7.9 | 17.5 | <0.001 |
| Use of energy drinks, % | 58 | 57.4 | 66.7 | 0.014 |
| Psychological health | ||||
| Psychological distressb, % | 29.1 | 27.3 | 55.7 | <0.001 |
| Blood pressure | ||||
| Hypertensionc, % | 4.5 | 3.9 | 14 | <0.001 |
| Elevated BPd, % | 12.9 | 11.9 | 29.2 | <0.001 |
| Anthropometric measurements | ||||
| BMI, kg/m2, mean (SD) | 21.6 (3.8) | 21.4 (3.4) | 25.9 (6.1) | <0.001 |
| WHO criteria of obesity, % | ||||
| Normal weight (BMI<25 kg/m2) | 85.9 | 87.9 | 55.6 | <0.001 |
| Overweight (BMI: 25–29 kg/m2) | 9.6 | 9.5 | 11.6 | |
| Obese (BMI≥30 kg/m2) | 4.5 | 2.6 | 32.8 | |
| Asian criteria of obesity, % | ||||
| Normal weight (BMI<23 kg/m2) | 73.6 | 75.6 | 43.7 | <0.001 |
| Overweight (BMI: 23–26 kg/m2) | 18.4 | 18.4 | 18.6 | |
| Obese (BMI≥27 kg/m2) | 8.0 | 6.1 | 37.7 | |
| Abdominal obesitye, % | 12.3 | 10.3 | 41.5 | <0.001 |
| Waist circumference, cm, mean (SD) | 73.2 (9.4) | 72.6 (8.5) | 83.3 (14.3) | <0.001 |
| Hip circumference, cm, mean (SD) | 91.2 (7.7) | 90.7 (7.1) | 98.7 (11.3) | <0.001 |
| Waist-to-hip ratio, mean (SD) | 0.80 (0.05) | 0.80 (0.05) | 0.84 (0.06) | <0.001 |
| Waist-to-hip ratio≥Q3 (0.83), % | 25.0 | 23.5 | 47.2 | <0.001 |
Abbreviations: OSA, obstructive sleep apnea; SD, standard deviation; PA, physical activity; BP, blood pressure; BMI, body mass index.
Student t-test for continuous variables; Chi-square for categorical variables.
Evaluated by the General Health Questionnaire 12-item scale (GHQ-12).
Hypertension defined as: Systolic BP≥140 mmHg or diastolic BP≥90 mmHg.
Elevated BP defined as: Systolic BP≥120 mmHg or diastolic BP≥80 mmHg.
Based on the International Diabetes Federation (IDF) criteria for the definition of abdominal obesity among South Asians: waist circumference ≥ 90 cm for men; waist circumference ≥ 80 cm for women.
Table 2 presents the results about OSA and anthropometric measures from the linear regression models. OSA was significantly associated with multiple measures of general obesity as measured by BMI as well as measures of fat distribution including abdominal adiposity. The associations were robust across obesity measures and persisted even after adjustment for possible confounders. For example, OSA was significantly related to a higher BMI (beta: 4.20; standard error: 0.27; P<0.001), after adjustment for age, sex, education, alcohol drinking, cigarette smoking, use of energy drinks, BP, and psychological distress. Further adjustment for WHR showed a significant and consistent association between OSA and a high BMI (beta: 3.64; standard error: 0.25; P<0.001). Similar results were found for other measures of abdominal obesity including WHR, WC, and HC.
Table 2.
Linear regression analyses: associations between obstructive sleep apnea and anthropometric measurements among 2911 college students in Thailand
| Model | BMI, kg/m2 | WHR | WC, cm | HC, cm | ||||
|---|---|---|---|---|---|---|---|---|
| Beta (SE) | P value | Beta (SE) | P value | Beta (SE) | P value | Beta (SE) | P value | |
| Model 1a | 4.55 (0.28) | <0.001 | 0.041 (0.004) | <0.001 | 10.75 (0.69) | <0.001 | 7.98 (0.57) | <0.001 |
| Model 2b | 4.31 (0.28) | <0.001 | 0.033 (0.004) | <0.001 | 9.40 (0.66) | <0.001 | 7.29 (0.57) | <0.001 |
| Model 3c | 4.31 (0.28) | <0.001 | 0.033 (0.004) | <0.001 | 9.41 (0.66) | <0.001 | 7.29 (0.56) | <0.001 |
| Model 4d | 4.06 (0.27) | <0.001 | 0.031 (0.004) | 0.018 | 8.83 (0.66) | <0.001 | 6.85 (0.56) | <0.001 |
| Model 5e | 4.20 (0.27) | <0.001 | 0.031 (0.004) | 0.018 | 9.04 (0.66) | <0.001 | 7.09 (0.57) | <0.001 |
| Model 6f | 3.64 (0.25) | <0.001 | 0.007 (0.004) | 0.072 | 1.25 (0.49) | 0.011 | 0.73 (0.43) | 0.092 |
Abbreviations: BMI, body mass index; WHR, waist-hip ratio; WC, waist circumference; HC, hip circumference; SE, standard error.
Model 1: Unadjusted
Model 2: adjusted for demographic factors including age, sex, and education level.
Model 3: adjusted for demographic & lifestyle factors including age, sex, education level, recreational physical activity participation, alcohol consumption, use of energy drinks, and cigarette smoking.
Model 4: Model 3+blood pressure
Model 5: Model 4+psychological distress (evaluated by the General Health Questionnaire 12-item scale).
Model 6+WHR/BMI (when BMI was the outcome variable, waist-to-hip ratio (WHR) but not BMI was adjusted for. When WHR, WC, or HC was the outcome variable, BMI but not WHR was adjusted for).
Table 3 shows the associations of OSA with overweight and obesity based on the multinomial logistic regression analyses. OSA was significantly associated with overweight and obesity, independent of potential confounders. The adjusted OR was 1.72 (95% CI: 1.04–1.85) for overweight and 24.23 (95% CI: 15.20–38.61) for obesity after adjustment for demographic and lifestyle factors, BP, and psychological distress. Further adjustment for WHR did not change the results substantially. We found similar results using the Asian criteria of overweight and obesity.
Table 3.
Multinomial logistic regression analyses: associations of obstructive sleep apnea with overweight and obesity among 2911 college students in Thailand
| Model | WHO criteriaa | Asian criteriab | ||
|---|---|---|---|---|
| Overweight (BMI: 25–29) |
Obesity (BMI≥30) |
Overweight (BMI: 23–26) |
Obesity (BMI≥27) |
|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Model 1c | 1.91 (1.17, 3.10) | 19.58 (13.18, 29.09) | 1.75 (1.16, 2.64) | 10.78 (7.53, 15.43) |
| Model 2d | 1.63 (1.00, 2.66) | 19.45 (12.89, 29.34) | 1.59 (1.05, 2.41) | 9.91 (6.86, 14.32) |
| Model 3e | 1.63 (0.99, 2.67) | 20.29 (13.37, 30.78) | 1.58 (1.04, 2.40) | 10.09 (6.96, 14.63) |
| Model 4f | 1.62 (0.98, 2.66) | 20.09 (12.98, 31.08) | 1.56 (1.02, 2.37) | 9.64 (6.58, 14.14) |
| Model 5g | 1.72 (1.04, 2.85) | 24.23 (15.20, 38.61) | 1.66 (1.09, 2.55) | 10.97 (7.36, 16.35) |
| Model 6h | 1.69 (0.99, 2.88) | 26.46 (15.54, 45.04) | 1.68 (1.08, 2.61) | 11.21 (7.09, 17.74) |
Abbreviations: BMI, body mass index; OR, odds ratio; CI, confidence interval; WHR, waist-to-hip ratio.
BMI<25 kg/m2 served as the reference group in the WHO criteria.
BMI<23 kg/m2 served as the reference group in the Asian criteria.
Model 1: Unadjusted
Model 2: adjusted for demographic factors including age, sex, and education level.
Model 3: adjusted for demographic & lifestyle factors including age, sex, education level, recreational physical activity participation, alcohol consumption, use of energy drinks, and cigarette smoking.
Model 4: Model 3+blood pressure
Model 5: Model 4+psychological distress (evaluated by the General Health Questionnaire 12-item scale).
Model 6: Model 5+WHR.
As shown in Table 4, OSA was strongly associated with general obesity as reflected by BMI, regardless of criteria used. Similar results were observed in terms of abdominal obesity defined by the upper quartile of WHR or the IDF criteria. After adjustment for potential confounders including BMI, students with OSA were more likely to have abdominal obesity compared with those without OSA (OR=2.09; 95% CI 1.19–3.67).
Table 4.
Logistic regression analyses: associations of obstructive sleep apnea with overweight and obesity among 2911 college students in Thailand
| Model | WHO criteria | Asian criteria | WHO criteria | Abdominal obesity | |
|---|---|---|---|---|---|
| BMI≥25 vs. BMI<25 |
BMI≥27 vs. BMI<27 |
BMI≥30 vs. BMI<30 |
WHR≥Q3 vs. <Q3 |
IDF Criteriaa |
|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Model 1b | 5.75 (4.21, 7.87) | 9.40 (6.71, 13.18) | 17.99 (12.22, 26.51) | 2.88 (2.13, 3.90) | 6.19 (4.50, 8.51) |
| Model 2c | 5.18 (3.76, 7.12) | 8.83 (6.24, 12.49) | 18.24 (12.18, 27.32) | 2.28 (1.66, 3.14) | 6.87 (4.92, 9.58) |
| Model 3d | 5.25 (3.80, 7.24) | 9.01 (6.35, 12.79) | 19.02 (12.63, 28.65) | 2.29 (1.66, 3.15) | 6.96 (4.98, 9.74) |
| Model 4e | 4.94 (3.56, 6.87) | 8.61 (6.00, 12.36) | 18.77 (12.22, 28.81) | 2.17 (1.57, 3.00) | 6.59 (4.68, 9.28) |
| Model 5f | 5.42 (3.86, 7.60) | 9.65 (6.62, 14.09) | 22.46 (14.21, 35.50) | 2.16 (1.56, 3.00) | 6.92 (4.87, 9.83) |
| Model 6g | 5.02 (3.44, 7.32) | 9.36 (6.12, 14.31) | 23.49 (14.05, 39.28) | 1.10 (0.76, 1.61) | 2.09 (1.19, 3.67) |
Abbreviations: BMI, body mass index; WHR, waist-hip ratio; IDF, International Diabetes Federation; OR, odds ratio; 95% CI, 95% confidence interval.
Abdominal obesity was defined by the International Diabetes Federation (IDF) criteria: WC ≥ 90 cm for men and WC ≥ 80 cm for women.
Model 1: Unadjusted
Model 2: adjusted for demographic factors including age, sex, and education level.
Model 3: adjusted for demographic & lifestyle factors including age, sex, education level, recreational physical activity participation, alcohol consumption, use of energy drinks, and cigarette smoking.
Model 4: Model 3+blood pressure
Model 5: Model 4+psychological distress (evaluated by the General Health Questionnaire 12-item scale).
Model 6: Model 5+WHR/BMI (When overweight/obesity or obesity (defined from BMI) was the outcome variable, waist-to-hip ratio (WHR) but not BMI was adjusted for. When abdominal obesity was the outcome variable, BMI but not WHR was adjusted for.
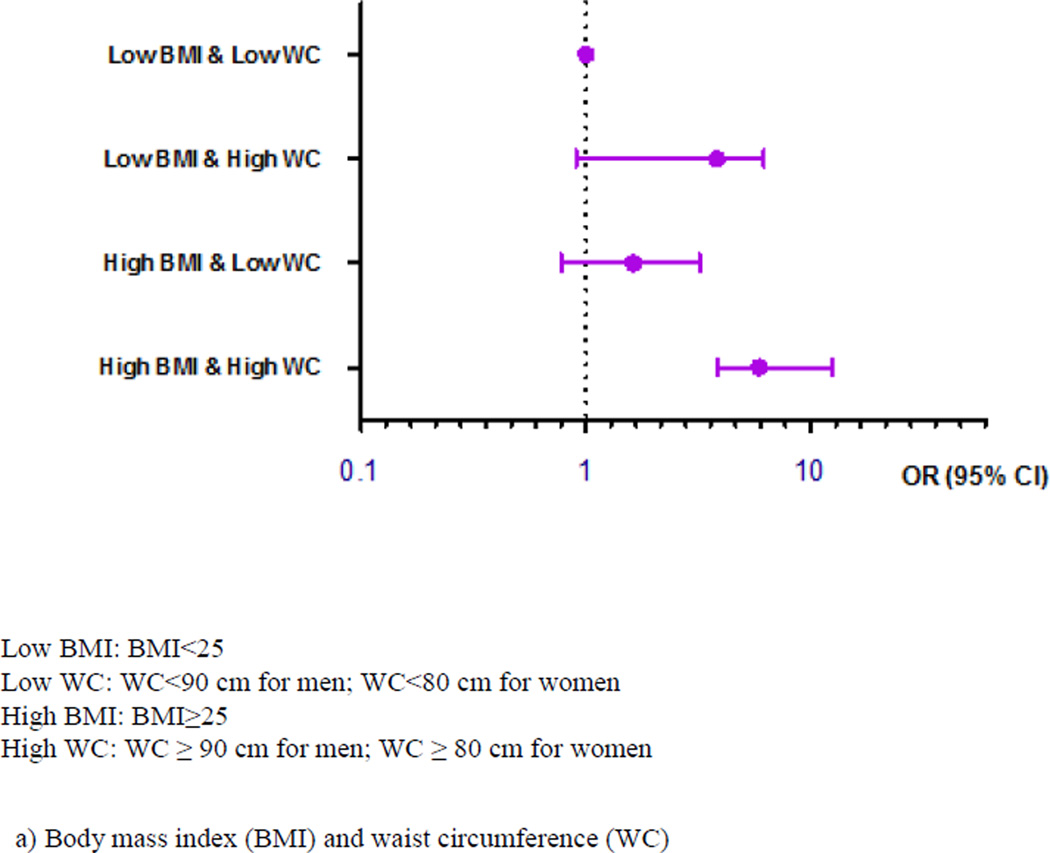
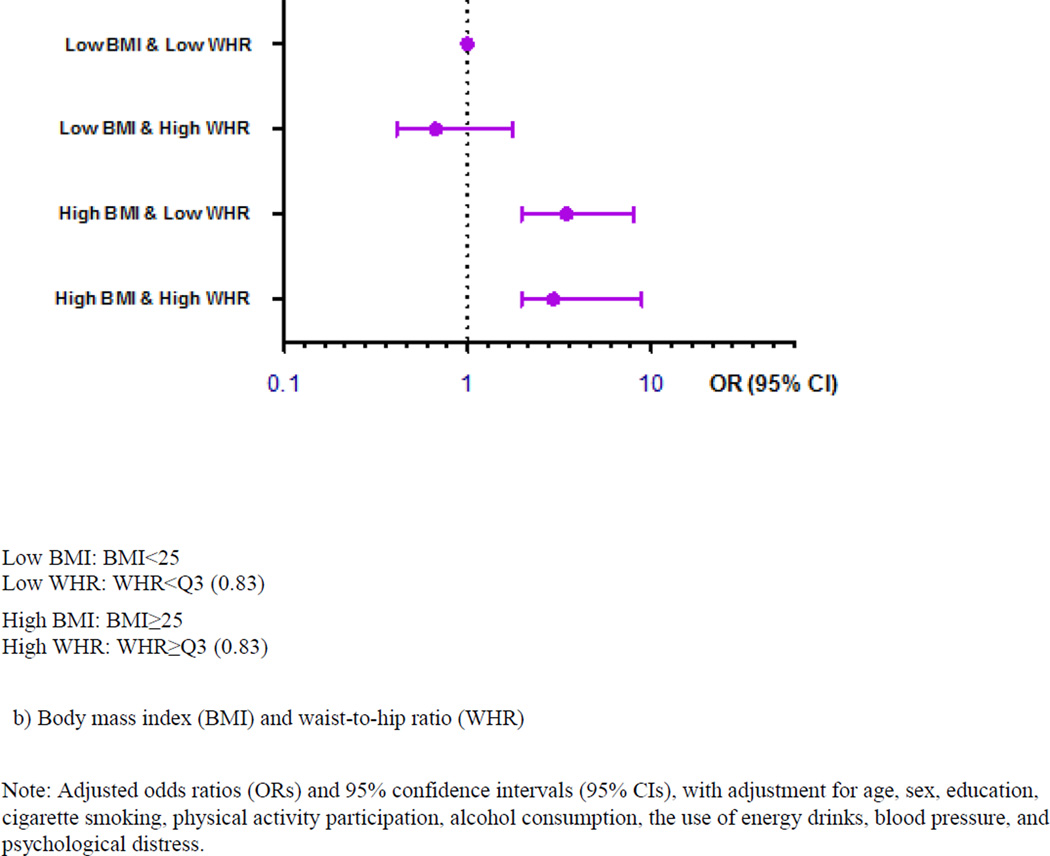
Figure 1 presents the associations of OSA with the joint effects of general obesity and abdominal obesity after adjustment for potential confounders. Compared to students without OSA, those with OSA had an 8-fold higher odds of having both general obesity assessed by BMI and abdominal obesity evaluated by WC (Figure 1a). When using WHR to define abdominal obesity (WHR≥Q3), we found consistent and robust associations between OSA and joint effects of general and abdominal obesity (Figure 1b).
Figure 1.
Associations of obstructive sleep apnea with the joint effects of general and abdominal obesity among 2911 college students in Thailand.
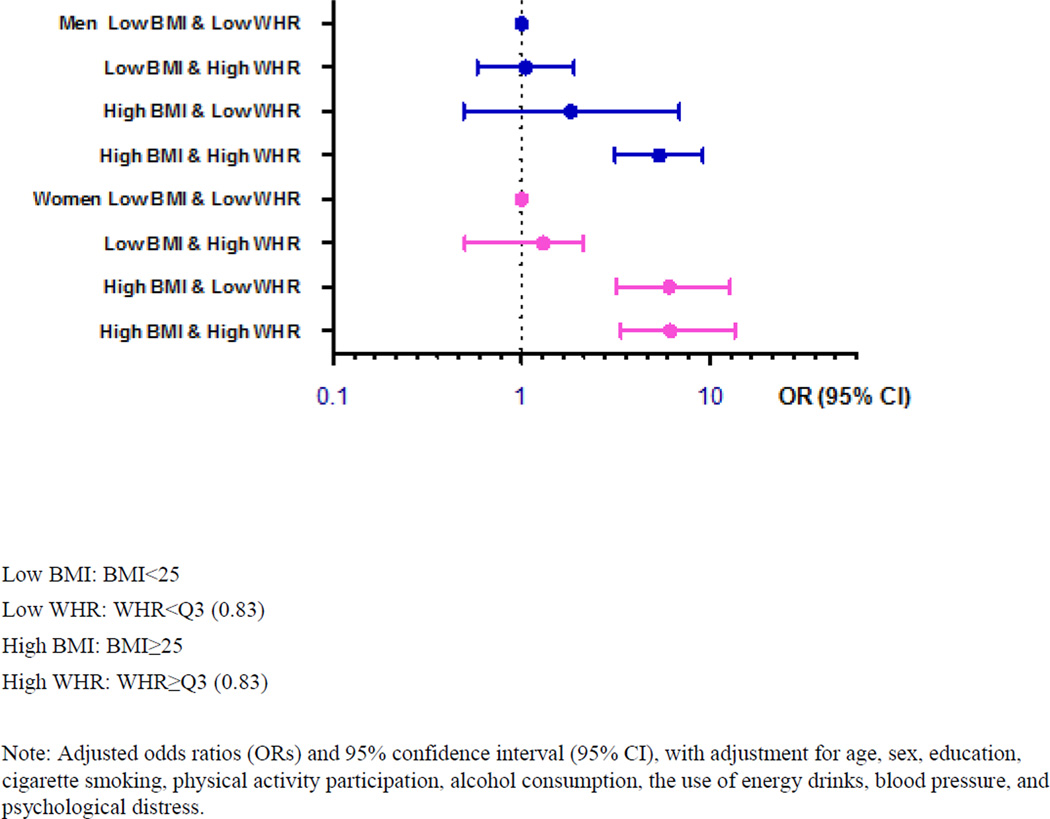
Figure 2 presents the gender-specific associations of OSA with the joint effects of general and abdominal obesity. The consistent and robust associations between OSA and joint effects of general obesity and abdominal obesity were found in both males and females.
Figure 2.
Associations of obstructive sleep apnea with the joint effects of general and abdominal obesity among 2911 college students in Thailand, according to gender distribution
Our stratified analyses show that associations of OSA with general obesity and abdominal adiposity were robust and evident for both males and females, individuals with normal and elevated BP, and those with and without psychological distress (Table 5).
Table 5.
Stratified analysis for the associations of obstructive sleep apnea with overweight and obesity, by sex, blood pressure, and psychological distress
| Stratified variable | WHO criteria | Asian criteria | WHO criteria | IDF criteria | |
|---|---|---|---|---|---|
| BMI≥25 vs. <25 OR (95% CI)a |
BMI≥27 vs. <27 OR (95% CI)a |
BMI≥30 vs. <30 OR (95% CI)a |
Abdominal obesityb OR (95% CI)c |
||
| Sex | |||||
| Male | 4.11 (2.35, 7.20) | 6.62 (3.39, 12.92) | 15.16 (6.62, 34.72) | 4.56 (1.94, 10.71) | |
| Female | 7.19 (4.23, 12.21) | 15.60 (8.79, 27.68) | 39.38 (20.04, 77.36) | 3.57 (1.70, 7.48) | |
| P value for interaction | 0.048 | 0.003 | 0.004 | 0.937 | |
| Elevated blood pressure | |||||
| No | 4.88 (3.28, 7.28) | 9.77 (6.24, 15.30) | 24.84 (14.40, 42.85) | 3.44 (1.95, 6.09) | |
| Yes | 8.87 (1.94, 40.54) | 9.67 (2.16, 43.34) | 18.52 (3.37, 101.87) | 2.27 (0.21, 24.68) | |
| P value for interaction | 0.990 | 0.199 | 0.076 | 0.396 | |
| Psychological distressd | |||||
| No | 7.04 (4.15, 11.94) | 14.53 (8.08, 26.10) | 36.89 (18.87, 72.10) | 4.43 (2.10, 9.34) | |
| Yes | 3.70 (2.10, 6.52) | 6.44 (3.37, 12.29) | 14.14 (6.16, 32.43) | 3.62 (1.59, 8.22) | |
| P value for interaction | 0.052 | 0.024 | 0.015 | 0.541 | |
Abbreviations: BMI, body mass index; IDF, International Diabetes Federation; OR, odds ratio; 95% CI, 95% confidence interval.
Except for the stratified variables, the following variables were adjusted for: age, sex, education, recreational physical activity participation, alcohol consumption, cigarette smoking, use of energy drinks, waist-to-hip ratio, and psychological distress.
Abdominal obesity was defined by the International Diabetes Federation (IDF) criteria for South Asians: WC ≥ 90 cm for men and waist circumference (WC) ≥ 80 cm for women.
Except for the stratified variables, the following variables were adjusted for: age, sex, education, recreational physical activity participation, alcohol consumption, cigarette smoking, use of energy drinks, body mass index, and psychological distress.
Psychological distress was evaluated by the General Health Questionnaire 12-item scale.
4. Discussion
In this large cross-sectional study, 6.3% of college students in Thailand were at a high risk of OSA. Although the prevalence of obesity in our study population was lower than that in US adults [1], we found consistent and robust associations of OSA with general and abdominal obesity, regardless of the obesity criteria used. To our knowledge, this is the first study to evaluate OSA and its association with both general obesity and abdominal obesity concurrently among young adults in Asia.
4.1 OSA Prevalence and OSA Related Risk Factors
OSA is a common disorder and remains underdiagnosed in the general population. The prevalence of OSA in our Thai college students was relatively low when compared with middle-aged and old adults in the US [25] but higher than other populations such as young college students in Hong Kong [28]. In the 2005 Sleep in America Poll of the National Sleep Foundation, the prevalence of OSA among middle-aged adults (mean age: 49 years) was 26% (men: 31%; women: 21%) [25]. The prevalence of OSA was 6% in a large, relatively healthy, community-based cross-sectional study of 22,389 volunteer blood donors aged 16–84 years in New Zealand [20]. A study conducted in a university student population in Hong Kong found that 25.7% of first-year college students reported snoring, while the estimated prevalence of sleep-disordered breathing (SDB) was only 0.1% among college students [28]. Although snoring was prevalent, SDB was uncommon in this Chinese college student population. The Hong Kong study reported that neck circumference did not predict the occurrence of SDB [28].
Predictors of high risk for OSA have been reported, including male gender, older age, alcohol consumption, and cigarette smoking [29]. Consistent with previous research, we found similar results. We also found that the use of energy drinks was related to a higher risk of OSA, which has not been reported substantially in the literature. The fact that most of the risk factors for OSA such as energy drinks and cigarette smoking are modifiable indicates the need to develop appropriate intervention programs among college students.
4.2 The Association between OSA and Obesity
Although obesity has been reported as a risk factor of OSA [12, 20], the bidirectional association between OSA and obesity is unclear. The Wisconsin Sleep Cohort Study of 690 middle-aged US adults reported that obesity was associated with a significantly increased prevalence of OSA [12]. A small cross-sectional survey of 370 young adults and adults in Nigeria [30] reported that overall 19% of participants had a high risk of OSA defined by the Berlin Questionnaire, and adults with OSA were more likely to be obese [30]. Another cross-sectional study in Korea reported that middle-aged adults with OSA had a higher BMI, WC, and percent body fat than those without OSA [21].
Consistent with the Korean study reporting a significant and independent association between OSA and visceral obesity [21], we found that Thai college students with OSA were more likely to have abdominal obesity than those without OSA independent of potential confounders. Furthermore, we found that OSA was related to a much higher risk of being both general obesity and abdominal obesity, indicating that early screening, diagnosis, and treatment of OSA could be important in reducing obesity and related comorbidities among young adults.
4.3 Potential Mechanism of the Association between OSA and Obesity
Data from animal and human studies provide a biological plausibility to the notion that OSA may activate pathways that lead to obesity, insulin resistance, atherosclerosis, hypertension, and cardiovascular disease [8]. OSA is associated with increased sympathetic activity, sleep disturbance, oxidative stress, systemic inflammation, insulin resistance, and changes in leptin, ghrelin, and orexin levels, which potentially lead to excess weight or obesity [8, 9]. Obesity can increase fat deposits around the upper airway, narrow the upper airway, and diminish the activity of the muscles in this region, collapsing the airway during sleep [8, 31]. Hormonal changes, changes in neuromuscular tone, and lifestyle factors such as energy drinks and alcohol consumption may contribute to the development of OSA among young adults [32, 33]. The lack of early diagnosis and treatment of OSA and obesity might form a vicious cycle where each aggravates the other [8].
4.4 Clinical and Public Health Implications
Our findings suggest that clinical and public health programs that target screening and treatment of OSA may help reduce the risk of obesity and its related cardiovascular morbidity among college students and other young adults. Identifying individuals at a high risk of OSA and targeting them for cardiovascular disease control may be important to mitigate risk for future morbidity and premature mortality [16, 17]. Effectively treated OSA was associated with a significant decline in health care use when compared to OSA patients who were not effectively treated [34], and undiagnosed OSA patients incurred much higher medical costs than controls without OSA [35]. Given the fact that OSA is an independent risk factor for many chronic diseases, our study emphasizes the need for recognition and appropriate management of OSA among young adults to reduce the prevalence of obesity and other cardiovascular diseases.
4.5 Strengths and Limitations
Our study has several strengths. First, this is a large population-based cross-sectional study of college students conducted in Thailand. Second, although polysomnography is the gold standard test for OSA diagnosis in clinical settings [36, 37]. it is expensive and time consuming, and is not available at most primary care centers or in general populations [38]. The Berlin Questionnaire is a validated instrument that has been used widely to identify individuals who are at risk for OSA [25]. Third, we used several robust statistical approaches and different definitions of obesity to evaluate the associations between OSA and general obesity and abdominal obesity. We also conducted stratified analyses and found strong and significant associations between OSA and obesity varied little by sex, blood pressure level and mental health status.
Our study has limitations. First, we did not use random sampling, but instead considered subjects who were willing to participate in the study, so it was a convenient sample. Hence, our findings may be subject to volunteer bias. Second, our study only included full-time Thai college students and did not include students taking classes in correspondence, extension, or night school programs. Thus, the results might not be generalized to those students. Third, as this was a cross-sectional study, we were unable to determine the causal association between OSA and obesity. In addition, although the Berlin Questionnaire has been validated as a screening tool for OSA in general and clinical populations, some researchers have reported a lower sensitivity for screening health care workers for OSA [39]. It is possible that the Berlin Questionnaire might have a high proportion of false negatives, the prevalence of OSA may have been underestimated in our study population. Future prospective studies are warranted to thoroughly elucidate bi-directional associations between OSA and obesity among college students and other young adults.
In conclusion, our study shows significant associations of OSA with general and abdominal obesity among Thai college students, suggesting that OSA could be a risk factor for obesity and related cardiovascular morbidities. Our findings have clinical and public health implications for OSA screening and treatment among young adults. Our study highlights the need for increased awareness of OSA risk and its adverse health consequences among young adults.
Acknowledgements
This work was supported by National Institutes of Health (NIH) and National Institute on Minority Health and Health Disparities (NIMHD) grants (T37-MD001449) and NIH/NCRR/NCATS (8UL1TR000170).
Footnotes
Competing Interests
The authors have no competing interests.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Journal of the American Medical Association. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. http://dx.doi.org/10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PW, Ghushchyan V, Ben-Joseph RH. The effect of obesity and cardiometabolic risk factors on expenditures and productivity in the United States. Obesity (Silver Spring) 2008;16(9):2155–2162. doi: 10.1038/oby.2008.325. http://dx.doi.org/10.1038/oby.2008.325. [DOI] [PubMed] [Google Scholar]
- 3.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obesity Reviews. 2001;2(3):173–182. doi: 10.1046/j.1467-789x.2001.00032.x. http://dx.doi.org/10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. The American Journal of Clinical Nutrition. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho T, Goel K, Correa de Sa D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of "normal weight central obesity". Journal of the American College of Cardiology. 2013;61(5):553–560. doi: 10.1016/j.jacc.2012.10.035. http://dx.doi.org/10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Ge KY, Da-Wei F. The magnitude and trends of under- and over-nutrition in Asian countries. Biomedical and Environmental Sciences. 2001;14(1–2):53–60. [PubMed] [Google Scholar]
- 7.Jitnarin N, Kosulwat V, Rojroongwasinkul N, Boonpraderm A, Haddock CK, Poston WS. Prevalence of overweight and obesity in Thai population: results of the National Thai Food Consumption Survey. Eating and Weight Disorders. 2011;16(4):e242–e249. doi: 10.1007/BF03327467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care 2008. 2008;31(Suppl 2):S303–S309. doi: 10.2337/dc08-0715. http://dx.doi.org/10.2337/dc08-s272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42(6):1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. http://dx.doi.org/10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 10.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep. 1997;20(1):65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46(2):85–90. doi: 10.1136/thx.46.2.85. http://dx.doi.org/10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. Journal of the American Medical Association. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. http://dx.doi.org/10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 13.Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med. 2013;13:10. doi: 10.1186/1471-2466-13-10. http://dx.doi.org/10.1186/1471-2466-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. Journal of the American Medical Association. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 15.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23(Suppl 4):S122–S126. [PubMed] [Google Scholar]
- 16.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. The New England Journal of Medicine. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 17.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, De la Cruz-Moron I, Perez-Ronchel J, De la Vega-Gallardo F, Fernandez-Palacin A. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128(2):624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 18.Pendlebury ST, Pepin JL, Veale D, Levy P. Natural evolution of moderate sleep apnoea syndrome: significant progression over a mean of 17 months. Thorax. 1997;52(10):872–878. doi: 10.1136/thx.52.10.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sforza E, Addati G, Cirignotta F, Lugaresi E. Natural evolution of sleep apnoea syndrome: a five year longitudinal study. The European Respiratory Journal. 1994;7(10):1765–1770. doi: 10.1183/09031936.94.07101765. http://dx.doi.org/10.1183/09031936.94.07101765. [DOI] [PubMed] [Google Scholar]
- 20.Wilsmore BR, Grunstein RR, Fransen M, Woodward M, Norton R, Ameratunga S. Sleep, blood pressure and obesity in 22,389 New Zealanders. Internal Medicine Journal 2012. 2012;42(6):634–641. doi: 10.1111/j.1445-5994.2012.02753.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim NH, Lee SK, Eun CR, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Yun CH, Shin C. Short Sleep Duration Combined with Obstructive Sleep Apnea is Associated with Visceral Obesity in Korean Adults. Sleep. 2013;36(5):723–729. doi: 10.5665/sleep.2636. http://dx.doi.org/10.5665/sleep.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohsoonthorn V, Khidir H, Casillas G, Lertmaharit S, Tadesse MG, Pensuksan WC, Rattananupong T, Gelaye B, Williams MA. Sleep quality and sleep patterns in relation to consumption of energy drinks, caffeinated beverages, and other stimulants among Thai college students. Sleep and Breathing. 2013;17(3):1017–1028. doi: 10.1007/s11325-012-0792-1. http://dx.doi.org/10.1007/s11325-012-0792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chierakul N, Chaipattarapol C, Ruttanaumpawan P, Nana A, Naruman C, Tangchityongsiva S. Comparison of clinical and polysomnographic characteristics of non-obese and obese patients with obstructive sleep apnea. Journal of the Medical Association of Thailand. 2007;90(Suppl 2):48–53. [PubMed] [Google Scholar]
- 24.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41(6):1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 25.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130(3):780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 26.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, Rutter C. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychological Medicine. 1997;27(1):191–197. doi: 10.1017/s0033291796004242. [DOI] [PubMed] [Google Scholar]
- 28.Hui DS, Chan JK, Ho AS, Choy DK, Lai CK, Leung RC. Prevalence of snoring and sleep-disordered breathing in a student population. Chest. 1999;116(6):1530–1536. doi: 10.1378/chest.116.6.1530. [DOI] [PubMed] [Google Scholar]
- 29.Koyama RG, Esteves AM, Oliveira ESL, Lira FS, Bittencourt LR, Tufik S, de Mello MT. Prevalence of and risk factors for obstructive sleep apnea syndrome in Brazilian railroad workers. Sleep Medicine. 2012;13(8):1028–1032. doi: 10.1016/j.sleep.2012.06.017. http://dx.doi.org/10.1016/j.sleep.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Adewole OO, Hakeem A, Fola A, Anteyi E, Ajuwon Z, Erhabor G. Obstructive sleep apnea among adults in Nigeria. Journal of the National Medical Association. 2009;101(7):720–725. doi: 10.1016/s0027-9684(15)30983-4. [DOI] [PubMed] [Google Scholar]
- 31.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiological Reviews. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. http://dx.doi.org/10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh V, Pandey S, Singh A, Gupta R, Prasad R, Singh Negi MP. Study pattern of snoring and associated risk factors among medical students. Bioscience trends. 2012;6(2):57–62. [PubMed] [Google Scholar]
- 33.Pullman AW, Masters RC, Zalot LC, Carde LE, Saraiva MM, Dam YY, Randall Simpson JA, Duncan AM. Effect of the transition from high school to university on anthropometric and lifestyle variables in males. Applied Physiology, Nutrition, and Metabolism. 2009;34(2):162–171. doi: 10.1139/H09-007. http://dx.doi.org/10.1139/H09-007. [DOI] [PubMed] [Google Scholar]
- 34.Bahammam A, Delaive K, Ronald J, Manfreda J, Roos L, Kryger MH. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999;22(6):740–747. doi: 10.1093/sleep/22.6.740. [DOI] [PubMed] [Google Scholar]
- 35.Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, Psaty BM. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22(6):749–755. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 36.Goncalves SC, Martinez D, Gus M, de Abreu-Silva EO, Bertoluci C, Dutra I, Branchi T, Moreira LB, Fuchs SC, de Oliveira AC, Fuchs FD. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132(6):1858–1862. doi: 10.1378/chest.07-1170. [DOI] [PubMed] [Google Scholar]
- 37.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. British Medical Journal. 2000;320(7233):479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang KP, Terris DJ. Screening for obstructive sleep apnea: an evidence-based analysis. Am J Otolaryngology. 2006;27(2):112–118. doi: 10.1016/j.amjoto.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Geiger-Brown J, Rogers VE, Han K, Trinkoff A, Bausell RB, Scharf SM. Occupational screening for sleep disorders in 12-h shift nurses using the Berlin Questionnaire. Sleep and Breathing. 2013;17(1):381–388. doi: 10.1007/s11325-012-0705-3. http://dx.doi.org/10.1007/s11325-012-0705-3. [DOI] [PubMed] [Google Scholar]